Possibility of Breast Cancer Prevention: Use of Soy Isoflavones and Fermented Soy Beverage Produced Using Probiotics
Abstract
:1. Soy Milk and Its Active Ingredients
2. Fermentation of Soy Milk
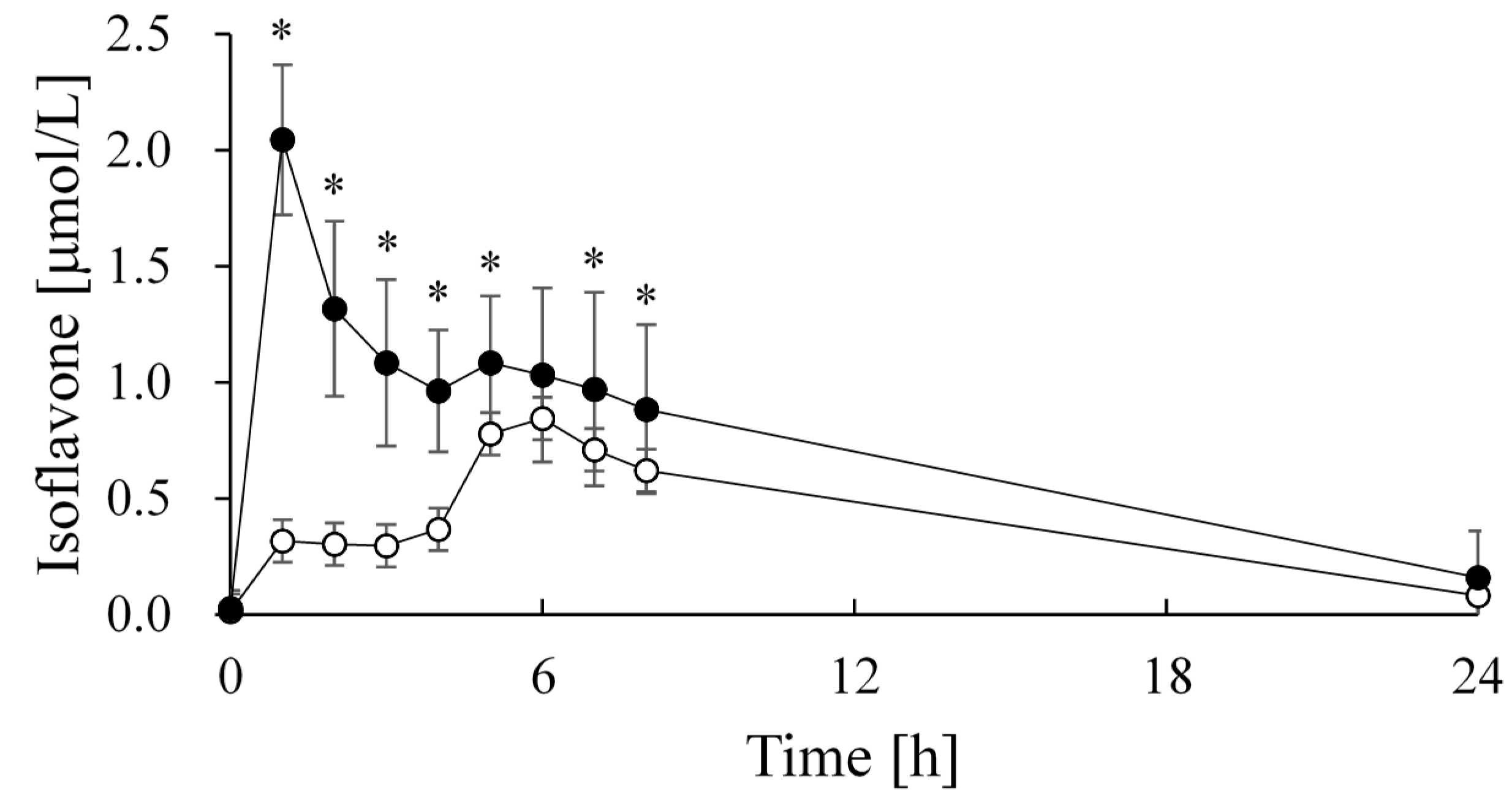
| Group | Cmax (μmol/L) | tmax (h) | AUC (μmol·24 h/L) |
|---|---|---|---|
| Soy milk | 0.95 ± 0.38 | 5.9 ± 1.3 | 9.55 ± 3.37 |
| FSM beverage | 2.04 ± 0.32 * | 1.0 ± 0.0 * | 17.30 ± 6.38 * |
3. Breast Cancer
3.1. Incidence: Differences between Western and Asian Countries
3.2. Risk Factors and Lifestyle
4. Epidemiological Study on Breast Cancer
5. Non-Clinical Study of Breast Cancer Prevention
| Group | Incidence (%) † | Multiplicity (tumors/rat) ‡ | Volume (cm3/tumor) § | Tumor Tissue Profile | |
|---|---|---|---|---|---|
| ER-α (%) | Ki-67 (%) | ||||
| Control | 73.8 (31/42) | 2.7 ± 0.5 | 1.3 ± 0.2 | 55.1 ± 1.7 | 25.9 ± 1.0 |
| Soy milk | 59.5 (25/42) | 1.6 ± 0.3 | 2.5 ± 1.1 | 57.1 ± 1.6 | 24.5 ± 1.2 |
| LcS | 73.8 (31/42) | 3.0 ± 0.5 | 0.8 ± 0.1 | 60.8 ± 1.5 | 24.5 ± 1.0 |
| LcS + Soy milk | 59.5 (25/42) | 1.2 ± 0.2 * | 0.8 ± 0.2 | 47.2 ± 2.0 | 21.8 ± 1.4 |


6. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Scheiber, M.D.; Liu, J.H.; Subbiah, M.T.; Rebar, R.W.; Setchell, K.D. Dietary inclusion of whole soy foods results in significant reductions in clinical risk factors for osteoporosis and cardiovascular disease in normal postmenopausal women. Menopause 2001, 8, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.J.; Persky, V.; Setchell, K.D.; Barnes, S. Soy intake and cancer risk: A review of the in vitro and in vivo data. Nutr. Cancer 1994, 21, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Cassidy, A. Dietary isoflavones: Biological effects and relevance to human health. J. Nutr. 1999, 129, 758S–767S. [Google Scholar] [PubMed]
- Molteni, A.; Brizio-Molteni, L.; Persky, V. In vitro hormonal effects of soybean isoflavones. J. Nutr. 1995, 125, 751S–756S. [Google Scholar] [PubMed]
- Akiyama, T.; Ishida, J.; Nakagawa, S.; Ogawara, H.; Watanabe, S.; Itoh, N.; Shibuya, M.; Fukami, Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987, 262, 5592–5595. [Google Scholar] [PubMed]
- Su, S.J.; Yeh, T.M.; Chuang, W.J.; Ho, C.L.; Chang, K.L.; Cheng, H.L.; Liu, H.S.; Cheng, H.L.; Hsu, P.Y.; Chow, N.H. The novel targets for anti-angiogenesis of genistein on human cancer cells. Biochem. Pharmacol. 2005, 69, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.L.; McCay, J.A.; Zhang, L.X.; Brown, R.D.; You, L.; Karrow, N.A.; Germolec, D.R.; White, K.L., Jr. Genistein modulates immune responses and increases host resistance to B16F10 tumor in adult female B6C3F1 mice. J. Nutr. 2001, 131, 3251–3258. [Google Scholar] [PubMed]
- Dijsselbloem, N.; Vanden, B.W.; De, N.A.; Haegeman, G. Soy isoflavone phyto-pharmaceuticals in interleukin-6 affections. Multi-purpose nutraceuticals at the crossroad of hormone replacement, anti-cancer and anti-inflammatory therapy. Biochem. Phamacol. 2004, 68, 1171–1185. [Google Scholar] [CrossRef]
- Husain, D.; Khanna, K.; Puri, S.; Haghighizadeh, M. Supplementation of soy isoflavones improved sex hormones, blood pressure, and postmenopausal symptoms. J. Am. Coll. Nutr. 2015, 34, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Wangen, K.E.; Duncan, A.M.; Xu, X.; Kurzer, M.S. Soy isoflavones improve plasma lipids in normocholesterolemic and mildly hypercholesterolemic postmenopausal women. Am. J. Clin. Nutr. 2001, 73, 225–231. [Google Scholar] [PubMed]
- Lagari, V.S.; Levis, S. Phytoestrogens in the prevention of postmenopausal bone loss. J. Clin. Densitom. 2013, 16, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Lethaby, A.; Marjoribanks, J.; Kronenberg, F.; Roberts, H.; Eden, J.; Brown, J. Phytoestrogens for menopausal vasomotor symptoms. Cochrane Database Syst. Rev. 2013, 10, CD001395. [Google Scholar]
- Nagata, C.; Mizoue, T.; Tanaka, K.; Tsuji, I.; Tamakoshi, A.; Matsuo, K.; Wakai, K.; Inoue, M.; Tsugane, S.; Sasazuki, S.; et al. Soy intake and breast cancer risk: An evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn. J. Clin. Oncol. 2014, 44, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Chen, M.L.; Qin, Y.; Zhang, Q.Y.; Xu, H.X.; Zhou, Y.; Mi, M.T.; Zhu, J.D. Isoflavone consumption and risk of breast cancer: A dose-response meta-analysis of observational studies. Asia Pac. J. Clin. Nutr. 2013, 22, 118–127. [Google Scholar] [PubMed]
- Wang, H.G.; Murphy, P.A. Isoflavone content in commercial soybean foods. J. Agric. Food Chem. 1994, 42, 1666–1673. [Google Scholar] [CrossRef]
- Coward, L.; Barnes, N.C.; Setchell, K.D.R.; Barnes, S. Genistein, daidzein and their β-glycocide conjugates: Antitumor isoflavones in soybean foods from American and Asian diets. J. Agric. Food Chem. 1993, 41, 1961–1967. [Google Scholar] [CrossRef]
- Setchell, K.D.; Brown, N.M.; Zimmer-Nechemias, L.; Brashear, W.T.; Wolfe, B.E.; Kirschner, A.S.; Heubi, J.E. Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role of intestinal metabolism for bioavailability. Am. J. Clin. Nutr. 2002, 76, 447–453. [Google Scholar] [PubMed]
- Atkinson, C.; Frankenfeld, C.L.; Lampe, J.W. Gut bacterial metabolism of the soy isoflavone daidzein: Exploring the relevance to human health. Exp. Biol. Med. (Maywood) 2005, 230, 155–170. [Google Scholar]
- Bressani, R.; Elías, L.G. Processed vegetable protein mixtures for human consumption in developing countries. Adv. Food Res. 1968, 66, 1–103. [Google Scholar]
- Murti, T.W.; Bouillanne, C.; Landon, M.; Desmazeaud, M.J. Bacterial growth and volatile compounds in yoghurt-type products from soymilk containing Bifidobacterium spp. J. Food Sci. 1992, 1, 153–157. [Google Scholar]
- Shimakawa, Y.; Matsubara, S.; Yuki, N.; Ikeda, M.; Ishikawa, F. Evaluation of Bifidobacterium breve strain Yakult-fermented soymilk as a probiotic food. Int. J. Food Microbiol. 2003, 81, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.L.; Huang, H.Y.; Chou, C.C. Transformation of isoflavone phytoestrogens during the fermentation of soymilk with lactic acid bacteria and bifidobacteria. Food Microbiol. 2006, 23, 772–778. [Google Scholar] [CrossRef]
- Kano, M.; Takayanagi, T.; Harada, K.; Sawada, S.; Ishikawa, F. Bioavailability of isoflavones after ingestion of soy beverages in healthy adults. J. Nutr. 2006, 136, 2291–2296. [Google Scholar] [PubMed]
- Kano, M.; Ishikawa, F. Nutrition and Alcohol—Linking Nutrient Interactions and Dietary Intake; Watson, R., Preedy, V.R., Eds.; CRC Press: New York, NY, USA, 2004; pp. 301–311. [Google Scholar]
- Kano, M.; Ishikawa, F.; Matsubara, S.; Kikuchi-Hayakawa, H.; Shimakawa, Y. Soymilk products affect ethanol absorption and metabolism in rats during acute and chronic ethanol intake. J. Nutr. 2002, 132, 238–244. [Google Scholar] [PubMed]
- Kikuchi-Hayakawa, H.; Onodera, N.; Matsubara, S.; Yasuda, E.; Chonan, O.; Talahashi, R.; Ishikawa, F. Effects of soy milk and Bifidobacterium fermented soy milk on lipid metabolism in aged ovariectomized rats. Biosci. Biotechnol. Biochem. 1998, 62, 1688–1692. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi-Hayakawa, H.; Onodera, N.; Matsubara, S.; Yasuda, E.; Shimakawa, Y.; Takahashi, R.; Ishikawa, F. Effects of soya milk and Bifidobacterium-fermented soya milk on plasma and liver lipids, and faecal steroids in hamsters fed on a cholesterol-free or cholesterol-enriched diet. Br. J. Nutr. 1998, 79, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi-Hayakawa, H.; Onodera-Masuoka, N.; Kano, M.; Matsubara, S.; Yasuda, E.; Ishikawa, F. Effect of soy milk and Bifidobacterium-fermented soy milk on plasma and liver lipids in ovariectomized syrian hamsters. J. Nutr. Sci. Vitaminol. 2000, 46, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.O.; Jakesz, R. Breast cancer issues in developing countries: An overview of the Breast Health Global Initiative. World J. Surg. 2008, 32, 2578–2585. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, Y.; Zhang, M. Comparison of time trends in breast cancer incidence (1973–2002) in Asia, from cancer incidence in five continents, Vols IV-IX. Jpn. J. Clin. Oncol. 2009, 39, 411–412. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.R.; Boniol, M.; Joubert, C.; Hery, C.; Haukka, J.; Autier, P.; Nishino, Y.; Sobue, T.; Chen, C.J.; You, S.L.; et al. Secular trends in breast cancer mortality in five East Asian populations: Hong Kong, Japan, Korea, Singapore and Taiwan. Cancer Sci. 2010, 101, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- About Cancer Statistics in Japan 2009. Center for Cancer controland information services, National Cancer Center. Available online: http://ganjoho.ncc.go.jp/public/statistics/backnumber/2009_en.html (accessed on 21 February 2011).
- Harkinson, S.; Tamimi, R.; Hunter, D. Textbooks of cancer epidemiology, 2nd Edition; Adami, H.O., Hunter, D., Trichopoulos, D., Eds.; Oxford University Press: New York, NY, USA, 2008; Chapter 16. [Google Scholar]
- Nagata, C.; Kawakami, N.; Shimizu, H. Trends in the incidence rate and risk factors for breast cancer in Japan. Breast Cancer Res. Treat. 1997, 44, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Amine, E.K.; Baba, N.H.; Belhadj, M.; Deurenberg-Yap, M.; Djazayery, A.; Forrester, T.; Galuska, D.A.; Herman, S.; James, W.P.T.; M’Buyamba Kabangu, J.R.; et al. Report of A Joint WHO/FAO Expert Consultation. Diet, Nutrition, and the Prevention of Chronic Diseases. In Presented at World Health Organization, Geneva, Switzerland, 28 January–1 February 2002.
- Parkin, S.L.; Whelan, S.L.; Ferlay, J.; Teppo, L.; Thomas, D.B. (Eds.) Cancer Incidence in Five Contrinents Vol VIII; IARC Scientific Publications: Lyon, France, 2002; No. 155.
- Toi, M.; Hirota, S.; Tomotaki, A.; Sato, N.; Nozumi, Y.; Anan, K.; Nagashima, T.; Tokuda, Y.; Masuda, N.; Ohsumi, N.; et al. Probiotic beverage with soy isoflavone consumption for breast cancer prevention: A case-control study. Curr. Nutr. Food Sci. 2013, 9, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Aso, Y.; Akaza, H.; Kotake, T.; Tsukamoto, T.; Imai, K.; Naito, S. Preventive effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer in a double-blind trial. The BLP Study Group. Eur. Urol. 1995, 27, 104–109. [Google Scholar] [PubMed]
- Ohashi, Y.; Nakai, S.; Tsukamoto, T.; Masumori, N.; Akaza, H.; Miyanaga, N.; Kitamura, T.; Kawabe, K.; Kotake, T.; Kuroda, M.; et al. Habitual intake of lactic acid bacteria and risk reduction of bladder cancer. Urol. Int. 2002, 68, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Naito, S.; Koga, H.; Yamaguchi, A.; Fujimoto, N.; Hasui, Y.; Kuramoto, H.; Iguchi, A.; Kinukawa, N.; Kyushu University Urological Oncology Group. Prevention of recurrence with epirubicin and Lactobacillus casei after transurethral resection of bladder cancer. J. Urol. 2008, 179, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Akedo, I.; Otani, T.; Suzuki, T.; Nakamura, T.; Takeyama, I.; Ishiguro, S.; Miyaoka, E.; Sobue, T.; Kakizoe, T. Randomized trial of dietary fiber and Lactobacillus casei administration for prevention of colorectal tumors. Int. J. Cancer 2005, 116, 762–767. [Google Scholar] [CrossRef] [PubMed]
- De Moreno de LeBlanc, A.; Matar, C.; LeBlanc, N.; Perdigón, G. Effects of milk fermented by Lactobacillus helveticus R389 on a murine breast cancer model. Breast Cancer Res. 2005, 7, R477–R486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takagi, A.; Ikemura, H.; Matsuzaki, T.; Sato, M.; Nomoto, K.; Morotomi, M.; Yokokura, T. Relationship between the in vitro response of dendritic cells to Lactobacillus and prevention of tumorigenesis in the mouse. J. Gastroenterol. 2008, 43, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.R.; Hsieh, S.C.; Huang, H.Y.; Chou, C.C. Effect of lactic fermentation on the total phenolic, saponin and phytic acid contents as well as anti-colon cancer cell proliferation activity of soymilk. J. Biosci. Bioeng. 2013, 115, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.H.; Liu, J.J.; Chen, C.H.; Huang, T.S.; Lu, F.J. Growth inhibition and induction of apoptosis in MCF-7 breast cancer cells by fermented soy milk. Nutr. Cancer 2002, 43, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Nakatsugi, S.; Watanabe, K.; Kawamori, T.; Ishikawa, F.; Morotomi, M.; Sugie, S.; Toda, T.; Sugimura, T.; Wakabayashi, K. Inhibitory effects of Bifidobacterium-fermented soy milk on 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine-induced rat mammary carcinogenesis, with a partial contribution of its component isoflavones. Carcinogenesis 2000, 21, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Kaga, C.; Takagi, A.; Kano, M.; Kado, S.; Kato, I.; Sakai, M.; Miyazaki, K.; Nanno, M.; Ishikawa, F.; Ohashi, Y.; et al. Lactobacillus casei Shirota enhances the preventive efficacy of soymilk in chemically induced breast cancer. Cancer Sci. 2013, 104, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Takagi, A.; Matsuzaki, T.; Sato, M.; Nomoto, K.; Morotomi, M.; Yokokura, T. Enhancement of natural killer cytotoxicity delayed murine carcinogenesis by a probiotic microorganism. Carcinogenesis 2001, 22, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Nagao, F.; Nakayama, M.; Muto, T.; Okumura, K. Effects of a fermented milk drink containing Lactobacillus casei strain Shirota on the immune system in healthy human subjects. Biosci. Biotechnol. Biochem. 2000, 64, 2706–2708. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Rowland, I.; Thomas, L.V.; Yaqoob, P. Immunomodulatory effects of a probiotic drink containing Lactobacillus casei Shirota in healthy older volunteers. Eur. J. Nutr. 2013, 52, 1853–1863. [Google Scholar] [CrossRef] [PubMed]
- Reale, M.; Boscolo, P.; Bellante, V.; Tarantelli, C.; di Nicola, M.; Forcella, L.; Li, Q.; Morimoto, K.; Muraro, R. Daily intake of Lactobacillus casei Shirota increases natural killer cell activity in smokers. Br. J. Nutr. 2012, 108, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Kaga, C.; Takagi, A.; Kano, M. Chemopreventive effect of soymilk fermented with probiotics. In Preparation.
- Fritz, H.; Seely, D.; Flower, G.; Skidmore, B.; Fernandes, R.; Vadeboncoeur, S.; Kennedy, D.; Cooley, K.; Wong, R.; Sagar, S.; et al. Soy, red clover, and isoflavones and breast cancer: A systematic review. PLoS ONE 2013, 8, e81968. [Google Scholar] [CrossRef] [PubMed]
- Yamori, Y. Food factors for atherosclerosis prevention: Asian perspective derived from analyses of worldwide dietary biomarkers. Exp. Clin. Cardiol. 2006, 11, 94–98. [Google Scholar] [PubMed]
- Shu, X.O.; Zheng, Y.; Cai, H.; Gu, K.; Chen, Z.; Zheng, W.; Lu, W. Soy food intake and breast cancer survival. JAMA 2009, 302, 2437–2443. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, R.K.; Anderson, W.F.; Yamamoto, S.; Tsukuma, H.; Pfeiffer, R.M.; Kobayashi, K.; Devesa, S.S.; Levine, P.H. Early- and late-onset breast cancer types among women in the United States and Japan. Cancer Eprdemiol. Biomark. Prev. 2007, 16, 1437–1442. [Google Scholar] [CrossRef]
- Prentice, R.L.; Garnet, L. The women’s health initiative: Lessons learned. Ann. Rev. Public Health 2008, 29, 131–150. [Google Scholar] [CrossRef]
- Prentice, R.L.; Caan, B.; Chlebowski, R.T.; Patterson, R.; Kuller, L.H.; Ockene, J.K.; Margolis, K.L.; Limacher, M.C.; Manson, J.E.; Parker, L.M.; et al. Low-fat dietary pattern and risk of invasive breast cancer: The women’s health initiative randomized controlled dietary modification trial. JAMA 2006, 295, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J.; Li, Q.; Melnichouk, O.; Greenberg, C.; Minkin, S.; Hislop, G.; Boyd, N.F. A randomized trial of dietary intervention for breast cancer prevention. Cancer Res. 2011, 71, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, R.T.; Blackburn, G.L.; Thomson, C.A.; Nixon, D.W.; Shapiro, A.; Hoy, M.K.; Goodman, M.T.; Giuliano, A.E.; Karanja, N.; McAndrew, P.; et al. Dietary fat reduction and breast cancer outcome: Interim efficacy results from the women’s intervention nutrition study. J. Natl. Cancer Inst. 2006, 98, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takagi, A.; Kano, M.; Kaga, C. Possibility of Breast Cancer Prevention: Use of Soy Isoflavones and Fermented Soy Beverage Produced Using Probiotics. Int. J. Mol. Sci. 2015, 16, 10907-10920. https://doi.org/10.3390/ijms160510907
Takagi A, Kano M, Kaga C. Possibility of Breast Cancer Prevention: Use of Soy Isoflavones and Fermented Soy Beverage Produced Using Probiotics. International Journal of Molecular Sciences. 2015; 16(5):10907-10920. https://doi.org/10.3390/ijms160510907
Chicago/Turabian StyleTakagi, Akimitsu, Mitsuyoshi Kano, and Chiaki Kaga. 2015. "Possibility of Breast Cancer Prevention: Use of Soy Isoflavones and Fermented Soy Beverage Produced Using Probiotics" International Journal of Molecular Sciences 16, no. 5: 10907-10920. https://doi.org/10.3390/ijms160510907





