Dihydroaustrasulfone Alcohol (WA-25) Impedes Macrophage Foam Cell Formation by Regulating the Transforming Growth Factor-β1 Pathway
Abstract
:1. Introduction
2. Results
2.1. Dihydroaustrasulfone Alcohol (WA-25) Suppresses the Inducible Nitric Oxide Synthase (iNOS) and Cyclooxygenase (COX)-2 Protein Expression in Lipopolysaccharide (LPS)-Induced RAW 264.7 Cells
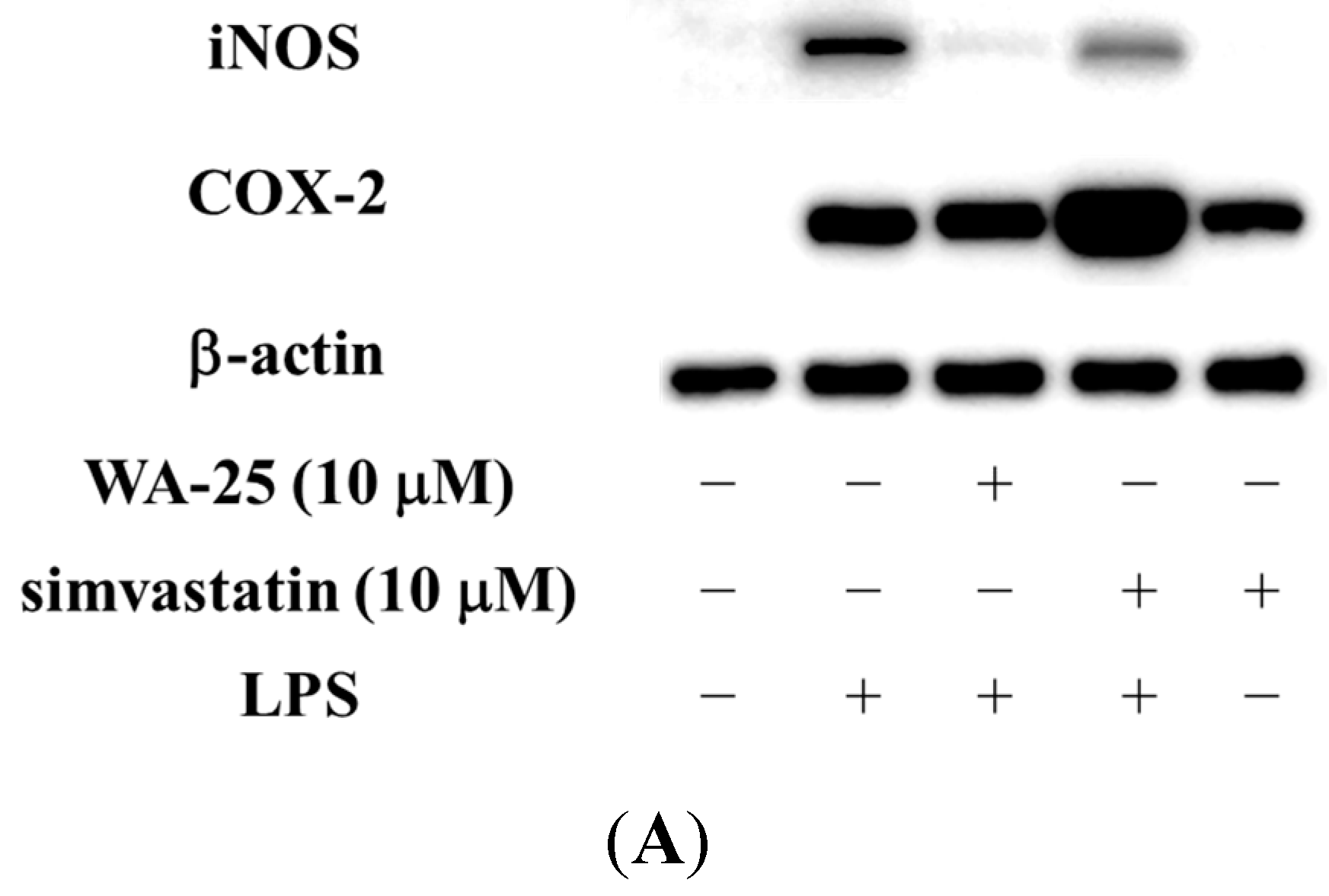
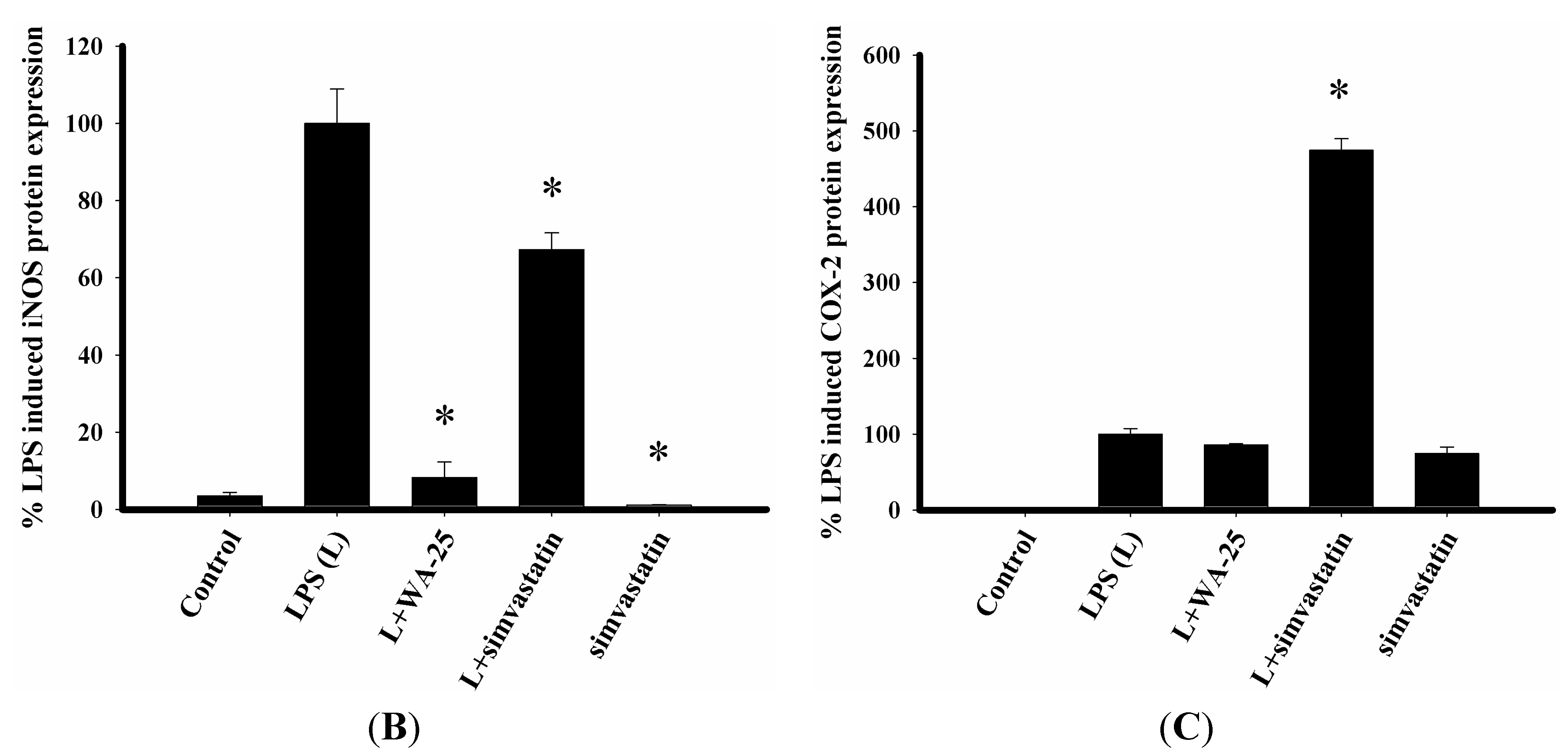
2.2. Effect of WA-25 on Lipolysis in Lipid-Laden Macrophages
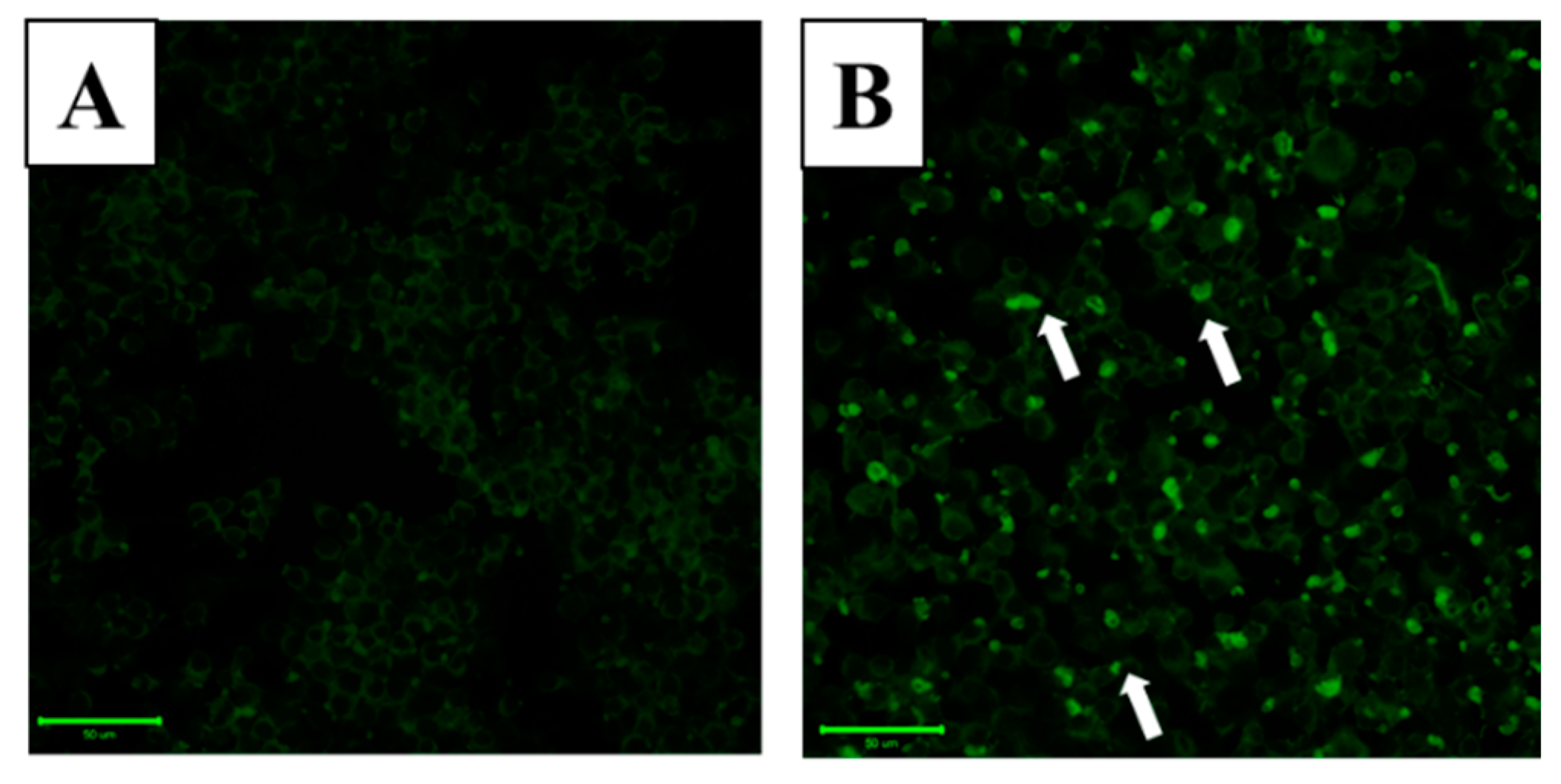
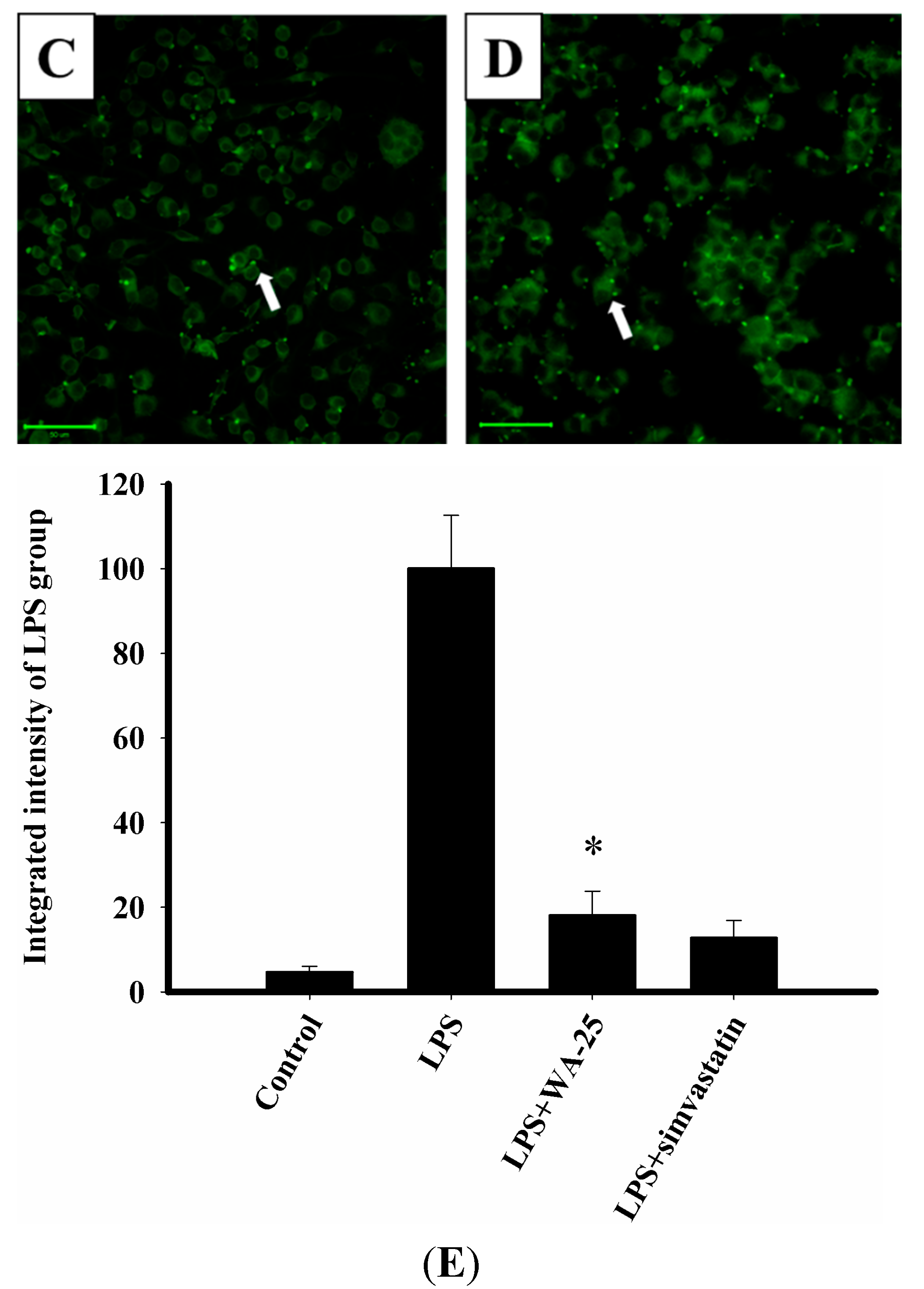
2.3. LAMP-1 and cAMP Involvement in the Effect of WA-25 on Lipolysis
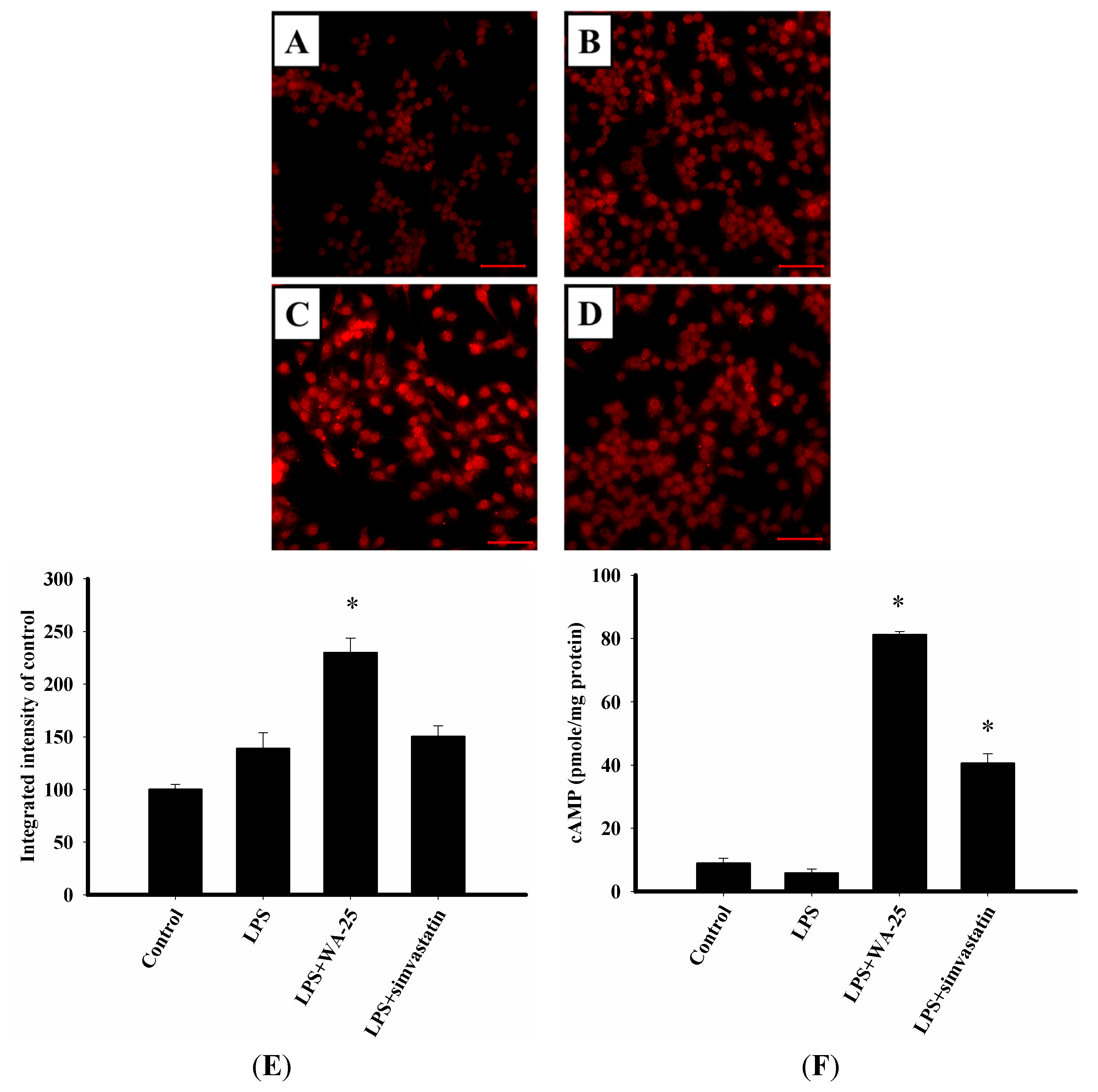
2.4. WA-25 Disrupts LPS-Induced Down-Regulation of TGF-β1 Protein

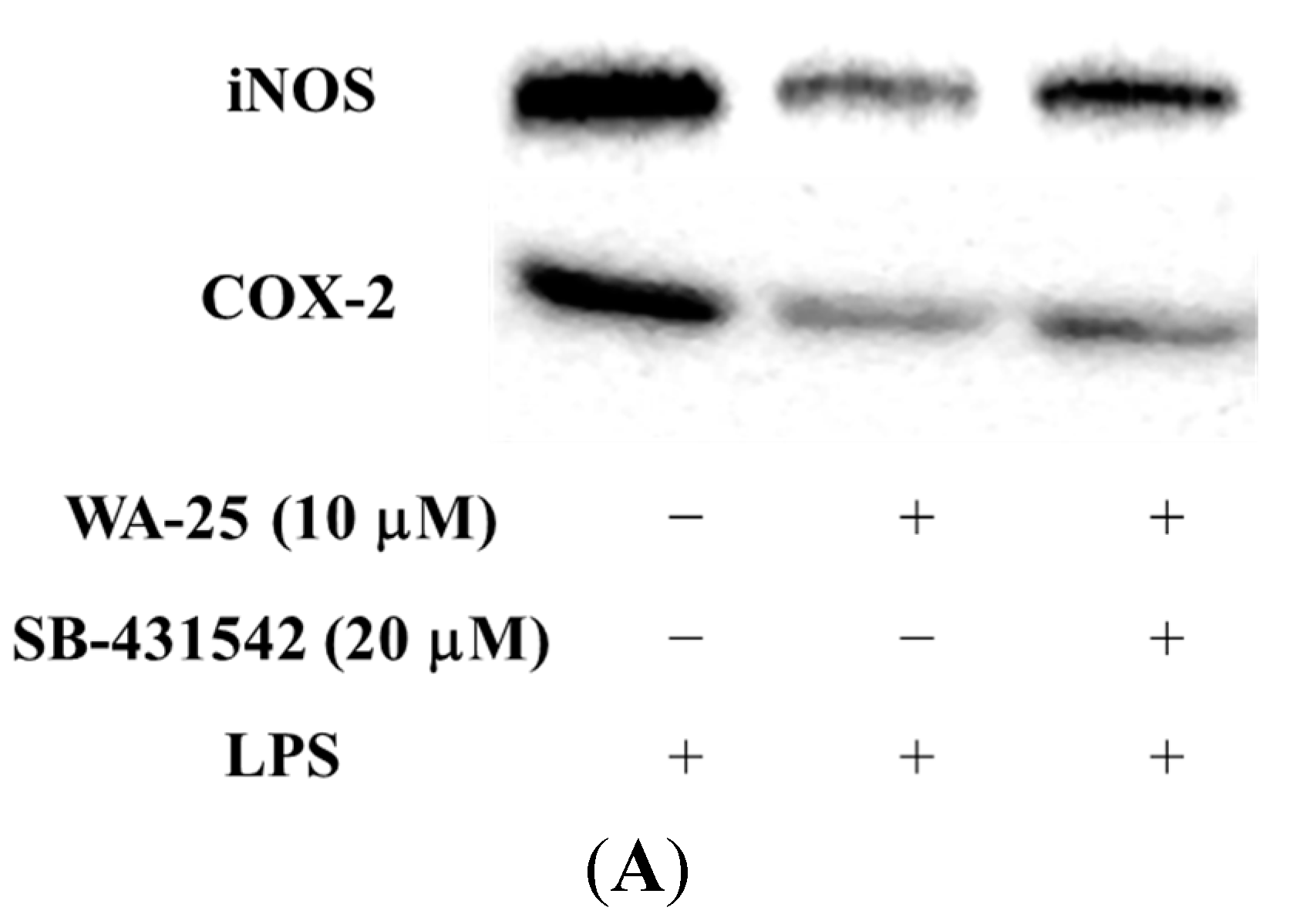
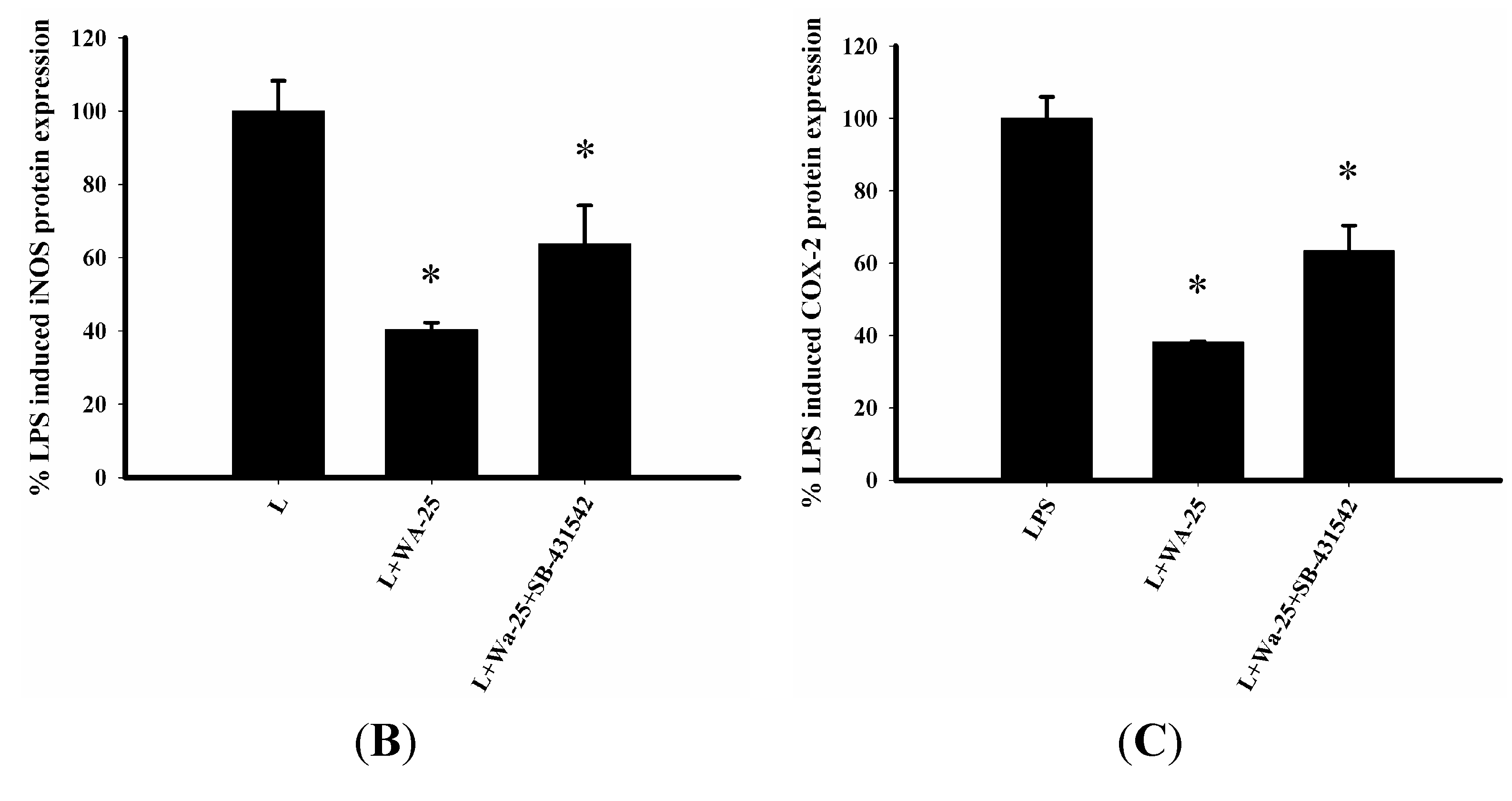
2.5. The Effect of TGF-β1 Inhibitor (SB-431542) on the Anti-Inflammatory Effect of WA-25
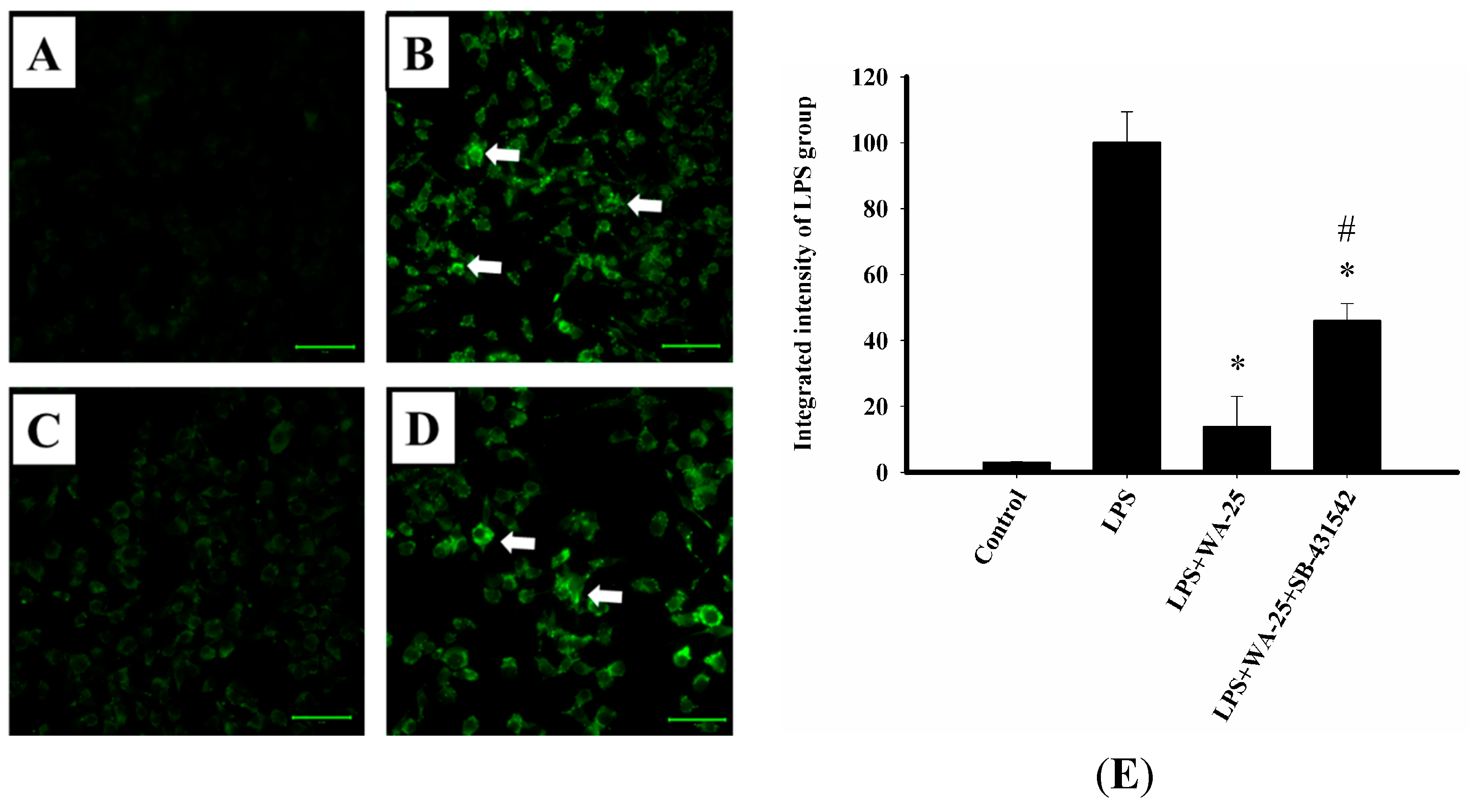
2.6. TGF-β1 Inhibition Blocked the Lipolytic Effect of WA-25 in RAW 264.7 Cells
2.7. WA-25 Does not Change Alter CD-36 Expression, But it Can Decreases the Accumulation of Oxidized LDL (oxLDL)

3. Discussion
4. Experimental Section
4.1. Chemicals
4.2. RAW 264.7 Macrophage Cell Line Culture
4.3. Anti-Inflammatory Assay
4.4. Western Blot Analysis
4.5. Lipid Droplets Fluorescence Assay
4.6. Immunocytochemistry
4.7. Measurement of cAMP Levels
4.8. LDL Uptake Cell-Based Assay
4.9. Data and Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alwan, A. Global Status Report on Non-Communicable Diseases 2010; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Heidenreich, P.A.; Trogdon, J.G.; Khavjou, O.A.; Butler, J.; Dracup, K.; Ezekowitz, M.D.; Finkelstein, E.A.; Hong, Y.; Johnston, S.C.; Khera, A.; et al. Forecasting the future of cardiovascular disease in the United States: A policy statement from the American Heart Association. Circulation 2011, 123, 933–944. [Google Scholar]
- Singh, R.B.; Mengi, S.A.; Xu, Y.J.; Arneja, A.S.; Dhalla, N.S. Pathogenesis of atherosclerosis: A multifactorial process. Exp. Clin. Cardiol. 2002, 7, 40–53. [Google Scholar] [PubMed]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation 2001, 104, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Stary, H.C.; Chandler, A.B.; Dinsmore, R.E.; Fuster, V.; Glagov, S.; Insull, W., Jr.; Rosenfeld, M.E.; Schwartz, C.J.; Wagner, W.D.; Wissler, R.W. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1995, 92, 1355–1374. [Google Scholar] [CrossRef] [PubMed]
- Formato, M.; Farina, M.; Spirito, R.; Maggioni, M.; Guarino, A.; Cherchi, G.M.; Biglioli, P.; Edelstein, C.; Scanu, A.M. Evidence for a proinflammatory and proteolytic environment in plaques from endarterectomy segments of human carotid arteries. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Schönbeck, U.; Mach, F.; Sukhova, G.K.; Murphy, C.; Bonnefoy, J.Y.; Fabunmi, R.P.; Libby, P. Regulation of matrix metalloproteinase expression in human vascular smooth muscle cells by T lymphocytes: A role for CD40 signaling in plaque rupture? Circ. Res. 1997, 81, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Amarenco, P.; Labreuche, J.; Lavallée, P.; Touboul, P.J. Statins in stroke prevention and carotid atherosclerosis: Systematic review and up-to-date meta-analysis. Stroke 2004, 35, 2902–2909. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.G.; McTaggart, F.; Raza, A. Rosuvastatin: A highly effective new HMG-CoA reductase inhibitor. Cardiovasc. Drug Rev. 2002, 20, 303–328. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S. Statins in atherosclerosis: Lipid-lowering agents with antioxidant capabilities. Atherosclerosis 2004, 173, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Golomb, B.A.; Evans, M.A. Statin adverse effects: A review of the literature and evidence for a mitochondrial mechanism. Am. J. Cardiovasc. Drugs 2008, 8, 373–418. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, D.; Wassenaar, W. Rhabdomyolysis and cerivastatin: Was it a problem of dose? Can. Med. Assoc. J. 2002, 167, 737. [Google Scholar]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Jean, Y.H.; Chen, W.F.; Duh, C.Y.; Huang, S.Y.; Hsu, C.H.; Lin, C.S.; Sung, C.S.; Chen, I.M.; Wen, Z.H. Inducible nitric oxide synthase and cyclooxygenase-2 participate in anti-inflammatory and analgesic effects of the natural marine compound lemnalol from Formosan soft coral Lemnalia cervicorni. Eur. J. Pharmacol. 2008, 578, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Jean, Y.H.; Chen, W.F.; Sung, C.S.; Duh, C.Y.; Huang, S.Y.; Lin, C.S.; Tai, M.H.; Tzeng, S.F.; Wen, Z.H. Capnellene, a natural marine compound derived from soft coral, attenuates chronic constriction injury-induced neuropathic pain in rats. Br. J. Pharmacol. 2009, 158, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L.; Qian, Y.; Meng, W.F.; Pang, J.Y.; Lin, Y.C.; Guan, Y.Y.; Chen, S.P.; Liu, J.; Pei, Z.; Wang, G.L. A novel marine compound xyloketal B protects against oxidized LDL-induced cell injury in vitro. Biochem. Pharmacol. 2009, 78, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.H.; Chao, C.H.; Wu, M.H.; Sheu, J.H. A neuroprotective sulfone of marine origin and the in vivo anti-inflammatory activity of an analogue. Eur. J. Med. Chem. 2010, 45, 5998–6004. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Chien, Y.C.; Pan, C.H.; Sheu, J.H.; Chen, C.Y.; Wu, C.H. Inhibitory effect of dihydroaustrasulfone alcohol on the migration of human non-small cell lung carcinoma A549 cells and the antitumor effect on a Lewis lung carcinoma-bearing tumor model in C57BL/6J mice. Mar. Drugs 2014, 12, 196–213. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Chen, J.S. Nitric oxide-independent lipid metabolism in RAW 264.7 macrophages loaded with oleic acid. Cell Biol. Int. 2006, 30, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.N.; Frei, B.; Vita, J.A.; Keaney, J.F., Jr. Antioxidants and atherosclerotic heart disease. N. Engl. J. Med. 1997, 337, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Miyakawa, H.; Kuramitsu, H.K. Porphyromonas gingivalis induces murine macrophage foam cell formation. Microb. Pathog. 2003, 35, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Greenberg, A.S.; Tseng, Y.Z.; Wang, S.M. Possible involvement of protein kinase C in the induction of adipose differentiation-related protein by sterol ester in RAW 264.7 macrophages. J. Cell. Biochem. 2001, 83, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.S.; Hall, R.J.; Evans, T.J.; Pomerance, A.; Maclouf, J.; Creminon, C.; Yacoub, M.H.; Polak, J.M. Cyclooxygenase-2 is widely expressed in atherosclerotic lesions affecting native and transplanted human coronary arteries and colocalizes with inducible nitric oxide synthase and nitrotyrosine particularly in macrophages. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Holm, C. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem. Soc. Trans. 2003, 31, 1120–1124. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, X.; Yang, Y.; Wu, L.; Li, F.; Zhang, R.; Yuan, G.; Wang, N.; Chen, M.; Ning, G. Berberine attenuates cAMP-induced lipolysis via reducing the inhibition of phosphodiesterase in 3T3-L1 adipocytes. Biochim. Biophys. Acta 2011, 1812, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Molloy, R.G.; Mannick, J.A.; Rodrick, M.L. Cytokines, sepsis and immunomodulation. Br. J. Surg. 1993, 80, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, J.; Wu, H.; Jiang, C.; Zheng, Q.; Li, Z. Inhibition of RAW264.7 macrophage inflammatory cytokines release by small haparin RNAi targeting TLR4. J. Huazhong Univ. Sci. Technol. 2006, 26, 500–503. [Google Scholar] [CrossRef]
- Chen, J.S.; Chen, Y.L.; Greenberg, A.S.; Chen, Y.J.; Wang, S.M. Magnolol stimulates lipolysis in lipid-laden RAW 264.7 macrophages. J. Cell. Biochem. 2005, 94, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile red: A selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 1985, 100, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Rios, F.J.; Koga, M.M.; Pecenin, M.; Ferracini, M.; Gidlund, M.; Jancar, S. Oxidized LDL induces alternative macrophage phenotype through activation of CD36 and PAFR. Mediators Inflamm. 2013, 2013, 198193. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Witztum, J.L. Atherosclerosis. The road ahead. Cell 2001, 104, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Chen, F.; Kuang, C.; Chen, Y. Suppression of matrix metalloproteinase-9 transcription by transforming growth factor-β is mediated by a nuclear factor-κB site. Biochem. J. 2004, 381, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Kim, B.C. Lipopolysaccharide inhibits transforming growth factor-β1-stimulated Smad6 expression by inducing phosphorylation of the linker region of Smad3 through a TLR4-IRAK1-ERK1/2 pathway. FEBS Lett. 2011, 585, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Agah, R.; Xiao, M.; Frutkin, A.D.; Kremen, M.; Shi, H.; Dichek, D.A. In vivo expression of a conditional TGF-β1 transgene: No evidence for TGF-β1 transgene expression in SM22α-tTA transgenic mice. J. Mol. Cell Cardiol. 2006, 40, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Jamkhande, P.G.; Chandak, P.G.; Dhawale, S.C.; Barde, S.R.; Tidke, P.S.; Sakhare, R.S. Therapeutic approaches to drug targets in atherosclerosis. Saudi Pharm. J. 2014, 22, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Charo, I.F.; Taub, R. Anti-inflammatory therapeutics for the treatment of atherosclerosis. Nat. Rev. Drug Discov. 2011, 10, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.H.; Hur, S.K.; Oh, O.J.; Kim, S.S.; Nam, K.A.; Lee, S.K. Evaluation of natural products on inhibition of inducible cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) in cultured mouse macrophage cells. J. Ethnopharmacol. 2002, 83, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Paraskevas, K.I.; Tzovaras, A.A.; Briana, D.D.; Mikhailidis, D.P. Emerging indications for statins: A pluripotent family of agents with several potential applications. Curr. Pharm. Des. 2007, 13, 3622–3636. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Itabe, H.; Higashi, Y.; Fujimoto, Y.; Shiomi, M.; Yoshizumi, M.; Ouchi, Y.; Takano, T. Foam cell formation containing lipid droplets enriched with free cholesterol by hyperlipidemic serum. J. Lipid Res. 2001, 42, 1771–1781. [Google Scholar] [PubMed]
- Gbelcová, H.; Svéda, M.; Laubertová, L.; Varga, I.; Vítek, L.; Kolář, M; Strnad, H.; Zelenka, J.; Böhmer, D.; Ruml, T. The effect of simvastatin on lipid droplets accumulation in human embryonic kidney cells and pancreatic cancer cells. Lipids Health Dis. 2013, 12, 126. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Bobryshev, Y.V.; Shchelkunova, T.A.; Morozov, I.A.; Rubtsov, P.M.; Sobenin, I.A.; Orekhov, A.N.; Smirnov, A.N. Changes of lysosomes in the earliest stages of the development of atherosclerosis. J. Cell. Mol. Med. 2013, 17, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Emanuel, R.; Sergin, I.; Bhattacharya, S.; Turner, J.N.; Epelman, S.; Settembre, C.; Diwan, A.; Ballabio, A.; Razani, B. Induction of lysosomal biogenesis in atherosclerotic macrophages can rescue lipid-induced lysosomal dysfunction and downstream sequelae. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1942–1952. [Google Scholar] [CrossRef] [PubMed]
- Carmen, G.Y.; Víctor, S.M. Signalling mechanisms regulating lipolysis. Cell. Signal. 2006, 18, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Frutkin, A.D.; Otsuka, G.; Stempien-Otero, A.; Sesti, C.; Du, L.; Jaffe, M.; Dichek, H.L.; Pennington, C.J.; Edwards, D.R.; Nieves-Cintrón, M.; et al. TGF-β1 limits plaque growth, stabilizes plaque structure, and prevents aortic dilation in apolipoprotein E-null mice. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Youssef, S.; Stüve, O.; Patarroyo, J.C.; Ruiz, P.J.; Radosevich, J.L.; Hur, E.M.; Bravo, M.; Mitchell, D.J.; Sobel, R.A.; Steinman, L.; et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature 2002, 420, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Collot-Teixeira, S.; Martin, J.; McDermott-Roe, C.; Poston, R.; McGregor, J.L. CD36 and macrophages in atherosclerosis. Cardiovasc. Res. 2007, 75, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Wang, C.; Xiang, X.R.; Chen, F.C.; Yang, C.M.; Wu, J. Porphyromonas gingivalis lipopolysaccharide increases lipid accumulation by affecting CD36 and ATP-binding cassette transporter A1 in macrophages. Oncol. Rep. 2013, 30, 1329–1336. [Google Scholar] [PubMed]
- Dong, M.; Yang, X.; Lim, S.; Cao, Z.; Honek, J.; Lu, H.; Zhang, C.; Seki, T.; Hosaka, K.; Wahlberg, E.; et al. Cold exposure promotes atherosclerotic plaque growth and instability via UCP1-dependent lipolysis. Cell Metab. 2013, 18, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.H.; Wen, Z.H.; Wu, Y.C.; Yeh, H.C.; Sheu, J.H. Cytotoxic and anti-inflammatory cembranoids from the soft coral Lobophytum crassum. J. Nat. Prod. 2008, 71, 1819–1824. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Wen, Z.H.; Wu, G.J.; Chang, Y.C.; Wang, J.J.; Wong, C.S. Dexamethasone modulates the development of morphine tolerance and expression of glutamate transporters in rats. Neuroscience 2005, 133, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.J.; Chen, W.F.; Hung, H.C.; Jean, Y.H.; Sung, C.S.; Chakraborty, C.; Lee, H.P.; Chen, N.F.; Wen, Z.H. Effects of propofol on proliferation and anti-apoptosis of neuroblastoma SH-SY5Y cell line: New insights into neuroprotection. Brain Res. 2011, 1384, 42–50. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-C.; Hung, H.-C.; Feng, C.-W.; Huang, S.-Y.; Chen, C.-H.; Lin, Y.-Y.; Chen, Y.-C.; Yang, S.-N.; Su, J.-H.; Sheu, J.-H.; et al. Dihydroaustrasulfone Alcohol (WA-25) Impedes Macrophage Foam Cell Formation by Regulating the Transforming Growth Factor-β1 Pathway. Int. J. Mol. Sci. 2015, 16, 10507-10525. https://doi.org/10.3390/ijms160510507
Wang Y-C, Hung H-C, Feng C-W, Huang S-Y, Chen C-H, Lin Y-Y, Chen Y-C, Yang S-N, Su J-H, Sheu J-H, et al. Dihydroaustrasulfone Alcohol (WA-25) Impedes Macrophage Foam Cell Formation by Regulating the Transforming Growth Factor-β1 Pathway. International Journal of Molecular Sciences. 2015; 16(5):10507-10525. https://doi.org/10.3390/ijms160510507
Chicago/Turabian StyleWang, Yi-Chen, Han-Chun Hung, Chien-Wei Feng, Shi-Ying Huang, Chun-Hong Chen, Yen-You Lin, Yao-Chang Chen, San-Nan Yang, Jui-Hsin Su, Jyh-Horng Sheu, and et al. 2015. "Dihydroaustrasulfone Alcohol (WA-25) Impedes Macrophage Foam Cell Formation by Regulating the Transforming Growth Factor-β1 Pathway" International Journal of Molecular Sciences 16, no. 5: 10507-10525. https://doi.org/10.3390/ijms160510507





