Potential Anti-Cancer Activities and Mechanisms of Costunolide and Dehydrocostuslactone
Abstract
:1. Introduction
2. General Pharmacology
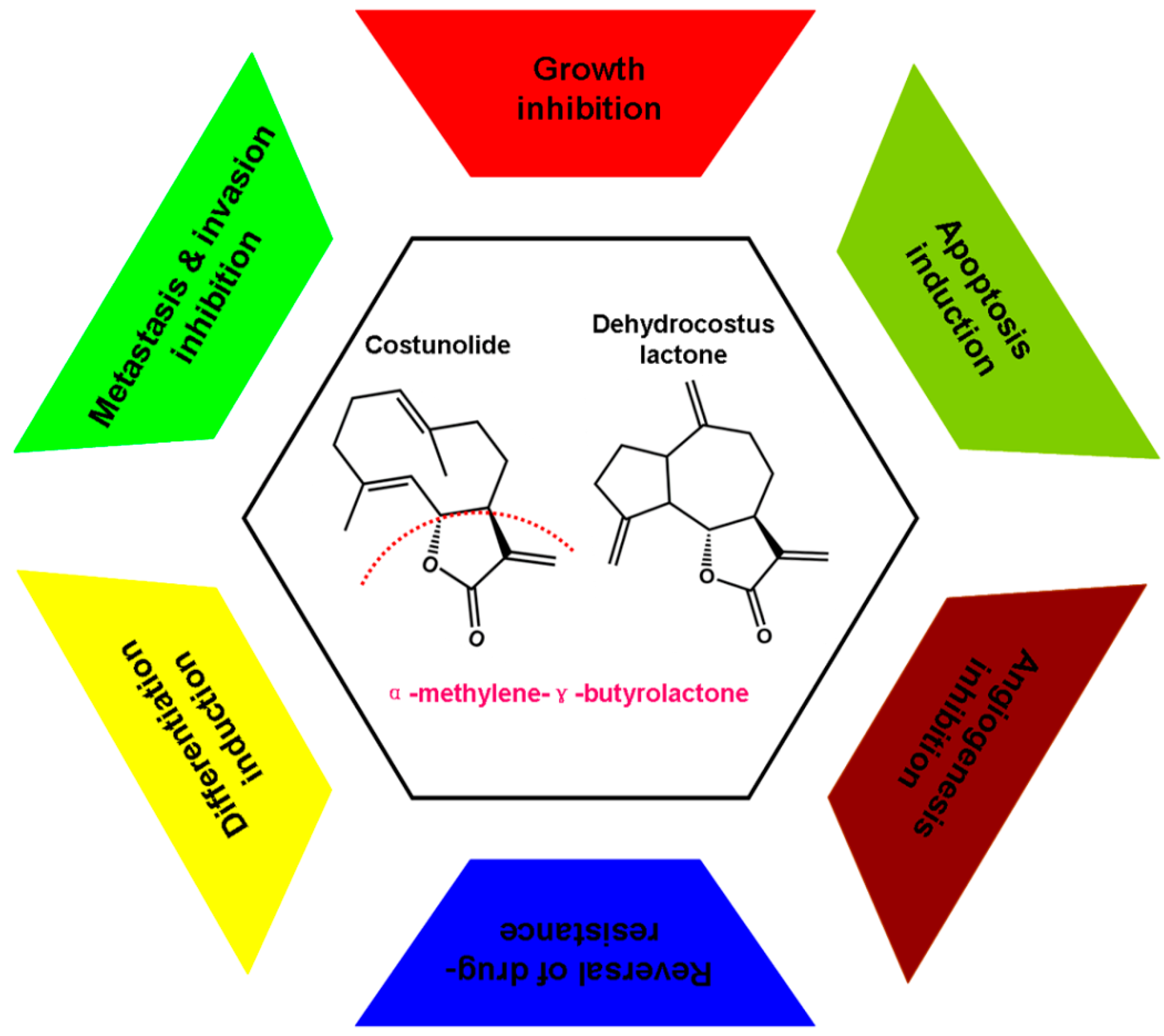
3. Experimental Anti-Cancer Activities and Associated Molecular Mechanisms
4. Inhibition Effect on Cancer Cell Proliferation
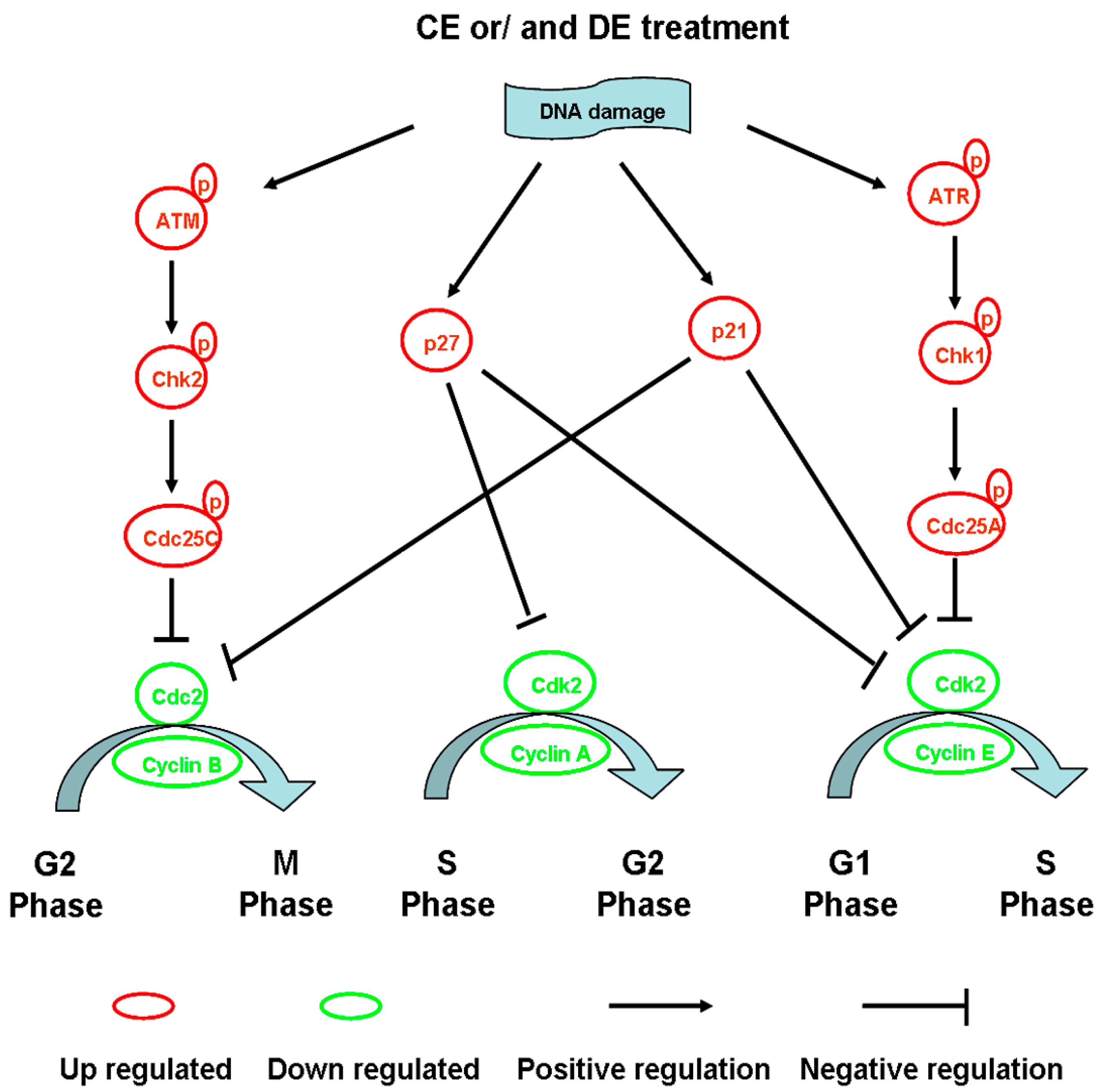
4.1. Modulation of Cell Cycle Progression
4.2. Influence of Tubulin Polymerization
4.3. Inhibition of Telomerase Activity
4.4. Induction of Cell Apoptosis
4.5. The Mitochondria-Dependent Intrinsic Pathway
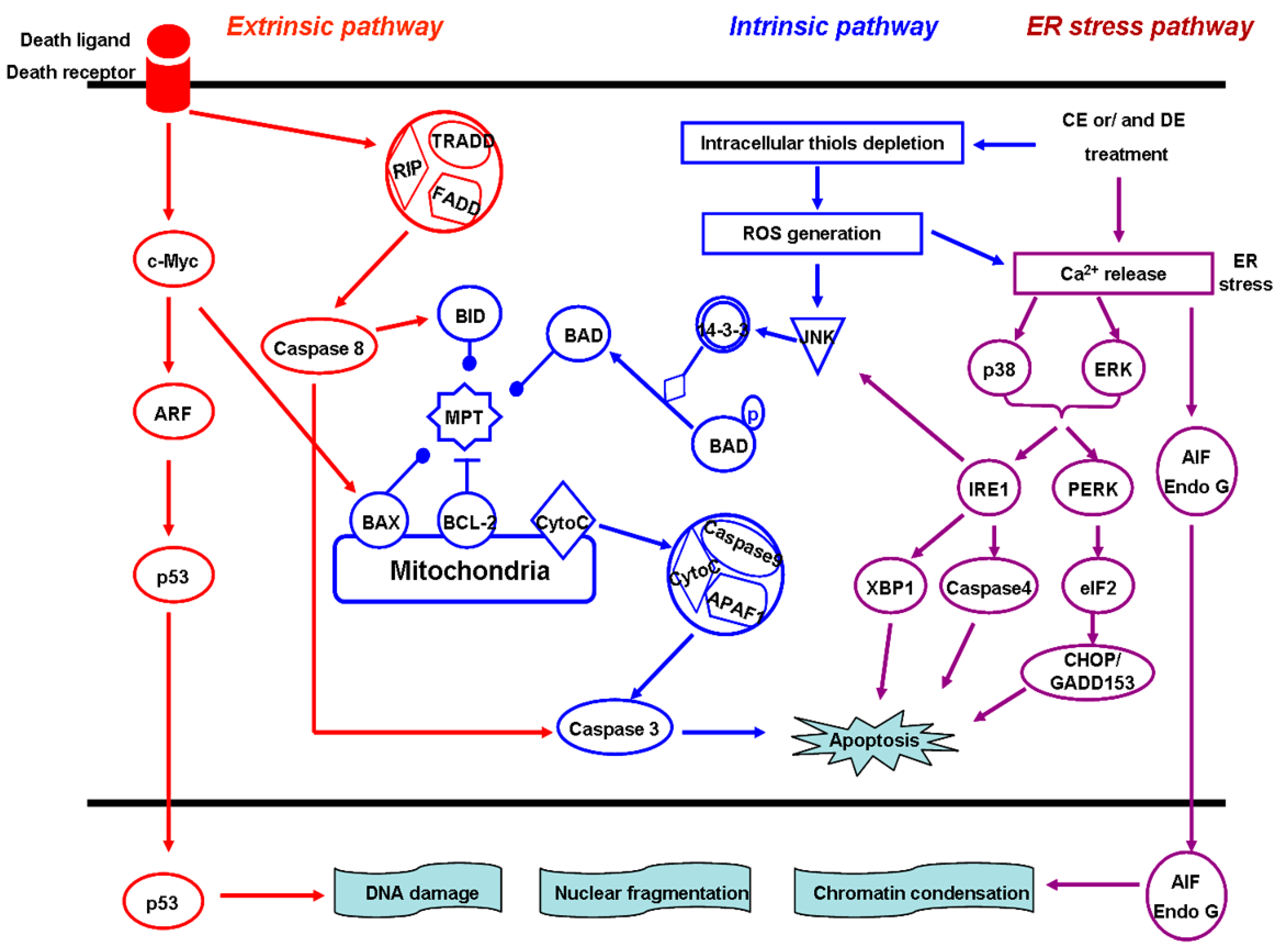
4.6. The Death Receptor-Mediated Extrinsic Pathway
4.7. The ER Stress Pathway
4.8. Anti-Cancer Metastasis and Invasion
5. Reversion of Multidrug Resistance
6. Anti-Angiogenic Activity
7. Induction of Cancer Cell Differentiation
8. Anti-Tumor Activity, Pharmacokinetics and Metabolism of CE and DE in Vivo
9. Conclusions and Future Prospects
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Chai, H.; Kinghorn, A.D. The continuing search for antitumor agents from higher plants. Phytochem. Lett. 2010, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- DeVita, V.T.; Hellman, S.; Rosenberg, S.A. Cancer: Principles and Practice of Oncology, 8th ed.; Lippincott-Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Butturini, E.; Cavalieri, E.; de Prati, A.C.; Darra, E.; Rigo, A.; Shoji, K.; Murayama, N.; Yamazaki, H.; Watanabe, Y.; Suzuki, H.; et al. Two naturally occurring terpenes, dehydrocostuslactone and costunolide, decrease intracellular GSH content and inhibit STAT3 activation. PLoS ONE 2011, 6, e20174. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Chang, H.S.; Chen, I.S.; Chen, C.J.; Hsu, M.L.; Fu, S.L.; Chen, Y.J. Costunolide causes mitotic arrest and enhances radiosensitivity in human hepatocellular carcinoma cells. Radiat. Oncol. 2011, 6, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.L.; Wu, L.Y.; Kuo, P.L. Dehydrocostuslactone, a medicinal plant-derived sesquiterpene lactone, induces apoptosis coupled to endoplasmic reticulum stress in liver cancer cells. J. Pharmacol. Exp. Ther. 2009, 329, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Kim, G.H. Evaluation of anticancer activity of dehydrocostuslactone in vitro. Mol. Med. Rep. 2010, 3, 185–188. [Google Scholar] [PubMed]
- Pitchai, D.; Roy, A.; Banu, S. In vitro and in silico evaluation of NF-κB targeted costunolide action on estrogen receptor-negative breast cancer cells-a comparison with normal breast cells. Phytother. Res. 2014, 28, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Ahn, W.S. Antiproliferative effects of dehydrocostuslactone through cell cycle arrest and apoptosis in human ovarian cancer SK-OV-3 cells. Int. J. Mol. Med. 2009, 23, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.M.; Syu, W.J.; Don, M.J.; Lu, J.J.; Lee, G.H. Cytotoxic sesquiterpene lactones from the root of Saussurea lappa. J. Nat. Prod. 2003, 66, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Lim, S.S.; Park, S.Y.; Shin, H.K.; Kim, J.S.; Park, J.H. Apoptosis of DU145 human prostate cancer cells induced by dehydrocostus lactone isolated from the root of Saussurea lappa. Food Chem. Toxicol. 2008, 46, 3651–3658. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Hong, J.E.; Lim, S.S.; Kwon, G.T.; Kim, J.; Kim, J.S.; Lee, K.W.; Park, J.H. The hexane extract of Saussurea lappa and its active principle, dehydrocostus lactone, inhibit prostate cancer cell migration. J. Med. Food 2012, 15, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Rasul, A.; Bao, R.; Malhi, M.; Zhao, B.; Tsuji, I.; Li, J.; Li, X. Induction of apoptosis by costunolide in bladder cancer cells is mediated through ROS generation and mitochondrial dysfunction. Molecules 2013, 18, 1418–1433. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.; Kumar, T.V.; Sreedhar, E.; Naidu, V.G.; Krishna, S.R.; Babu, K.S.; Srinivas, P.V.; Rao, J.M. A new sesquiterpene lactone from the roots of Saussurea lappa: Structure–anticancer activity study. Bioorg. Med. Chem. Lett. 2008, 18, 4015–4017. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.X.; Wang, Y.; Gu, X.; Wen, Y.Y.; Yan, C. A platform for fast screening potential anti-breast cancer compounds in traditional Chinese medicines. Biomed. Chromatogr. 2013, 27, 1759–1766. [Google Scholar] [CrossRef] [PubMed]
- Cateni, F.; Zilic, J.; Zacchigna, M.; Bonivento, P.; Frausin, F.; Scarcia, V. Synthesis and biological properties of new α-methylene-γ-butyrolactones and α, β-unsaturated δ-lactones. Eur. J. Med. Chem. 2006, 41, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, R.; Baraldi, P.G.; Tabrizi, M.A.; Bermejo, J.; Estévez, F.; Borgatti, M.; Gambari, R. Design, synthesis, and biological evaluation of hybrid molecules containing α-methylene-γ-butyrolactones and α-bromoacryloyl moieties. J. Med. Chem. 2005, 48, 7906–7910. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, F.; He, E.Q.; Wang, S.; Xu, H.; Liu, K. Effects of eighteen sesquiterpenes from Saussurea lappa on the proliferation of six human cancer cell lines. Nat. Prod. Res. Dev. 2008, 20, 808–812. [Google Scholar]
- Cho, J.Y.; Park, J.; Yoo, E.S.; Baik, K.U.; Jung, J.H.; Lee, J.; Park, M.H. Inhibitory effect of sesquiterpene lactones from Saussurea lappa on tumor necrosis factor-α production in murine macrophage like cells. Planta Med. 1998, 64, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.S.; Pae, H.O.; Jeong, S.O.; Kim, Y.C.; Kwon, T.O.; Lee, H.S.; Kim, N.S.; Park, S.D.; Chung, H.T. The α-methylene-γ-butyrolactone moiety in dehydrocostus lactone is responsible for cytoprotective heme oxygenase-1 expression through activation of the nuclear factor E2-related factor 2 in HepG2 cells. Eur. J. Pharmacol. 2007, 565, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Butturini, E.; di Paola, R.; Suzuki, H.; Paterniti, I.; Ahmad, A.; Mariotto, S.; Cuzzocrea, S. Costunolide and dehydrocostuslactone, two natural sesquiterpene lactones, ameliorate the inflammatory process associated to experimental pleurisyinmice. Eur. J. Pharmacol. 2014, 730, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Kassuya, C.A.; Cremoneze, A.; Barros, L.F.; Simas, A.S.; Lapa, F.R.; Mello-Silva, R.; Stefanello, M.E.; Zampronio, A.R. Antipyretic and anti-inflammatory properties of the ethanolic extract, dichloromethane fraction and costunolide from Magnolia ovata (Magnoliaceae). J. Ethnopharmacol. 2009, 124, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.L.; Ni, W.C.; Tsai, E.M.; Hsu, Y.L. Dehydrocostuslactone disrupts signal transducers and activators of transcription 3 through up-regulation of suppressor of cytokine signaling in breast cancer cells. Mol. Cancer Ther. 2009, 8, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Choi, S.U.; Lee, C.O.; Yoo, S.E.; Yoon, S.K.; Kim, Y.K.; Ryu, S.Y. Costunolide, a sesquiterpene from the stem bark of Magnolia sieboldii, inhibits the RAS-farnesyl-proteintransferase. Planta Med. 2001, 67, 358–359. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Chou, C.K.; Lee, S.D.; Wang, J.C.; Yeh, S.F. Active compounds from Saussurea lappa Clarks that suppress hepatitis B virus surface antigen gene expression in human hepatoma cells. Antivir. Res. 1995, 27, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Song, H.E.; Lee, H.B.; Kim, C.S.; Koketsu, M.; Ngan, L.T.; Ahn, Y.J. Growth inhibitory, bactericidal, and morphostructural effects of dehydrocostus lactone from Magnolia sieboldii Leaves on antibiotic-susceptible and -resistant strains of Helicobacter pylori. PLoS ONE 2014, 9, e95530. [Google Scholar] [CrossRef] [PubMed]
- Duraipandiyan, V.; Al-Harbi, N.A.; Ignacimuthu, S.; Muthukumar, C. Antimicrobial activity of sesquiterpene lactones isolated from traditional medicinal plant, Costus speciosus (Koen ex.Retz.) Sm. BMC Complement. Altern. Med. 2012, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Barrero, A.F.; Oltra, J.E.; Alvarez, M.; Raslan, D.S.; Saude, D.A.; Akssira, M. New sources and antifungal activity of sesquiterpene lactones. Fitoterapia 2000, 71, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Eliza, J.; Daisy, P.; Ignacimuthu, S. Antioxidant activity of costunolide and eremanthin isolated from Costus speciosus (Koen ex.Retz) Sm. Chem. Biol. Interact. 2010, 188, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.S.; Choi, E.M. The effects of dehydrocostus lactone on osteoblastic MC3T3-E1 cells in redox changes and PI3K/Akt/CREB. Immunopharmacol. Immunotoxicol. 2012, 34, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, O.P.; Singh, R.H.; Dutta, S.K. Studies on antidiabetic medicinal plants used in Indian folklore. Aryavaidyan 1996, 9, 159–167. [Google Scholar]
- Sutar, N.; Garai, R.; Sharma, U.S.; Singh, N.; Roy, S.D. Antiulcerogenic activity of Saussurea lappa root. Int. J. Pharm. Life Sci. 2011, 2, 516–520. [Google Scholar]
- Seki, K.; Hashimoto, A.; Kobayashi, H.; Kawahara, Y.; Yamahara, J. Motility inhibitory effect on Anchusan and Jintan and its active components in Anisakis type larvae. Yakuri Chiryo 1991, 19, 265–289. [Google Scholar]
- Triana, J.; López, M.; Rico, M.; González-Platas, J.; Quintana, J.; Estévez, F.; León, F.; Bermejo, J. Sesquiterpenoid derivatives from Gonospermum elegans and their cytotoxic activity for HL-60 human promyelocytic cells. J. Nat. Prod. 2003, 66, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Triana, J.; Eiroa, J.L.; Ortega, J.J.; León, F.; Brouard, I.; Torres, F.; Quintana, J.; Estévez, F.; Bermejo, J. Sesquiterpene lactones from Gonospermum gomerae and G. fruticosum and their cytotoxic activities. J. Nat. Prod. 2008, 71, 2015–2020. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, K.; Agarwal, R. Multitargeted therapy of cancer by silymarin. Cancer Lett. 2008, 269, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, Y.; Ji, W.; Li, X.; Sun, B.; Gao, Q.; Su, C. Anti-tumor activities of matrine and oxymatrine: Literature review. Tumour Biol. 2014, 35, 5111–5119. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kang, S.N.; Kim, H.J.; Kim, T.S. Potentiation of 1,25-dihydroxyvitamin D3-induced differentiation of human promyelocytic leukemia cells into monocytes by costunolide, a germacranolide sesquiterpene lactone. Biochem. Pharmacol. 2002, 64, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.I.; Kim, J.H.; Lee, K.T.; Choi, J.H. Costunolide induces apoptosis in platinum-resistant human ovarian cancer cells by generating reactive oxygen species. Gynecol. Oncol. 2011, 123, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.J.; Zhao, F.; Gao, Z.T.; Xu, H.; Liu, K. Inhibitory efects of sesquiterpenes from Saussurealappa on the vascular endothelial growth factor. Nat. Prod. Res. Dev. 2010, 22, 687–691. [Google Scholar]
- Obaya, A.J.; Sedivy, J.M. Regulation of cyclin-Cdk activity in mammalian cells. Cell. Mol. Life Sci. 2002, 59, 126–142. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Seo, H.S.; Choi, H.S.; Choi, H.S.; Kim, S.R.; Shin, Y.C.; Ko, S.G. Induction of Fas-mediated extrinsic apoptosis, p21WAF1-related G2/M cell cycle arrest and ROS generation by costunolide in estrogen receptor-negative breast cancer cells, MDA-MB-231. Mol. Cell. Biochem. 2012, 363, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Rasul, A.; Yu, B.; Yang, L.; Arshad, M.; Khan, M.; Ma, T.; Yang, H. Costunolide, a sesquiterpene lactone induces G2/M phase arrest and mitochondria-mediated apoptosis in human gastric adenocarcinoma SGC-7901 cells. J. Med. Plants Res. 2012, 6, 1191–1200. [Google Scholar]
- Kretschmer, N.; Rinner, B.; Stuendl, N.; Kaltenegger, H.; Wolf, E.; Kunert, O.; Boechzelt, H.; Leithner, A.; Bauer, R.; Lohberger, B. Effect of costunolide and dehydrocostus lactone on cell cycle, apoptosis, and ABC transporter expression in human soft tissue sarcoma. Planta Med. 2012, 78, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Lohberger, B.; Rinner, B.; Stuendl, N.; Kaltenegger, H.; Steinecker-Frohnwieser, B.; Bernhart, E.; Rad, B.E.; Weinberg, A.M.; Leithner, A.; Bauer, R.; et al. Sesquiterpene lactones downregulate G2/M cell cycle regulator proteins and affect the invasive potential of human soft tissue sarcoma cells. PLoS ONE 2013, 8, e66300. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Tsai, A.C.; Peng, C.Y.; Chang, Y.L.; Lee, K.H.; Teng, C.M.; Pan, S.L. Dehydrocostuslactone suppresses angiogenesis in vitro and in vivo through inhibition of Akt/GSK-3β and mTOR signaling pathways. PLoS ONE 2012, 7, e31195. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.L.; Pan, S.L.; Ho, Y.F.; Hwang, T.L.; Kung, F.L.; Guh, J.H. Costunolide induces apoptosis through nuclear calcium2+ overload and DNA damage response in human prostate cancer. J. Urol. 2011, 185, 1967–1974. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.J.; Kim, K.A.; Kim, K.C. p53 down-regulates SETDB1 gene expression during paclitaxel induced-cell death. Biochem. Biophys. Res. Commun. 2014, 28, 43–48. [Google Scholar] [CrossRef]
- Gismondi, A.; Canuti, L.; Impei, S.; di Marco, G.; Kenzo, M.; Colizzi, V.; Canini, A. Antioxidant extracts of African medicinal plants induce cell cycle arrest and differentiation in B16F10 melanoma cells. Int. J. Oncol. 2013, 43, 956–964. [Google Scholar] [PubMed]
- Gismondi, A.; Canuti, L.; Grispo, M.; Canini, A. Biochemical composition and antioxidant properties of Lavandula angustifolia Miller essential oil are shielded by propolis against UV radiations. Photochem. Photobiol. 2014, 90, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Forni, C.; Braglia, R.; Mulinacci, N.; Urbani, A.; Ronci, M.; Gismondi, A.; Tabolacci, C.; Provenzano, B.; Lentini, A.; Beninati, S. Antineoplastic activity of strawberry (Fragaria × ananassa Duch.) crude extracts on B16-F10 melanoma cells. Mol. Biosyst. 2014, 10, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.G.; Lee, K.T.; Chi, S.G.; Park, J.H. Costunolide induces apoptosis by ROS-mediated mitochondrial permeability transition and cytochrome C release. Biol. Pharm. Bull. 2001, 24, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Coqueret, O. New roles for p21 and p27 cell-cycle inhibitors: A function for each cell compartment? Trends Cell Biol. 2003, 13, 65–70. [Google Scholar] [CrossRef] [PubMed]
- De Forges, H.; Bouissou, A.; Perez, F. Interplay between microtubule dynamics and intracellular organization. Int. J. Biochem. Cell Biol. 2012, 44, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Nogales, E. Structural insights into microtubule function. Annu. Rev. Biochem. 2000, 69, 277–302. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.L.; Kavallaris, M.; McCarroll, J.A. Microtubules and their role in cellular stress in cancer. Front. Oncol. 2014, 4, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Dall Acqua, S. Natural products as antimitotic agents. Curr. Top. Med. Chem. 2014, 14, 2274–2285. [Google Scholar] [CrossRef]
- Whipple, R.A.; Vitolo, M.I.; Boggs, A.E.; Charpentier, M.S.; Thompson, K.; Martin, S.S. Parthenolide and costunolide reduce microtentacles and tumor cell attachment by selectively targeting detyrosinated tubulin independent from NF-κB inhibition. Breast Cancer Res. 2013, 15, R83. [Google Scholar] [CrossRef] [PubMed]
- Bocca, C.; Gabriel, L.; Bozzo, F.; Miglietta, A. A sesquiterpene lactone, costunolide, interacts with microtubule protein and inhibits the growth of MCF-7 cells. Chem. Biol. Interact. 2004, 147, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Counter, C.M.; Avilion, A.A.; LeFeuvre, C.E.; Stewart, N.G.; Greider, C.W.; Harley, C.B.; Bacchetti, S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992, 11, 1921–1929. [Google Scholar] [PubMed]
- Morin, G.B. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell 1989, 59, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.W.; Piatyszek, M.A.; Prowse, K.R.; Harley, C.B.; West, M.D.; Ho, P.L.; Coviello, G.M.; Wright, W.E.; Weinrich, S.L.; Shay, J.W. Specific association of human telomerase activity with immortal cells and cancer. Science 1994, 266, 2011–2015. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.W.; Wright, W.E. Telomerase activity in human cancer. Curr. Opin. Oncol. 1996, 8, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Im, E.; Kang, H.K.; Lee, J.H.; Kwak, H.S.; Bae, Y.T.; Park, H.J.; Kim, N.D. Inhibitory effects of costunolide on the telomerase activity in human breast carcinoma cells. Cancer Lett. 2005, 227, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Kanno, S.; Kitajima, Y.; Kakuta, M.; Osanai, Y.; Kurauchi, K.; Ujibe, M.; Ishikawa, M. Costunolide-induced apoptosis is caused by receptor-mediated pathway and inhibition of telomerase activity in NALM-6 cells. Biol. Pharm. Bull. 2008, 31, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Degterev, A.; Boyce, M.; Yuan, J. A decade of caspases. Oncogene 2003, 22, 8543–8567. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Spencer, S.L.; Sorger, P.K. Measuring and modeling apoptosis in single cells. Cell 2011, 144, 926–939. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, S.; Chen, J.; Ren, P.; Hu, Y.; Cao, Z.; Sun, H.; Ding, Y. Oxymatrine induces mitochondria dependent apoptosis in human osteosarcoma MNNG/HOS cells through inhibition of PI3K/Akt pathway. Tumour Biol. 2014, 35, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Saelens, X.; Festjens, N.; Vande Walle, L.; van Gurp, M.; van Loo, G.; Vandenabeele, P. Toxic proteins released from mitochondria in cell death. Oncogene 2004, 23, 2861–2874. [Google Scholar] [CrossRef] [PubMed]
- Garrido, C.; Galluzzi, L.; Brunet, M.; Puig, P.E.; Didelot, C.; Kroemer, G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006, 13, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Ekert, P.G.; Vaux, D.L. The mitochondrial death squad: Hardened killers or innocent bystanders? Curr. Opin. Cell Biol. 2005, 17, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Susin, S.A.; Daugas, E.; Ravagnan, L.; Samejima, K.; Zamzami, N.; Loeffler, M.; Costantini, P.; Ferri, K.F.; Irinopoulou, T.; Prévost, M.C.; et al. Two distinct pathways leading to nuclear apoptosis. J. Exp. Med. 2000, 192, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Cory, S.; Adams, J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Bröker, L.E.; Kruyt, F.A.; Giaccone, G. Cell death independent of caspases: A review. Clin. Cancer Res. 2005, 11, 3155–3162. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.S.; Pae, H.O.; Chung, H.T.; Kwon, J.W.; Lee, J.H.; Kwon, T.O.; Kwon, S.Y.; Chon, B.H.; Yun, Y.G. Dehydrocostus lactone enhances tumor necrosis factor-α-induced apoptosis of human leukemia HL-60 cells. Immunopharmacol. Immunotoxicol. 2004, 26, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.G.; Oh, H.; Oh, G.S.; Pae, H.O.; Choi, B.M.; Kwon, J.W.; Kwon, T.O.; Jang, S.I.; Chung, H.T. In vitro cytotoxicity of Mokko lactone in human leukemia HL-60 cells: Induction of apoptotic cell death by mitochondrial membrane potential collapse. Immunopharmacol. Immunotoxicol. 2004, 26, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Lee, K.T. Costunolide-induced apoptosis in human leukemia cells: Involvement of c-jun N-terminal kinase activation. Biol. Pharm. Bull. 2009, 32, 1803–1808. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yang, Y.I.; Lee, K.T.; Park, H.J.; Choi, J.H. Costunolide induces apoptosis in human endometriotic cells through inhibition of the prosurvival Akt and nuclear factor κB signaling pathway. Biol. Pharm. Bull. 2011, 34, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Chicheportiche, Y.; Bourdon, P.R.; Xu, H.; Hsu, Y.M.; Scott, H.; Hession, C.; Garcia, I.; Browning, J.L. TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J. Biol. Chem. 1997, 272, 32401–32410. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A.; Dixit, V.M. Death receptors: Signaling and modulation. Science 1998, 281, 1305–1308. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Moscardo, F.; Blesa, D.; Mestre, C.; Siebert, R.; Balasas, T.; Benito, A.; Rosenwald, A.; Climent, J.; Martinez, J.I.; Schilhabel, M.; et al. Characterization of 8p21.3 chromosomal deletions in B-cell lymphoma: TRAIL-R1and TRAIL-R2 as candidate dosage-dependent tumor suppressor genes. Blood 2005, 106, 3214–3222. [Google Scholar] [CrossRef] [PubMed]
- Wajant, H. The Fas signaling pathway: More than a paradigm. Science 2002, 296, 1635–1636. [Google Scholar] [CrossRef] [PubMed]
- Kischkel, F.C.; Hellbardt, S.; Behrmann, I.; Germer, M.; Pawlita, M.; Krammer, P.H.; Peter, M.E. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995, 14, 5579–5588. [Google Scholar] [PubMed]
- Feldman, D.E.; Chauhan, V.; Koong, A.C. The unfolded protein response: A novel component of the hypoxic stress response in tumors. Mol. Cancer Res. 2005, 3, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Moenner, M.; Pluquet, O.; Bouchecareilh, M.; Chevet, E. Integrated endoplasmic reticulum stress responses in cancer. Cancer Res. 2007, 67, 10631–10634. [Google Scholar] [CrossRef] [PubMed]
- Yung, H.W.; Korolchuk, S.; Tolkovsky, A.M.; Charnock-Jones, D.S.; Burton, G.J. Endoplasmic reticulum stress exacerbates ischemia-reperfusion-induced apoptosis through attenuation of Akt protein synthesis in human choriocarcinoma cells. FASEB J. 2007, 21, 872–884. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.Y.; Hsu, Y.L.; Ni, W.C.; Tsai, Y.M.; Yang, C.J.; Kuo, P.L.; Huang, M.S. Oxidative and endoplasmic reticulum stress signaling are involved in dehydrocostuslactone-mediated apoptosis in human non-small cell lung cancer cells. Lung Cancer 2010, 68, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Mehlen, P.; Puisieux, A. Metastasis: A question of life or death. Nat. Rev. 2006, 6, 449–458. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Bourboulia, D.; Stetler-Stevenson, W.G. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): Positive and negative regulators in tumor cell adhesion. Semin. Cancer Biol. 2010, 20, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Yang, J.; Moses, M.A. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J. Clin. Oncol. 2009, 27, 5287–5297. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, I. Matrix metalloproteinases in tumor invasion and metastasis. Semin. Cancer Biol. 2000, 10, 415–433. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Cho, S.G.; Woo, S.M.; Yun, Y.J.; Jo, J.; Kim, W.; Shin, Y.C.; Ko, S.G. Saussurea lappa clarke-derived costunolide prevents TNFα-induced breast cancer cell migration and invasion by inhibiting NF-κB activity. Evid. Based Complement. Altern. Med. 2013, 9, 362–357. [Google Scholar]
- Kuwano, M.; Toh, S.; Uchiumi, T.; Takano, H.; Kohno, K.; Wada, M. Multidrug resistance-associated protein subfamily transporters and drug resistance. Anticancer Drug Des. 1999, 14, 123–131. [Google Scholar] [PubMed]
- Allen, J.D.; Brinkhuis, R.F.; Wijnholds, J.; Schinkel, A.H. The mouse Bcrp1/Mxr/Abcp gene: Amplification and overexpression in cell lines selected for resistance to ropotecan, mitoxantrone, or doxorubicin. Cancer Res. 1999, 59, 4237–4241. [Google Scholar] [PubMed]
- Litman, T.; Druley, T.E.; Stein, W.D.; Bates, S.E. From MDR to MXR: New understanding of multidrug resistance systems, their properties and clinical significance. Cell. Mol. Life Sci. 2001, 58, 931–959. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.I.; Haber, M.; Henderson, M.J.; Norris, M.D. ABC transporters in cancer: More than just drug efflux pumps. Nat. Rev. Cancer 2010, 10, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Tumor angiogenesis: Therapeutic implication. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J.; Klagsbrun, M. Angiogenic factor. Science 1987, 235, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.; Fujita, T.; Kishimoto, S.; Sudo, K.; Kanamaru, T.; Brem, H.; Folkman, J. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature 1990, 348, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.J.; Itokawa, T.; Shibuya, M.; Kuwano, M.; Ono, M.; Higuchi, R.; Miyamoto, T. Costunolide, a sesquiterpene lactone from Saussurea lappa, inhibits the VEGFR KDR/Flk-1 signaling pathway. Cancer Lett. 2002, 187, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Beere, H.M.; Hickman, J.A. Differentiation: A suitable strategy for cancer chemotherapy? Anticancer Drug Des. 1993, 8, 299–322. [Google Scholar] [PubMed]
- Pan, Q.; Granger, J.; O’Connell, T.D.; Somerman, M.J.; Simpson, R.U. Promotion of HL-60 cell differentiation by 1,25-dihydroxyvitamin D3 regulation of protein kinase C levels and activity. Biochem. Pharmacol. 1997, 54, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Martell, R.E.; Simpson, R.U.; Hsu, T. Effects of protein kinase inhibitors 1(5-isoquinol inesulfonyl)-2-methylpiperazine dihydrochloride (H-7) and N-(2-guanidinoethyl)-5-isoquinolinesulfonamide hydrochloride (HA1004) on calcitriol-induced differentiation of HL-60 cells. Biochem. Pharmacol. 1988, 37, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Z.; Shao, G.Y.; Chen, S.; Wang, X.W.; Wang, Z.Y. Studies on the relationship between protein kinase C and differentiation of human promyelocytic leukemia cells induced by retinoic acid. Leuk. Res. 1989, 13, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Seo, B.R.; Seo, S.H.; Lee, K.T.; Park, J.H.; Park, H.J.; Choi, J.W.; Itoh, Y.; Miyamoto, K. Costunolide induces differentiation of human leukemia HL-60 cells. Arch. Pharm. Res. 2002, 25, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Danilenko, M.; Kim, T.S. Differential enhancement of leukaemia cell differentiation without elevation of intracellular calcium by plant-derived sesquiterpene lactone compounds. Br. J. Pharmacol. 2008, 155, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Wang, Y.; Gu, X.; Xue, Y.; Wu, Q.; Zhou, J.; Chan, Y. Metabolic transformation of breast cancer in a MCF-7 xenograft mouse model and inhibitory effect of volatile oil from Saussurea lappa Decne treatment. Metabolomics 2014. [Google Scholar] [CrossRef]
- Hu, F.; Feng, S.; Wu, Y.; Bi, Y.; Wang, C.; Li, W. Quantitative analysis of costunolide and dehydrocostuslactone in rat plasma by ultraperformance liquid chromatography–electrospray ionization–mass spectrometry. Biomed. Chromatogr. 2011, 25, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, X.; Gao, W.; Qu, Z.; Guo, H.; Liu, Z.; Liu, C. Pharmacokinetic study on costunolide and dehydrocostuslactone after oral administration of traditional medicine Aucklandia lappa Decne. by LC/MS/MS. J. Ethnopharmacol. 2014, 151, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Wang, Y.; Gu, X.; Guo, X.; Yan, C. Study on the pharmacokinetics andmetabolism of costunolide and dehydrocostus lactone in rats by HPLC-UV and UPLC-Q-TOF/MS. Biomed. Chromatogr. 2014, 28, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Peng, Z.; Su, C. Potential Anti-Cancer Activities and Mechanisms of Costunolide and Dehydrocostuslactone. Int. J. Mol. Sci. 2015, 16, 10888-10906. https://doi.org/10.3390/ijms160510888
Lin X, Peng Z, Su C. Potential Anti-Cancer Activities and Mechanisms of Costunolide and Dehydrocostuslactone. International Journal of Molecular Sciences. 2015; 16(5):10888-10906. https://doi.org/10.3390/ijms160510888
Chicago/Turabian StyleLin, Xuejing, Zhangxiao Peng, and Changqing Su. 2015. "Potential Anti-Cancer Activities and Mechanisms of Costunolide and Dehydrocostuslactone" International Journal of Molecular Sciences 16, no. 5: 10888-10906. https://doi.org/10.3390/ijms160510888






