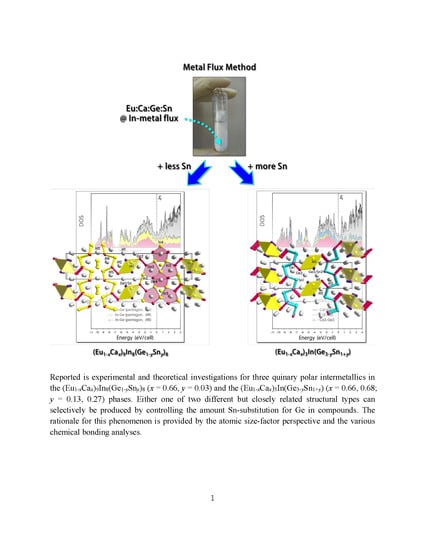
Crystal Structure, Chemical Bonding and Magnetism Studies for Three Quinary Polar Intermetallic Compounds in the (Eu1−xCax)9In8(Ge1−ySny)8 (x = 0.66, y = 0.03) and the (Eu1−xCax)3In(Ge3−ySn1+y) (x = 0.66, 0.68; y = 0.13, 0.27) Phases
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crystal Structure Descriptions
2.1.1. (Eu1−xCax)9In8(Ge1−ySny)8 Phase

| Empirical Formula | Eu3.04(4)Ca5.96In8Ge7.77(2)Sn0.23 | Eu1.02(1)Ca1.98InGe2.87(1)Sn1.13 | Eu0.95(1)Ca2.05InGe2.73(1)Sn1.27 |
|---|---|---|---|
| fw, g·mol−1 | 2211.05 | 691.24 | 690.25 |
| space group, Z | Pmmn (No. 59), 2 | Pnma (No. 62), 4 | |
| unit cell dimension, Å | a = 36.963(2) | a = 7.4974(8) | a = 7.5096(3) |
| b = 4.5176(2) | b = 4.4847(4) | b = 4.4959(2) | |
| c = 7.5155(3) | c = 23.819(3) | c = 23.8518(9) | |
| volume, Å3 | 1254.97(9) | 800.88(14) | 805.29(6) |
| density (ρcalcd), g·cm−3 | 5.851 | 5.733 | 5.693 |
| absorption coefficient (μ), cm−1 | 252.39 | 259.44 | 252.39 |
| GOF on F2 | 1.074 | 1.057 | 1.059 |
| R a [I > 2σ(I)] | R1 = 0.0348 | R1 = 0.0264 | R1 = 0.0256 |
| wR2 = 0.0595 | wR2 = 0.0462 | wR2 = 0.0400 | |
| R [all data] | R1 = 0.0540 | R1 = 0.0399 | R1 = 0.0411 |
| wR2 = 0.0637 | wR2 = 0.0541 | wR2 = 0.0472 | |
| largest diff. peak and hole, e−·Å−3 | 2.423 and −2.527 | 1.180 and −1.821 | 1.859 and −1.585 |
| Atom | Wyckoff Site | Occupation Factor | x | y | z | Ueq a (Å2) | |
|---|---|---|---|---|---|---|---|
| Eu3.04(4)Ca5.96In8Ge7.77(2)Sn0.23 | |||||||
| M1 b | 4f | 0.298(4)/0.702 | 0.0456 (1) | 1/4 | 0.0079(2) | 0.0066(5) | |
| M2 b | 4f | 0.506(4)/0.494 | 0.0755(1) | 1/4 | 0.5209(1) | 0.0053(3) | |
| M3 b | 4f | 0.271(4)/0.729 | 0.1443(1) | 1/4 | 0.1498(2) | 0.0055(5) | |
| M4 b | 4f | 0.284(4)/0.716 | 0.1740(1) | 1/4 | 0.6668(2) | 0.0060(5) | |
| M5 b | 2a | 0.325(6)/0.675 | 1/4 | 1/4 | 0.0310(3) | 0.0068(6) | |
| In1 | 4f | 1 | 0.5172(1) | 1/4 | 0.6502(1) | 0.0052(2) | |
| In2 | 4f | 1 | 0.6110(1) | 1/4 | 0.1632(1) | 0.0077(2) | |
| In3 | 4f | 1 | 0.7039(1) | 1/4 | 0.6868(1) | 0.0055(2) | |
| In4 | 2b | 1 | 3/4 | 1/4 | 0.2854(2) | 0.0068(3) | |
| In5 | 2a | 1 | 1/4 | 1/4 | 0.4544(2) | 0.0074(3) | |
| Ge1/Sn | 4f | 0.887(12)/0.113 | 0.5251(1) | 1/4 | 0.2668(2) | 0.0072(4) | |
| Ge2 | 4f | 1 | 0.5867(1) | 1/4 | 0.7882(2) | 0.0050(3) | |
| Ge3 | 4f | 1 | 0.6344(1) | 1/4 | 0.5427(2) | 0.0050(3) | |
| Ge4 | 4f | 1 | 0.6898(1) | 1/4 | 0.0519(2) | 0.0043(3) | |
| Eu1.02(1)Ca1.98InGe2.87(1)Sn1.13 | |||||||
| M1 b | 4c | 0.302(3)/0.698 | 0.0096(1) | 1/4 | 0.0735(1) | 0.0070(3) | |
| M2 b | 4c | 0.483(3)/0.517 | 0.0273(1) | 1/4 | 0.3814(1) | 0.0066(2) | |
| M3 b | 4c | 0.232(3)/0.768 | 0.1640(1) | 1/4 | 0.2267(1) | 0.0080(3) | |
| In1 | 4c | 1 | 0.1497(1) | 1/4 | 0.5283(1) | 0.0074(1) | |
| Sn1 | 4c | 1 | 0.1623(1) | 1/4 | 0.8244(1) | 0.0096(1) | |
| Ge1 | 4c | 1 | 0.0391(1) | 1/4 | 0.7108(1) | 0.0060(2) | |
| Ge2 | 4c | 1 | 0.2833(1) | 1/4 | 0.6371(1) | 0.0063(2) | |
| Ge3/Sn2 | 4c | 0.871(7)/0.129 | 0.7643(1) | 1/4 | 0.5375(1) | 0.0065(2) | |
| Eu0.95(1)Ca2.05InGe2.73(1)Sn1.27 | |||||||
| M1 b | 4c | 0.274(2)/0.726 | 0.0106(1) | 1/4 | 0.0740(1) | 0.0077(2) | |
| M2 b | 4c | 0.453(2)/0.547 | 0.0264(1) | 1/4 | 0.3811(1) | 0.0064(2) | |
| M3 b | 4c | 0.220(2)/0.780 | 0.1642(1) | 1/4 | 0.2269(1) | 0.0070(2) | |
| In1 | 4c | 1 | 0.1505(1) | 1/4 | 0.5287(1) | 0.0077(1) | |
| Sn1 | 4c | 1 | 0.1624 (1) | 1/4 | 0.8243(1) | 0.0088(1) | |
| Ge1 | 4c | 1 | 0.0395(1) | 1/4 | 0.7108(1) | 0.0062(1) | |
| Ge2 | 4c | 1 | 0.2837(1) | 1/4 | 0.6374(1) | 0.0064(1) | |
| Ge3/Sn2 | 4c | 0.734(7)/0.266 | 0.7635(1) | 1/4 | 0.5377(1) | 0.0067(2) | |
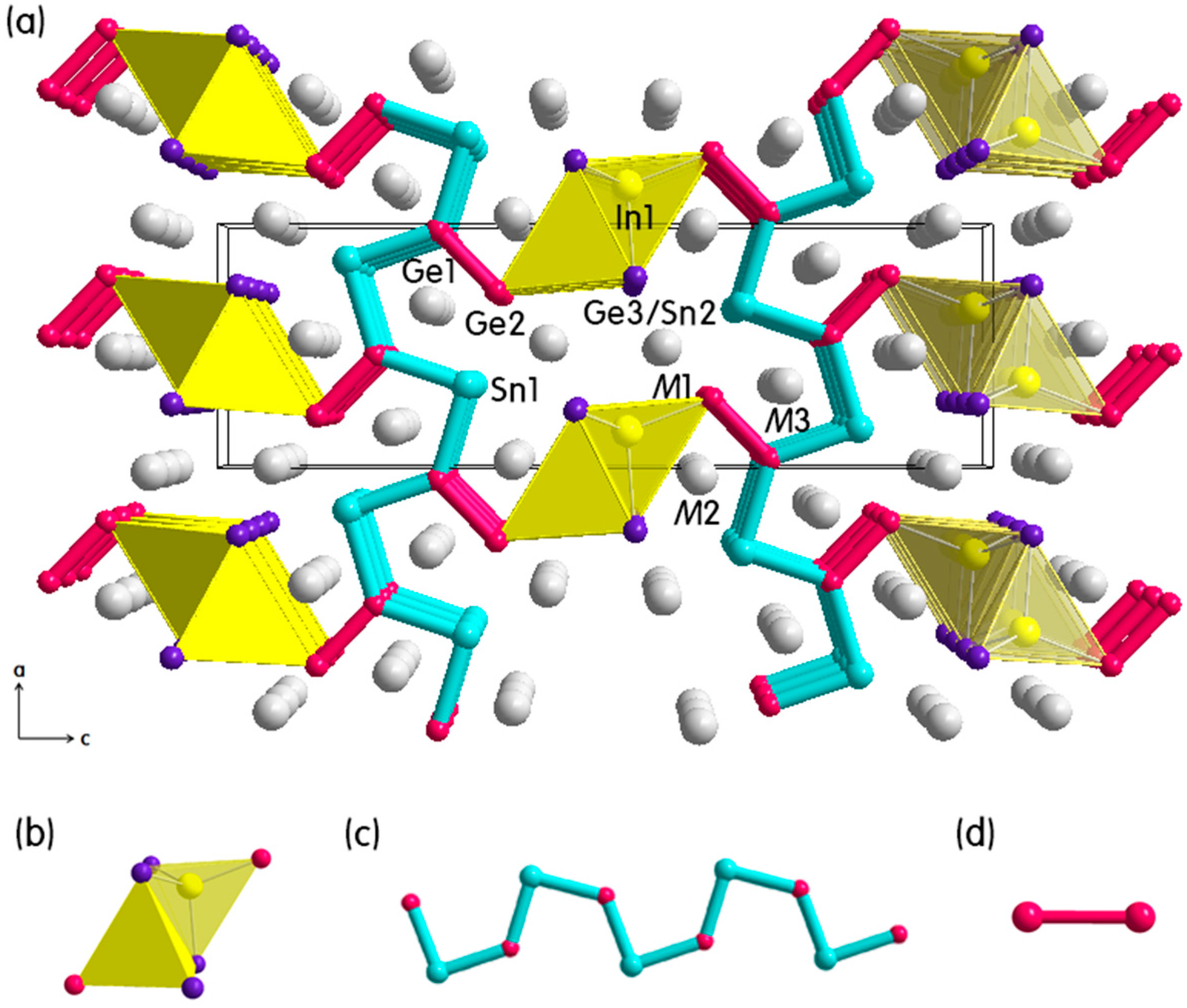
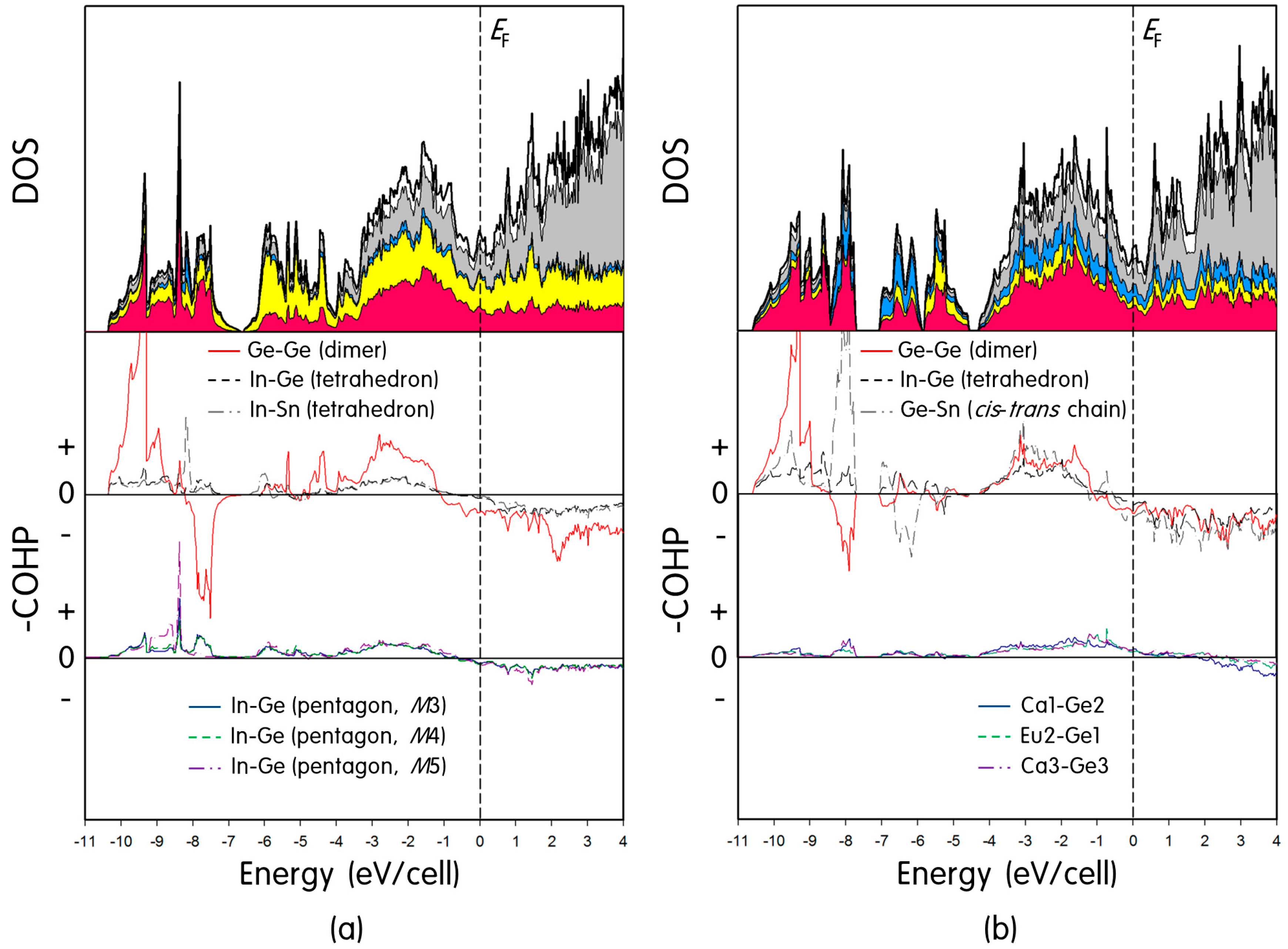
2.2. Electronic Structure Calculations
2.2.1. Eu3Ca6In8Ge7Sn
2.2.2. EuCa2InGe3Sn
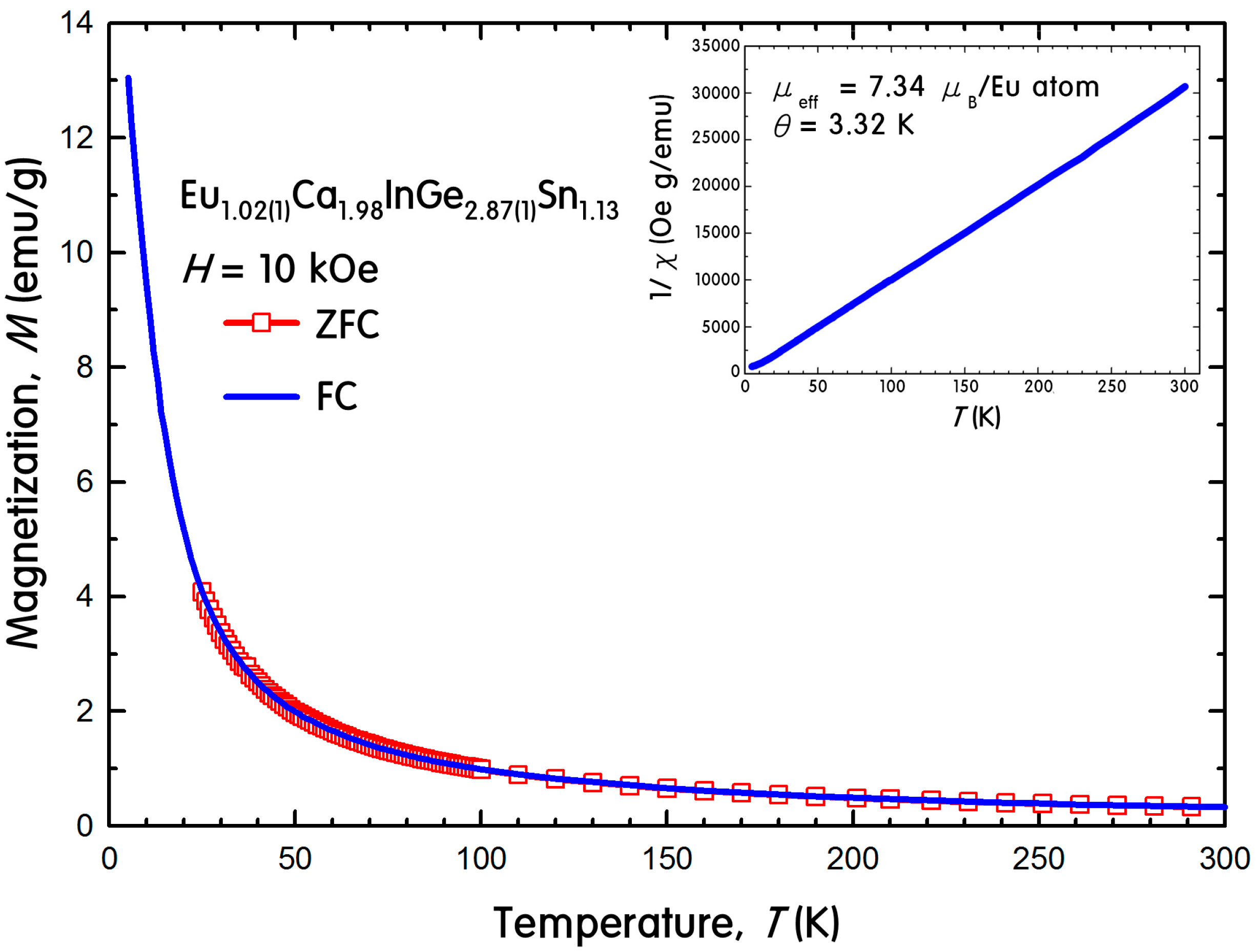
2.3. Physical Property Measurements
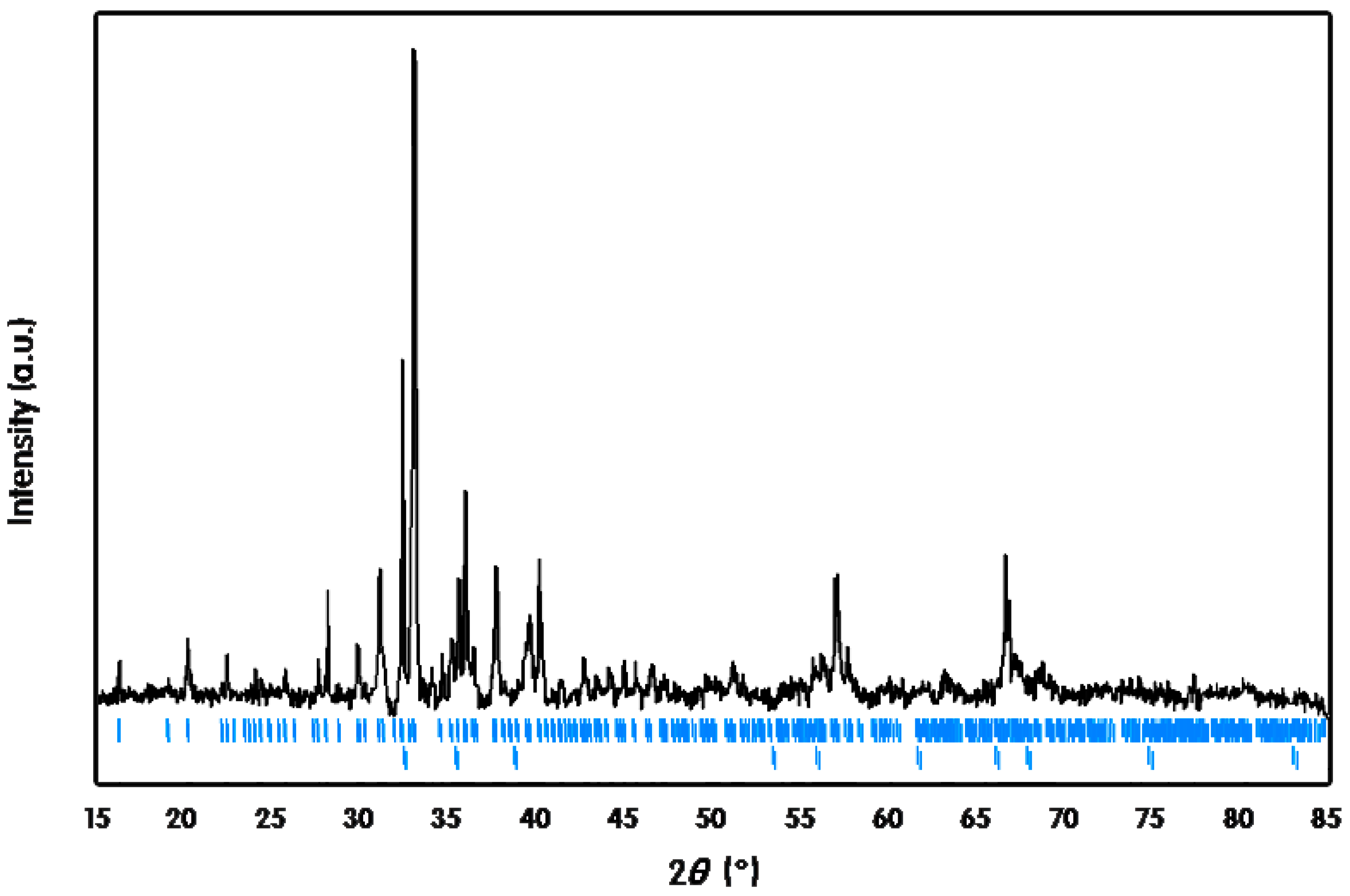
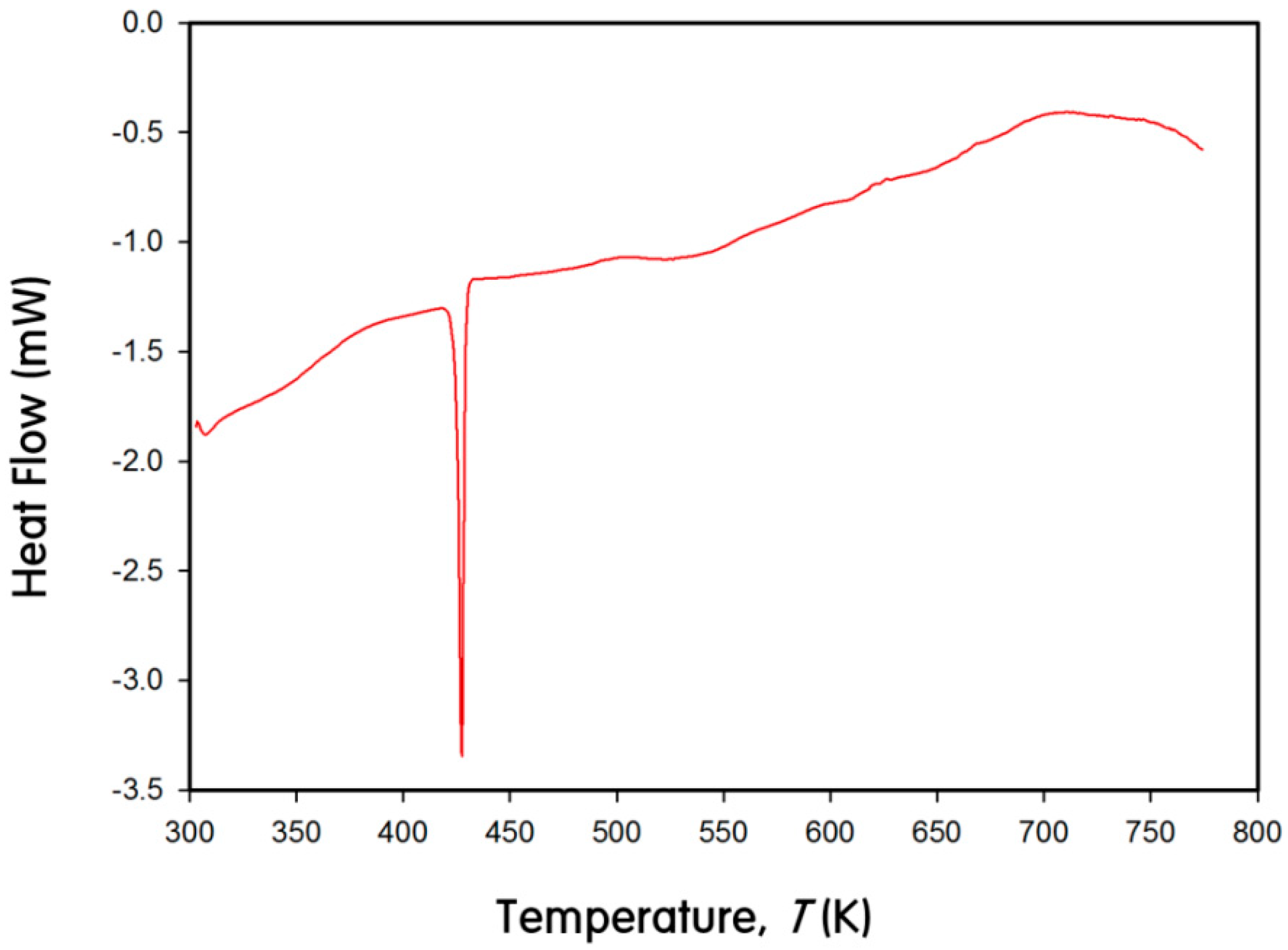
3. Experimental Section
3.1. Synthesis
3.2. Crystal Structure Determinations
| Atomic Pair | Distance | Atomic Pair | Distance | |
|---|---|---|---|---|
| Eu3.04(4)Ca5.96In8Ge7.77(2)Sn0.23 | Eu1.02(1)Ca1.98InGe2.87(1)Sn1.13 | Eu0.95(1)Ca2.05InGe2.73(1)Sn1.27 | ||
| In1-Ge1/Sn(×2) | 2.816(1) | In1-Ge2 | 2.779(1) | 2.779(1) |
| In1-Ge1/Sn(×1) | 2.896(2) | In1-Ge3/Sn2(×2) | 2.811(1) | 2.825(1) |
| In1-Ge2 | 2.770(1) | In1-Ge3/Sn2(×1) | 2.898(1) | 2.914(1) |
| In2-Ge1/Sn | 3.270(1) | Sn1-Ge1 | 2.859(1) | 2.861(1) |
| In2-Ge2 | 2.959(2) | Sn1-Ge1 | 2.947(1) | 2.953(1) |
| In2-Ge3 | 2.981(2) | Sn1-Ge2 | 2.986(1) | 2.987(1) |
| In2-Ge4 | 3.030(1) | Sn1-Ge3/Sn2 | 3.377(1) | 3.377(1) |
| In3-Ge3 | 2.787(1) | |||
| In3-Ge4 | 2.793(2) | |||
| In3-In5 | 3.022(1) | |||
| In4-Ge4 | 2.834(1) | |||
| In4-In5 | 2.988(1) | |||
| Ge2-Ge3 | 2.554(2) | Ge1-Ge2 | 2.536(1) | 2.534(1) |
3.3. Electronic Structure Calculations
3.4. EDS and SEM Images Analyses
3.5. DSC Measurement
3.6. Magnetic Property Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kanatzidis, M.G. Recent Trends in Thermoelectric Materials Research I; Series: Semiconductors and Semimetals; Academic: San Diego, CA, USA, 2001; Volume 69, p. 51. [Google Scholar]
- Condron, C.L.; Kauzlarich, S.M.; Nolas, G.S. Structure and thermoelectric characterization of AxBa8−xAl14Si31 (A = Sr, Eu) Single crystals. Inorg. Chem. 2007, 46, 2556–2562. [Google Scholar] [CrossRef] [PubMed]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Gschneidner, K.A., Jr.; Pecharsky, V.K.; Tsokol, A.O. Recent developments in magnetocaloric materials. Rep. Prog. Phys. 2005, 68, 1479–1539. [Google Scholar] [CrossRef]
- Morrelon, L.; Blasco, J.; Algarabel, P.A.; Ibarra, M.R. Nature of the first-order antiferromagnetic-ferromagnetic transition in the Ge-rich magnetocaloric compounds Gd5(SixGe1−x)4. Phys. Rev. B 2000, 62, 1022–1026. [Google Scholar] [CrossRef]
- Morellon, L.; Stankiewicz, J.; Garcia-Landa, B.; Algarabel., P.; Ibarra, M.R. Giant magnetoresistance near the magnetostructural transition in Gd5(Si1.8Ge2.2). Appl. Phys. Lett. 1998, 73, 3462. [Google Scholar] [CrossRef]
- Sarkar, S.; Peter, S. Structural phase transitions in a new compound Eu2AgGe3. Inorg. Chem. 2013, 52, 9741–9748. [Google Scholar] [CrossRef] [PubMed]
- Aynajian, P.; da Silva Neto, E.H.; Gyenis, A.; Baumbach, R.E.; Thompson, J.D.; Fisk, Z.; Bauer, E.D.; Yazdani, A. Visualizing heavy fermions emerging in a quantum critical Kondo lattice. Nature 2012, 486, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Custers, J.; Lorenzer, K.-A.; Müller, M.; Prokofiev, A.; Sidorenko, A.; Winkler, H.; Strydom, A.M.; Shimura, Y.; Sakakibara, T.; Yu, R.; et al. Destruction of the Kondo effect in the cubic heavy-fermion compound Ce3Pd20Si6. Nat. Mater. 2012, 11, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.H.; Kaczorowski, D.; Khan, R.T.; Bauer, E. Superconductivity and non-Fermi-liquid behavior of Ce2PdIn8. Phys. Rev. B 2011, 83, 064504. [Google Scholar] [CrossRef]
- Shim, J.H.; Haule, K.; Kotliar, G. Modeling the localized-to-itinerant electronic transition in the heavy fermion system CeIrIn5. Science 2007, 318, 1615–1617. [Google Scholar] [CrossRef] [PubMed]
- You, T.-S.; Tobash, P.H.; Bobev, S. Mixed cations and structural complexity in (Eu1−xCax)4In3Ge4 and (Eu1−xCax)3In2Ge3—The first two members of the homologous series A2[n+m]In2n+mGe2[n+m] (n, m = 1, 2, … ∞; A = Ca, Sr, Ba, Eu, or Yb). Inorg. Chem. 2010, 49, 1773–1783. [Google Scholar] [CrossRef] [PubMed]
- Sumanta, S.; Matthias, J.G.; Sebastian, C.P. Crystal structure and magnetic properties of indium flux grown EuAu2In4 and EuAuIn4. Cryst. Growth Des. 2013, 13, 4285–4294. [Google Scholar] [CrossRef]
- Woo, H.; Nam, G; Jang, E.; Kim, J.; Lee, Y.; Ahn, K.; You, T.-S. Single-crystal growth and size control of three novel polar intermetallics: Eu2.94(2)Ca6.06In8Ge8, Eu3.13(2)Ca5.87In8Ge8, and Sr3.23(3)Ca5.77In8Ge8 with crystal structure, chemical bonding, and magnetism studies. Inorg. Chem. 2014, 53, 4669–4677. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.; Jeon, J.; Lee, J.; Kim, J.; You, T.-S. Experimental and theoretical investigations for site-preference and anisotropic size-change of RE11Ge4In6-xMx (RE = La, Ce; M = Li, Ge; x = 1, 1.96). J. Alloys Compd. 2015, 620, 269–276. [Google Scholar] [CrossRef]
- Nam, G.; Jang, E.; You, T.-S. Site-preference among three anions in the quaternary BaAl4-type structure: Experimental and computational investigations for BaLi1.09(1)In0.91Ge2. Bull. Korean Chem. Soc. 2013, 34, 3847–3850. [Google Scholar] [CrossRef]
- Nam, G.; Jeon., J.; Kim, Y.; Kang, S.; Ahn, K.; You, T.-S. Combined effect of chemical pressure and valence electron concentration through the electron-deficient Li-substitution on the RE4LiGe4 (RE = La, Ce, Pr and Sm) system. J. Solid State Chem. 2013, 205, 10–20. [Google Scholar] [CrossRef]
- You, T.-S.; Jung, Y.; Han, M.-K.; Miller, G. The Y5−xMg24+x (1.08(4) < x < 1.30(1)) series and a ternary derivative Ce6.9Y12.5(7)Mg92.2: A comparison of their crystal and electronic structures. J. Solid State Chem. 2013, 204, 170–177. [Google Scholar] [CrossRef]
- Jung, Y.; Nam, G.; Jeon, J.; Kim, Y.; You, T.-S. Crystal structure and chemical bonding of novel Li-containing polar intermetallic compound La11Li12Ge16. J. Solid State Chem. 2012, 196, 543–549. [Google Scholar] [CrossRef]
- You, T.-S.; Bobev, S. Synthesis and structural characterization of A3In2Ge4 and A5In3Ge6 (A = Ca, Sr, Eu, Yb)—New intermetallic compounds with complex structures, exhibiting Ge–Ge and In–In bonding. J. Solid State Chem. 2010, 183, 1258–1265. [Google Scholar] [CrossRef]
- Andersen, O.K. Linear methods in band theory. Phys. Rev. B 1975, 12, 3060–3083. [Google Scholar] [CrossRef]
- Andersen, O.K.; Jepsen, O. Explicit, First-principles tight-binding theory. Phys. Rev. Lett. 1984, 53, 2571–2574. [Google Scholar] [CrossRef]
- Lambrecht, W.R.L.; Andersen, O.K. Minimal basis sets in the linear muffin-tin orbital method: Application to the diamond structure crystals carbon, silicon, and germanium. Phys. Rev. B 1986, 34, 2439–2449. [Google Scholar] [CrossRef]
- Jepsen, O.; Burkhardt, A.; Andersen, O.K. The TB-LMTO-ASA Program, version 4.7; Max-Plank-Institut für Festkorperforschung: Stuttgart, Germany, 1999. [Google Scholar]
- Andersen, O.K.; Jepsen, O.; Glötzel, D. In Highlights of Condensed Matter Theory; Bassani, F., Fumi, F., Tosi, M., Eds.; North-Holland, Lambrecht: Worland, WY, USA, 1985. [Google Scholar]
- Dronskowski, R.; Blöchl, P. Crystal orbital Hamilton populations (COHP): Energy-resolved visualization of chemical bonding in solids based on density-functional calculations. J. Phys. Chem. 1993, 97, 8617–8624. [Google Scholar] [CrossRef]
- Emsley, J. The Elements; Clarendon Press: Oxford, UK, 1990. [Google Scholar]
- Putz, M.V. Markovian approach of the electron localization functions. Int. J. Quant. Chem. 2005, 105, 1–11. [Google Scholar] [CrossRef]
- Putz, M.V. Density functionals of chemical bonding. Int. J. Mol. Sci. 2008, 9, 1050–1095. [Google Scholar] [CrossRef] [PubMed]
- Putz, M.V. Path integrals for electronic densities, reactivity indices, and localization functions in quantum systems. Int. J. Mol. Sci. 2009, 10, 4816–4940. [Google Scholar] [CrossRef] [PubMed]
- Hunter, B.A. International Union of Crystallography, Commission for Powder Diffraction; Newsletter 20; IUCr: Chester, UK, 1998; p. 21. [Google Scholar]
- APEX2; Bruker Analytical X-ray Systems, Inc.: Madison, WI, USA, 2007.
- SAINT; Bruker Analytical X-ray Systems, Inc.: Madison, WI, USA, 2002.
- Sheldrick, G.M. SADABS; University of Göttingen: Göttingen, Germany, 2003. [Google Scholar]
- Sheldrick, G.M. SHELXTL; University of Göttingen: Göttingen, Germany, 2001. [Google Scholar]
- Gelato, L.M.; Parthe, E. STRUCTURE TIDY—A computer program to standardize crystal structure data. J. Appl. Crystallogr. 1987, 20, 139–143. [Google Scholar] [CrossRef]
- Jepsen, O.; Andersen, O.K. Calculated electronic structure of the sandwichd1 metals LaI2 and CeI2: Application of new LMTO techniques. Z. Phys. B 1995, 97, 35–47. [Google Scholar] [CrossRef]
- Blöchl, P.E.; Jepsen, O.; Andersen, O.K. Improved tetrahedron method for Brillouin-zone integrations. Phys. Rev. B 1994, 49, 16223–16233. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, H.; Jang, E.; Kim, J.; Lee, Y.; Kim, J.; You, T.-S. Crystal Structure, Chemical Bonding and Magnetism Studies for Three Quinary Polar Intermetallic Compounds in the (Eu1−xCax)9In8(Ge1−ySny)8 (x = 0.66, y = 0.03) and the (Eu1−xCax)3In(Ge3−ySn1+y) (x = 0.66, 0.68; y = 0.13, 0.27) Phases. Int. J. Mol. Sci. 2015, 16, 9017-9036. https://doi.org/10.3390/ijms16049017
Woo H, Jang E, Kim J, Lee Y, Kim J, You T-S. Crystal Structure, Chemical Bonding and Magnetism Studies for Three Quinary Polar Intermetallic Compounds in the (Eu1−xCax)9In8(Ge1−ySny)8 (x = 0.66, y = 0.03) and the (Eu1−xCax)3In(Ge3−ySn1+y) (x = 0.66, 0.68; y = 0.13, 0.27) Phases. International Journal of Molecular Sciences. 2015; 16(4):9017-9036. https://doi.org/10.3390/ijms16049017
Chicago/Turabian StyleWoo, Hyein, Eunyoung Jang, Jin Kim, Yunho Lee, Jongsik Kim, and Tae-Soo You. 2015. "Crystal Structure, Chemical Bonding and Magnetism Studies for Three Quinary Polar Intermetallic Compounds in the (Eu1−xCax)9In8(Ge1−ySny)8 (x = 0.66, y = 0.03) and the (Eu1−xCax)3In(Ge3−ySn1+y) (x = 0.66, 0.68; y = 0.13, 0.27) Phases" International Journal of Molecular Sciences 16, no. 4: 9017-9036. https://doi.org/10.3390/ijms16049017