Molecular Characterization of Sec2 Loci in Wheat—Secale africanum Derivatives Demonstrates Genomic Divergence of Secale Species
Abstract
:1. Introduction
2. Results
2.1. Identification of Wheat—S. africanum Amphiploid and Substitution
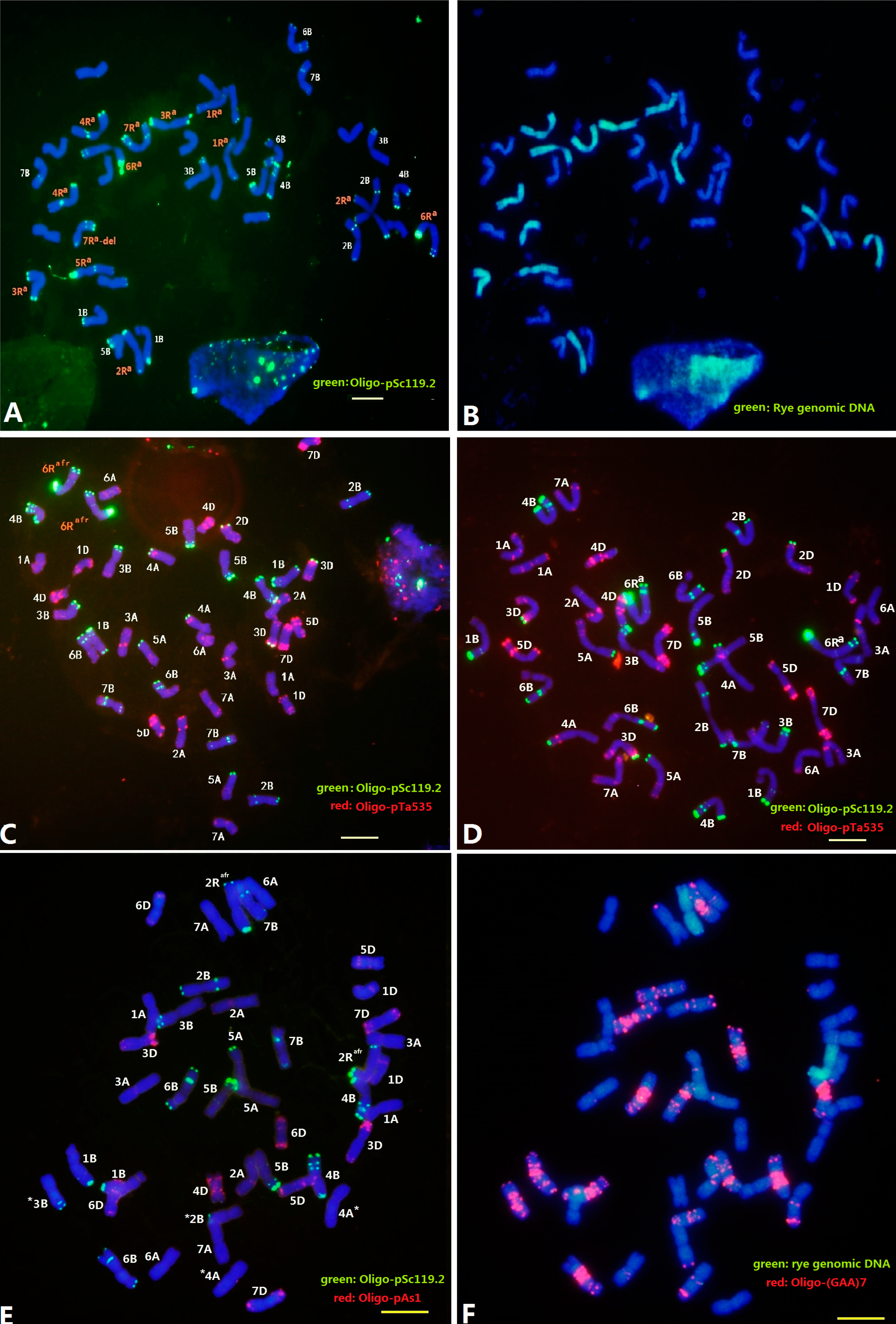
2.2. Localization of Sec2 in the S. africanum Chromosome
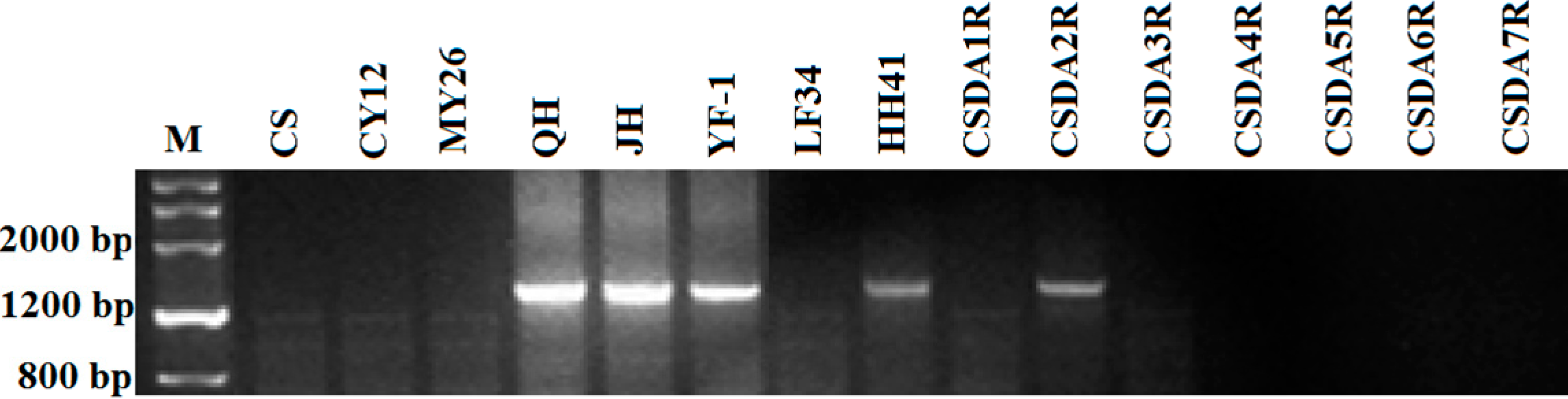
2.3. The Sequence Homology of Sec2 Genes
| Materials | Full-ORF | Pseudogenes |
|---|---|---|
| CSDA2R | JX877767 (1434 bp, 54.19 kD), JX877768 (1416 bp, 53.58 kD), JX877769 (1416 bp, 53.42 kD), JX877770 (1416 bp, 53.58 kD), JX877771 (1395 bp, 52.64 kD), JX877772 (1215 bp, 45.73 kD), JX877773 (1416 bp, 53.58 kD), JX877774 (1434 bp, 54.22 kD), JX877775 (1395 bp, 52.64 kD), JX877776 (1395 bp, 52.64 kD) | |
| S. sylvestre | JX877738 (1503 bp, 56.73 kD), JX877739 (1503 bp, 56.71 kD), JX877740 (1503 bp, 56.71 kD), JX877741 (1503 bp, 56.71 kD), JX877742 (1203 bp, 45.03 kD), JX877743 (1503 bp, 56.70 kD), JX877744 (1503 bp, 56.58 kD), JX877745 (1503 bp, 56.71 kD), JX877746 (1503 bp, 56.73 kD) | |
| YF-1 | JX877747 (1428 bp, 53.98 kD), JX877748 (1428 bp, 54.03 kD), JX877749 (1260 bp, 47.38 kD), JX877750 (1260 bp, 47.38 kD) | JX877751(1371 bp), JX877752(1356 bp), JX877753 (1356 bp), JX877754 (1356 bp), JX877755 (1356 bp) |
| HH41 | JX877756 (1428 bp, 54.39 kD), JX877757 (1464 bp, 55.23 kD), JX877758 (1428 bp, 53.90 kD), JX877759 (1452 bp, 54.64 kD), JX877760 (1311 bp, 49.04 kD) | JX877761 (1320 bp), JX877762( 1332 bp), JX877763 (1332 bp), JX877764 (1332 bp), JX877765 (1320 bp), JX877766 (1332 bp) |
2.4. The Amino Acid Sequences of Sec2 Genes

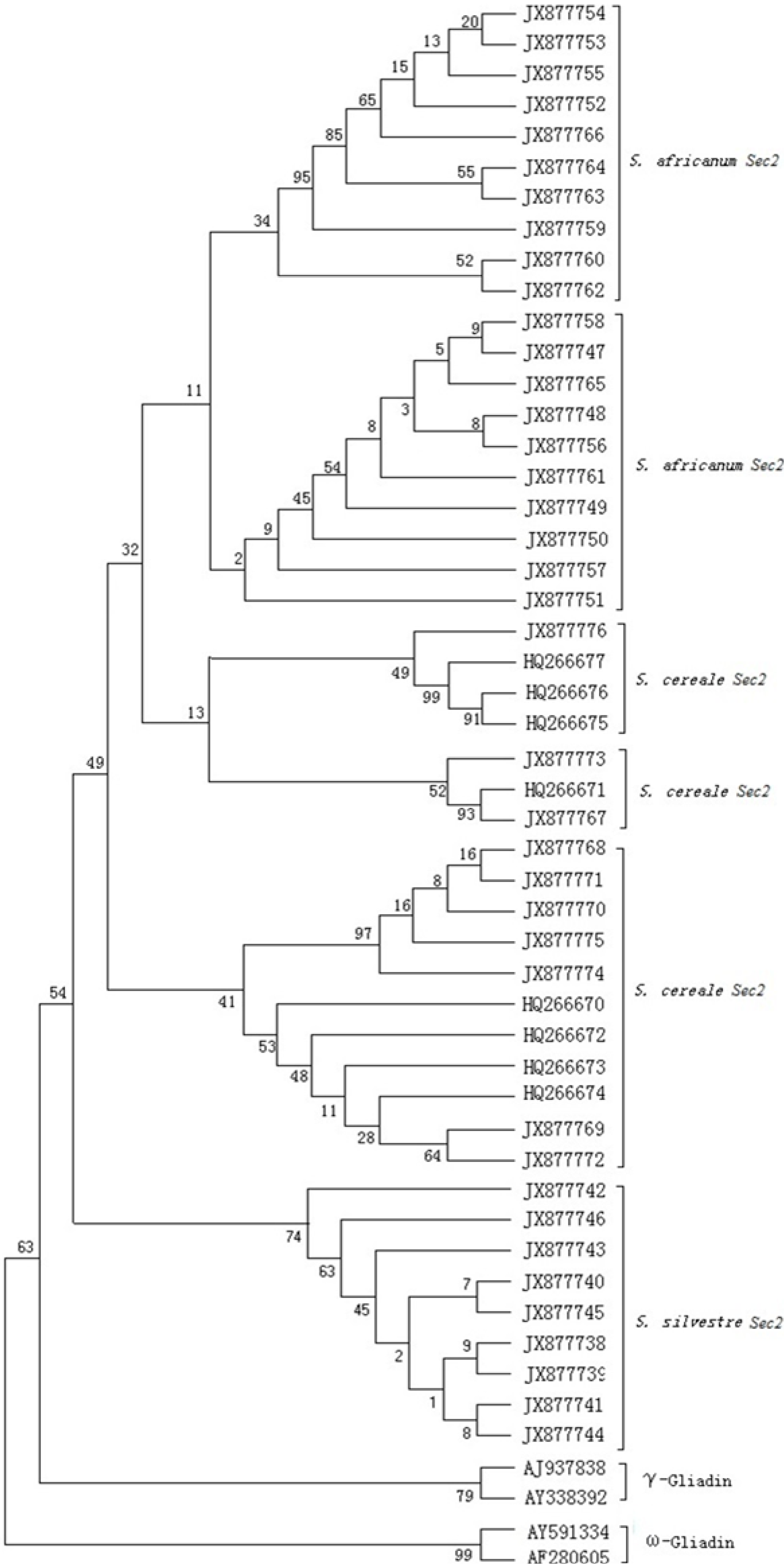
2.5. Phylogenetic Tree and the Evolutionary Analyses
3. Discussion
4. Experimental Section
4.1. Plant Materials
4.2. Genomic in Situ Hybridization and Fluorescence in Situ Hybridization
4.3. Primer Design, PCR Cloning and Sequencing
4.4. Phylogenetic Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vavilov, N.I. Studies on the origin of cultivated plants. Bull. Appl. Bot. Plant Breed. 1926, 2, 1–248. [Google Scholar]
- Tang, Z.X.; Ross, K.; Ren, Z.L.; Yang, Z.J.; Zhang, H.Y.; Chikmawati, T.; Miftahudin; Gustafson, J.P. Wealth of wild species: Role in plant genome elucidation and improvement—Secale. In Wild Crop Relatives: Genomic and Breeding Resources Cereals; Kole, C., Ed.; Springer: Berlin, Germany, 2011; pp. 367–395. [Google Scholar]
- Cuadrado, A.; Jouve, N. Evolutionary trends of different repetitive DNA sequences during speciation in the genus Secale. J. Hered. 2002, 93, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Riley, R. The cytogenetics of the differences between some Secale species. J. Agric. Sci. 1955, 46, 377–383. [Google Scholar] [CrossRef]
- Stutz, H.C. On the origin of cultivated rye. Am. J. Bot. 1972, 59, 59–70. [Google Scholar] [CrossRef]
- Devos, K.M.; Atkinson, M.D.; Chinoy, C.N.; Francis, H.A.; Harcourt, R.L.; Koebner, R.M.D.; Liu, C.J.; Masojc, P.; Xie, D.X.; Gale, M.D. Chromosomal rearrangements in the rye genome relative to that of wheat. Theor. Appl. Genet. 1993, 85, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Martis, M.M.; Zhou, R.; Haseneyer, G.; Schmutzer, T.; Vrána, J.; Kubaláková, M.; König, S.; Kugler, K.G.; Scholz, U.; Hackauf, B.; et al. Reticulate evolution of the rye genome. Plant Cell 2013, 25, 3685–3698. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.; Khoshbakht, K. Towards a “red list” for crop plant species. Genet. Resour. Crop. Evol. 2005, 52, 249–265. [Google Scholar] [CrossRef]
- Lukaszewski, A.J.; Gustafson, J.P. Cytogenetics of triticale. In Plant Breeding Reviews; Janick, J., Ed.; AVI Publishing: New York, NY, USA, 1987; Volume 5, pp. 41–93. [Google Scholar]
- Yang, Z.J.; Li, G.R.; Jia, J.Q.; Zeng, X.; Lei, M.P.; Zeng, Z.X.; Zhang, T.; Ren, Z.L. Molecular cytogenetic characterization of wheat—Secale africanum amphiploids and derived introgression lines with stripe rust resistance. Euphytica 2009, 167, 197–202. [Google Scholar] [CrossRef]
- Lei, M.P.; Li, G.R.; Liu, C.; Yang, Z.J. Characterization of new wheat—Secale africanum derivatives reveals evolutionary aspects of chromosome 1R in rye. Genome 2012, 55, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.P.; Li, G.R.; Zhang, S.F.; Liu, C.; Yang, Z.J. Molecular cytogenetic characterization of a new wheat—Secale africanum 2Ra (2D) substitution line for resistant to stripe rust. J. Genet. 2011, 90, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.P.; Li, G.R.; Zhou, L.; Li, C.H.; Liu, C.; Yang, Z.J. Identification of wheat—Secale africanum chromosome 2Rafr introgression lines with novel disease resistance and agronomic characteristics. Euphytica 2013, 194, 197–205. [Google Scholar] [CrossRef]
- Yang, Z.J.; Li, G.R.; Ren, Z.L. Identification of amphiploid between Triticum durum cv. Ailanmai native to Sichuan, China and Secale africanum. Wheat Inform. Serv. 2000, 91, 20–24. [Google Scholar]
- Yang, Z.J.; Li, G.R.; Ren, Z.L. Identification of Triticum durum-Secale africanum amphiploid and its crossability with common wheat. J. Genet. Breed. 2001; 55, 45–50. [Google Scholar]
- Lei, M.P.; Li, G.R.; Liu, C.; Yang, Z.J. Molecular cytogenetic characterization of a Triticum durum—Secale africanum substitution line for resistance to stripe rust (Puccinia striiformis Eriks. f. sp. tritici). J. Agric. Biotechnol. 2013, 21, 263–271. [Google Scholar]
- Shewry, P.R.; Parmar, S.; Miller, T.E. Chromosomal location of the structural genes for the Mr 75,000 γ-secalins in Secale montanum Guss: Evidence for a translocation involving chromosomes 2R and 6R in cultivated rye (Secale. cereale L.). Heredity 1985, 54, 381–383. [Google Scholar] [CrossRef]
- Shewry, P.R.; Field, J.M. The purification and characterization of two groups of storage proteins (secalins) from rye (Secale cereale L.). J. Exp. Bot. 1982, 33, 261–268. [Google Scholar] [CrossRef]
- Shewry, P.R.; Kreis, M.; Burgess, S.R.; Parmer, S.; Miflin, B.J. The synthesis and deposition of the prolamin storage proteins (secalins) of rye. Planta 1983, 159, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Gellrich, C.; Schieberle, P.; Wieser, H. Studies of partial amino acid sequences of γ-40k secalins of rye. Cereal Chem. 2005, 82, 541–545. [Google Scholar] [CrossRef]
- Lawrence, G.J.; Shepherd, K.W. Chromosomal location of genes controlling seed proteins in species related to wheat. Theor. Appl. Genet. 1981, 59, 25–31. [Google Scholar] [PubMed]
- Shewry, P.R.; Bradberry, D.; Franklin, J.; White, R.P. The chromosomal locations and linkage relationships of the structural genes for the prolamin storage proteins (secalins) of rye. Theor. Appl. Genet. 1984, 69, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.R.; Miflin, B.J.; Kasarda, D.D. The structural and evolutionary relationships of the prolamin storage proteins of barley, rye and wheat. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1984, 304, 297–308. [Google Scholar] [CrossRef]
- Hull, G.; Sabelli, P.A.; Shewry, P.R. Restriction fragment analysis of the secalin loci of rye. Biochem. Genet. 1992, 30, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Murray, F.R.; Skerritt, J.H.; Appels, R. A gene from the Sec2 (Gli-R2) locus of a wheat 2RS.2BL chromosomal translocation line. Theor. Appl. Genet. 2001, 102, 431–439. [Google Scholar] [CrossRef]
- Chen, Q.J.; Yuan, Z.W.; Zhang, L.Q.; Yan, Z.H.; Xiang, Z.G.; Wan, Y.F.; Zheng, Y.L.; Liu, D.C. Molecular characterization and comparative analysis of four new genes from Sec2 locus encoding 75K γ-secalin of rye species. J. Cereal Sci. 2008, 48, 111–116. [Google Scholar] [CrossRef]
- Allaby, R.G.; Banerjee, M.; Brown, T.A. Evolution of the high molecular weight glutenin loci of the A, B, D, and G genomes of wheat. Genome 1999, 42, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Gaut, B.S.; Morton, B.R.; McCraig, B.C.; Clegg, M.T. Substitution rate comparisons between grasses and palms: Synonymous rate differences at the nuclear gene Adh parallel rate differences at the plastid gene rbcL. Proc. Natl. Acad. Sci. USA 1996, 93, 10274–10279. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, B.; Liu, B.; Zhang, H.; Liu, D. Structure and evolutionary relationships among paralogous genes within the Sec2 locus in rye. J. Cereal Sci. 2012, 56, 282–288. [Google Scholar] [CrossRef]
- Middleton, C.P.; Senerchia, N.; Stein, N.; Akhunov, E.D.; Keller, B.; Wicker, T.; Kilian, B. Sequencing of chloroplast genomes from wheat, barley, rye and their relatives provides a detailed insight into the evolution of the Triticeae tribe. PLoS ONE 2014, 9, e85761. [Google Scholar] [CrossRef] [PubMed]
- Jenabi, T.; Saeidi, H.; Rahiminejad, M.R. Biodiversity of Secale strictum in Iran measured using microsatellites. Genet. Resour. Crop. Evol. 2011, 58, 497–505. [Google Scholar] [CrossRef]
- Huang, S.; Sirikhachornkit, A.; Faris, J.D.; Su, X.; Gill, B.S.; Haselkorn, R.; Gornicki, P. Phylogenetic analysis of the acetyl-CoA carboxylase and 3-phosphoglycerate kinase loci in wheat and other grasses. Plant Mol. Biol. 2002, 48, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Lv, Z.; Guo, X.; Zhang, X.; Han, F. Alteration of terminal heterochromatin and chromosome rearrangements in derivatives of wheat-rye hybrids. J. Genet. Genomics 2013, 40, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Yang, M.; Fei, Y.; Tan, F.; Ren, Z.; Yan, B.; Zhang, H.; Tang, Z. Alterations and abnormal mitosis of wheat chromosomes induced by wheat-rye monosomic addition lines. PLoS ONE 2013, 8, e70483. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Yang, Z.; Fu, S. Oligonucleotides replacing the roles of repetitive sequences pAs1, pSc119.2, pTa-535, pTa71, CCS1, and pAWRC.1 for FISH analysis. J. Appl. Genet. 2014, 55, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lang, T.; Dai, G.; Li, D.; Li, C.; Song, X.; Yang, Z. Precise identification of two wheat—Thinopyrum intermedium substitutions reveals the compensation and rearrangement between wheat and Thinopyrum chromosomes. Mol. Breed. 2015, 35, 1. [Google Scholar] [CrossRef]
- NCBI network service. Available online: http://www.ncbi.nlm.nih.gov/ (Accessed on 3 March 2015).
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef] [PubMed]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Nei, M. Molecular Evolutionary Genetics; Columbia University Press: New York, NY, USA, 1987. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Zhang, H.; Zhou, L.; Gao, D.; Lei, M.; Zhang, J.; Yang, Z. Molecular Characterization of Sec2 Loci in Wheat—Secale africanum Derivatives Demonstrates Genomic Divergence of Secale Species. Int. J. Mol. Sci. 2015, 16, 8324-8336. https://doi.org/10.3390/ijms16048324
Li G, Zhang H, Zhou L, Gao D, Lei M, Zhang J, Yang Z. Molecular Characterization of Sec2 Loci in Wheat—Secale africanum Derivatives Demonstrates Genomic Divergence of Secale Species. International Journal of Molecular Sciences. 2015; 16(4):8324-8336. https://doi.org/10.3390/ijms16048324
Chicago/Turabian StyleLi, Guangrong, Hongjun Zhang, Li Zhou, Dan Gao, Mengping Lei, Jie Zhang, and Zujun Yang. 2015. "Molecular Characterization of Sec2 Loci in Wheat—Secale africanum Derivatives Demonstrates Genomic Divergence of Secale Species" International Journal of Molecular Sciences 16, no. 4: 8324-8336. https://doi.org/10.3390/ijms16048324







