Quercetin Improves Postischemic Recovery of Heart Function in Doxorubicin-Treated Rats and Prevents Doxorubicin-Induced Matrix Metalloproteinase-2 Activation and Apoptosis Induction
Abstract
:1. Introduction
2. Results
2.1. Effects of Quercetin on Biometric Parameters
| Parameter | C | QCT | DOX | DOX–QCT |
|---|---|---|---|---|
| BW (g) | 432.7 ± 13.3 | 428.6 ± 21.3 | 384.6 ± 16.8 a | 355.4 ± 17.1 |
| Weight gain (g) | 154.8 ± 11.9 | 153.5 ± 14.4 | 107.2 ± 13.2 a | 89.3 ± 16.3 |
| HW (g) | 1.43 ± 0.09 | 1.37 ± 0.12 | 1.26 ± 0.07 | 1.11 ± 0.06 |
| HW/BW | 3.325 ± 0.196 | 3.162 ± 0.211 | 3.282 ± 0.176 | 3.170 ± 0.174 |
| LV (mg) | 870.6 ± 51.3 | 841.7 ± 86.3 | 822.7 ± 39.5 | 740.8 ± 34.0 |
| SBP (mm·Hg) | 113 ± 4 | 120 ± 3 | 137 ± 4 a | 118 ± 5 b |
| HR (beats/min) | 369 ± 5 | 376 ± 16 | 392 ± 6 a | 368 ± 9 b |
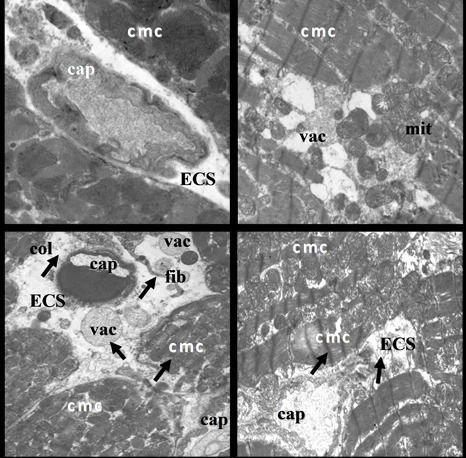
2.2. Electron Microscopic Analysis of Quercetin Effects on Ultrastructural Changes Induced by Doxorubicin
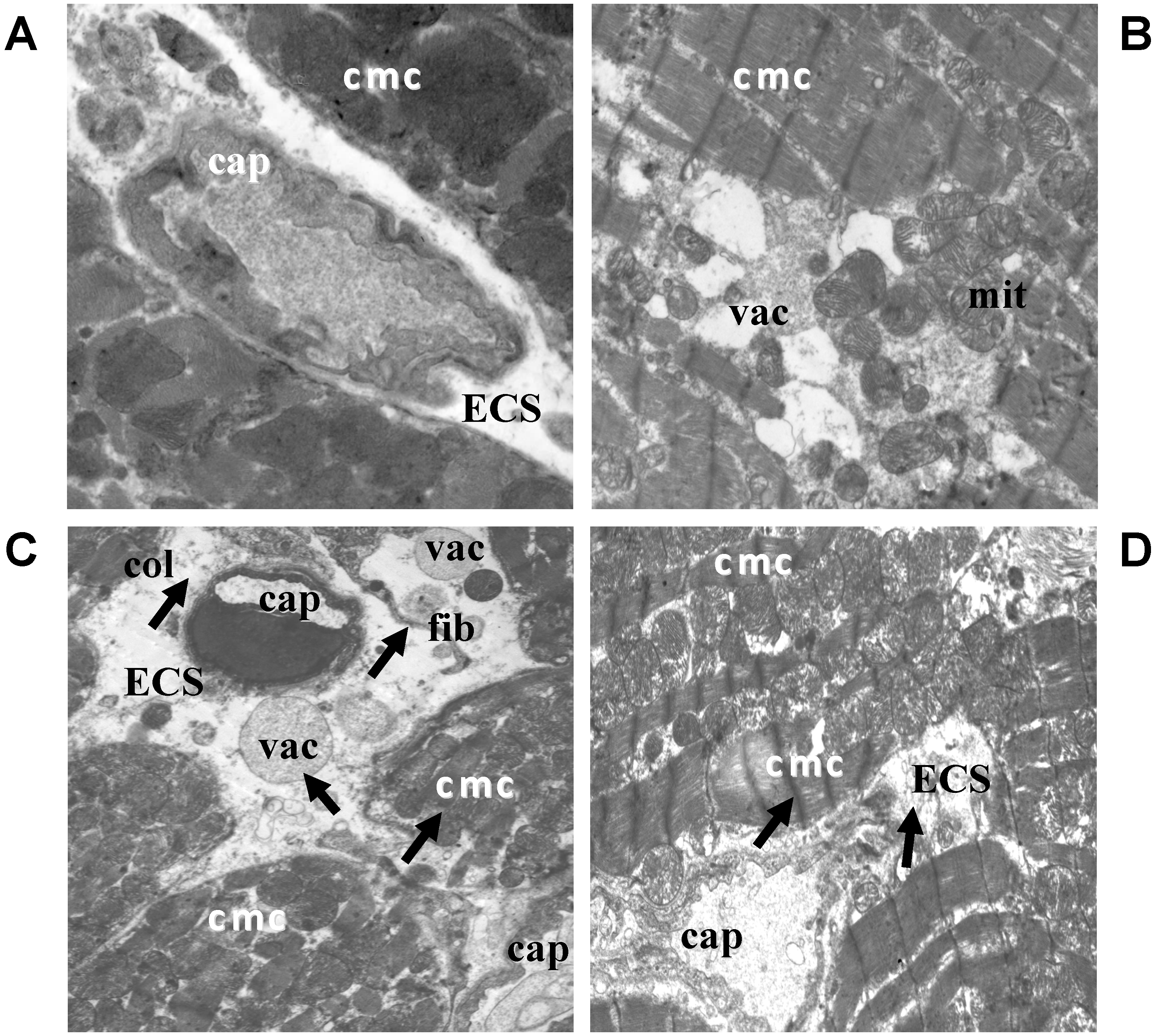
2.3. Quercetin Treatment Modulates the Doxorubicin-Induced Effects on Matrix Metalloproteinase-2
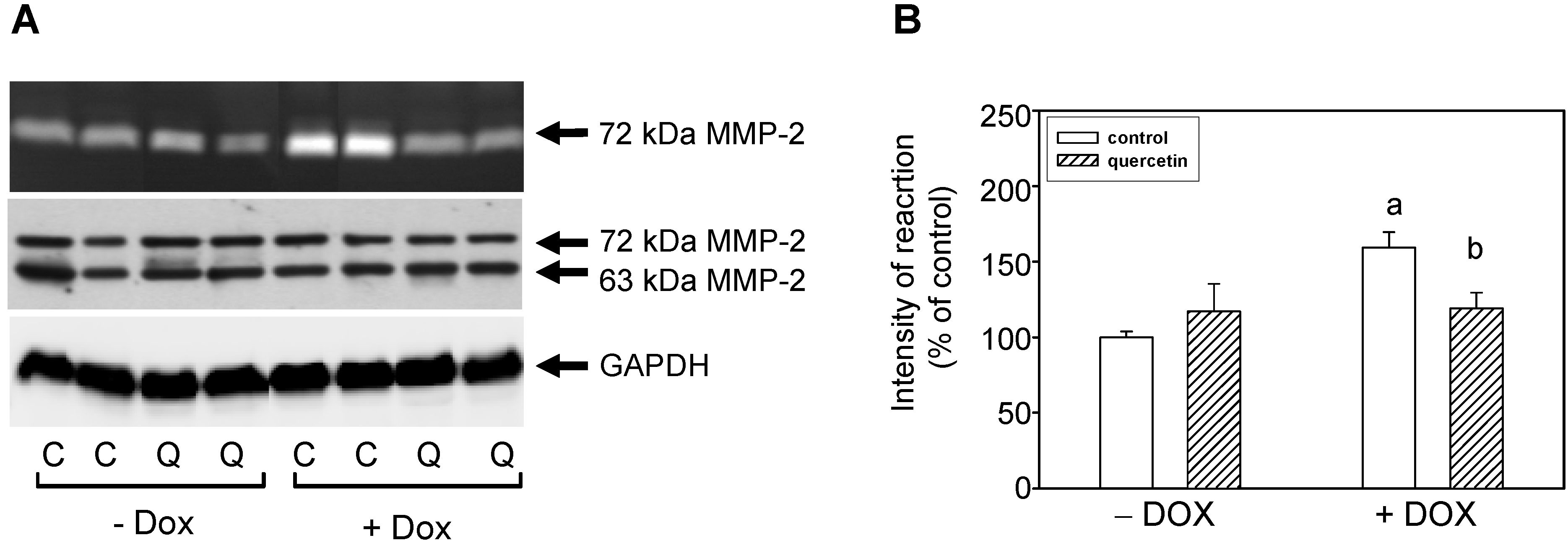

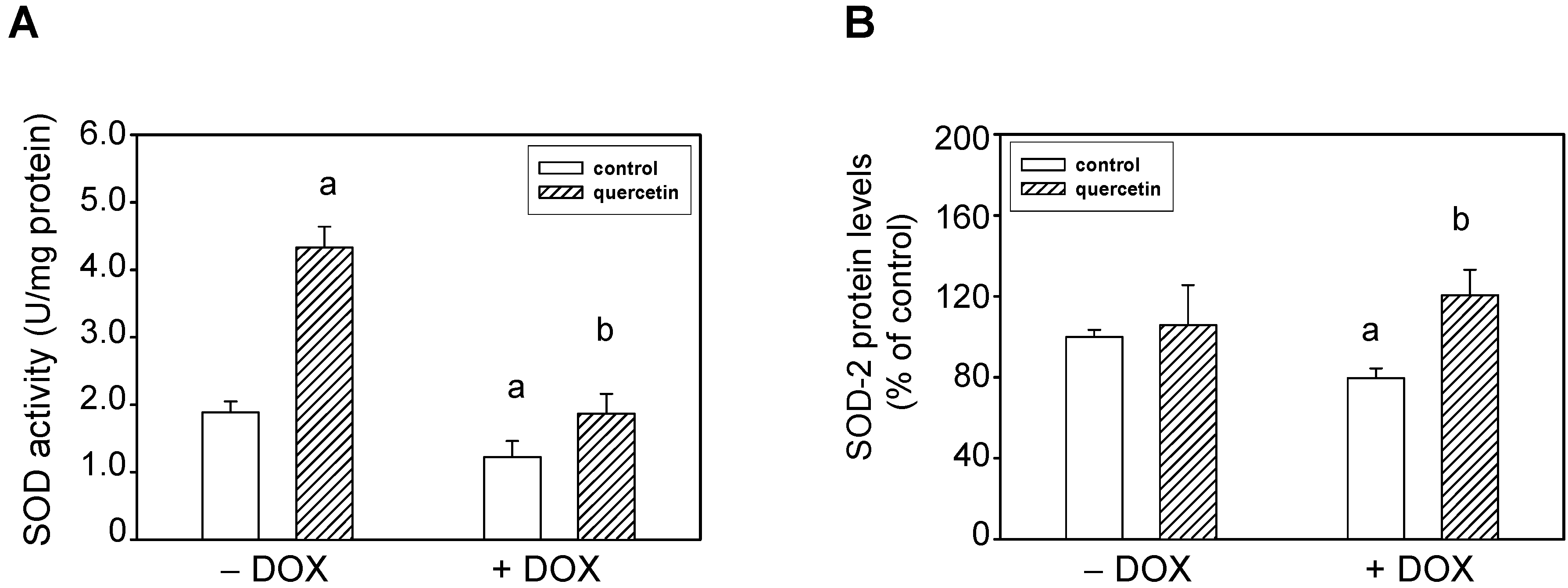
2.4. Quercetin Prevents the Negative Effects of Doxorubicin on Apoptosis Induction and Superoxide Dismutase Inhibition

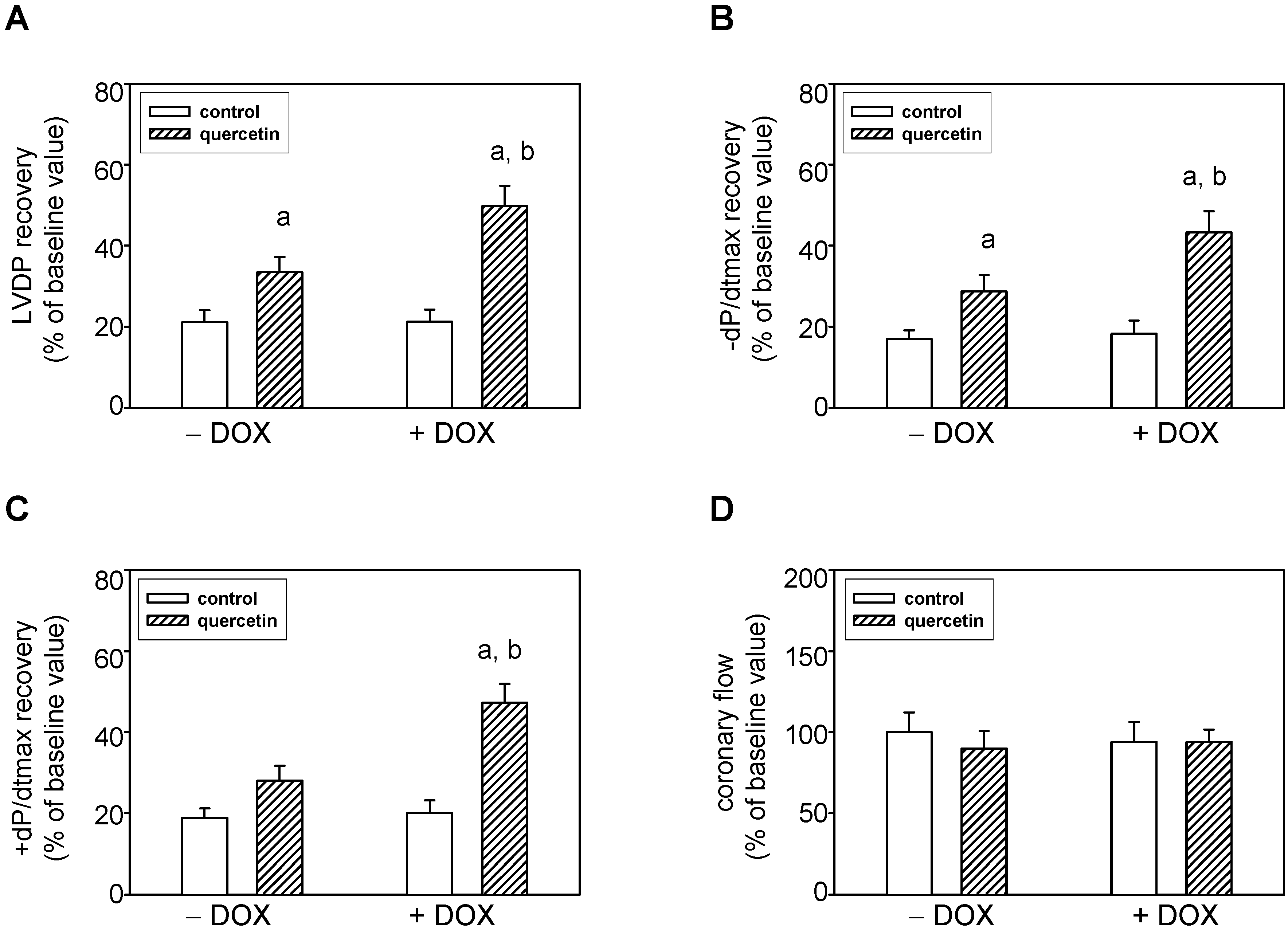
2.5. Prolonged Application of Quercetin Improves Postischemic Recovery of Cardiac Function of Isolated Rat Hearts
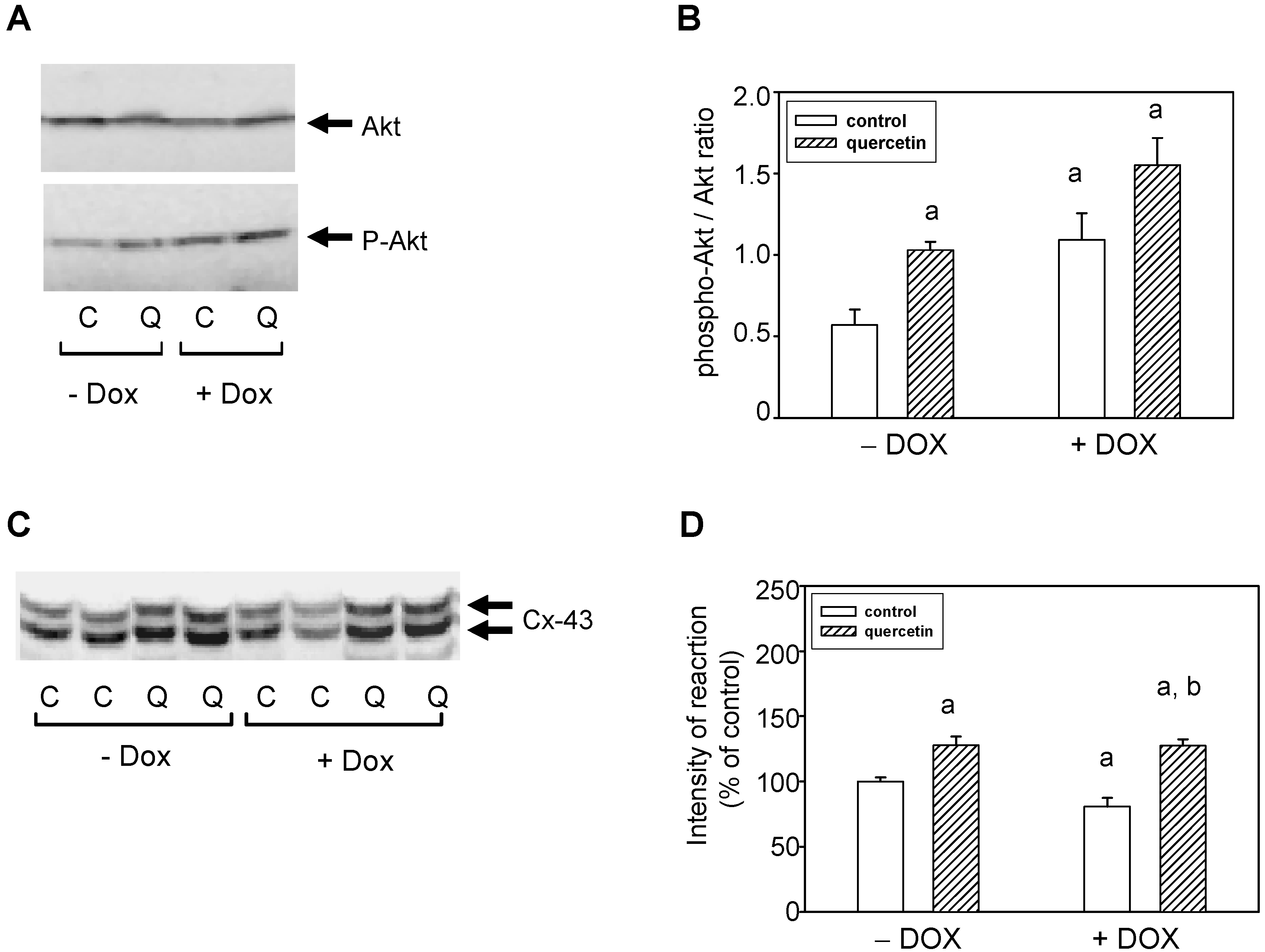
2.6. Cardioprotective Effects of Quercetin Are Associated with Akt Kinase Activation and Connexin-43 Up-Regulation
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Experimental Model
4.3. Systolic Blood Pressure and Heart Rate Measurement
4.4. Perfusion Technique and Determination of Heart Function
4.5. Samples Collection
4.6. Transmission Electron Microscopy
4.7. Preparation of Tissue Protein Fractions and Western Blot Analysis
4.8. Measurement of MMP-2 Activities by Gelatin Zymography
4.9. Determination of Superoxide Dismutase Activity
4.10. Statistical Evaluation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hertog, M.G.; Bueno-de-Mesquita, H.B.; Fehily, A.M.; Sweetnam, P.M.; Elwood, P.C.; Kromhout, D. Fruit and vegetable consumption and cancer mortality in the Caerphilly Study. Cancer Epidemiol. Biomark. Prev. 1996, 5, 673–677. [Google Scholar]
- Erden Inal, M.; Kahraman, A. The protective effect of flavonol quercetin against ultraviolet a induced oxidative stress in rats. Toxicology 2000, 154, 21–29. [Google Scholar]
- Wu, J.; Xu, X.; Li, Y.; Kou, J.; Huang, F.; Liu, B.; Liu, K. Quercetin, luteolin and epigallocatechin gallate alleviate TXNIP and NLRP3-mediated inflammation and apoptosis with regulation of AMPK in endothelial cells. Eur. J. Pharmacol. 2014, 745, 59–68. [Google Scholar]
- Yu, P.X.; Zhou, Q.J.; Zhu, W.W.; Wu, Y.H.; Wu, L.C.; Lin, X.; Chen, M.H.; Qiu, B.T. Effects of quercetin on LPS-induced disseminated intravascular coagulation (DIC) in rabbits. Thromb. Res. 2013, 131, 270–273. [Google Scholar]
- Duarte, J.; Perez-Palencia, R.; Vargas, F.; Ocete, M.A.; Perez-Vizcaino, F.; Zarzuelo, A.; Tamargo, J. Antihypertensive effects of the flavonoid quercetin in spontaneously hypertensive rats. Br. J. Pharmacol. 2001, 133, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.; Galisteo, M.; Vera, R.; Villar, I.C.; Zarzuelo, A.; Tamargo, J.; Pérez-Vizcaíno, F.; Duarte, J. Quercetin downregulates NADPH oxidase, increases eNOS activity and prevents endothelial dysfunction in spontaneously hypertensive rats. J. Hypertens. 2006, 24, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y; Oue, E. Antihypertensive effect of quercetin in rats fed with a high-fat high-sucrose diet. Biosci. Biotechnol. Biochem. 2006, 70, 933–939. [Google Scholar]
- Jin, H.B.; Yang, Y.B.; Song, Y.L.; Zhang, Y.C.; Li, Y.R. Protective roles of quercetin in acute myocardial ischemia and reperfusion injury in rats. Mol. Biol. Rep. 2012, 39, 11005–11009. [Google Scholar] [CrossRef] [PubMed]
- Bartekova, M.; Carnicka, S.; Pancza, D.; Ondrejcakova, M.; Breier, A.; Ravingerova, T. Acute treatment with polyphenol quercetin improves postischemic recovery of isolated perfused rat hearts after global ischemia. Can. J. Physiol. Pharmacol. 2010, 88, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Annapurna, A.; Reddy, C.S.; Akondi, R.B.; Rao, S.R. Cardioprotective actions of two bioflavonoids, quercetin and rutin, in experimental myocardial infarction in both normal and streptozotocin-induced type I diabetic rats. J. Pharmacy Pharmacol. 2009, 61, 1365–1374. [Google Scholar]
- Milton Prabu, S; Muthumani, M.; Shagirtha, K. Quercetin potentially attenuates cadmium induced oxidative stress mediated cardiotoxicity and dyslipidemia in rats. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 582–595. [Google Scholar] [PubMed]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef] [PubMed]
- Arola, O.J.; Saraste, A.; Pulkki, K.; Kallajoki, M.; Parvinen, M.; Voipio-Pulkki, L.M. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res. 2000, 60, 1789–1792. [Google Scholar] [PubMed]
- Singal, P.K.; Iliskovic, N. Doxorubicin-induced cardiomyopathy. N. Engl. J. Med. 1998, 339, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Gharanei, M.; Hussain, A.; Janneh, O.; Maddock, H.L. Doxorubicin induced myocardial injury is exacerbated following ischaemic stress via opening of the mitochondrial permeability transition pore. Toxicol. Appl. Pharmacol. 2013, 268, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Simoncíkova, P.; Ravingerova, T.; Barancik, M. The effect of chronic doxorubicin treatment on mitogen-activated protein kinases and heat stress proteins in rat hearts. Physiol. Res. 2008, 57, S97–S102. [Google Scholar] [PubMed]
- Mackraj, I.; Govender, T.; Ramesar, S. The antihypertensive effects of quercetin in a salt-sensitive model of hypertension. J. Cardiovasc. Pharmacol. 2008, 51, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Rendig, S.V.; Symons, J.D.; Longhurst, J.C.; Amsterdam, E.A. Effects of red wine, alcohol, and quercetin on coronary resistance and conductance arteries. J. Cardiovasc. Pharmacol. 2001, 38, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Adamcová, M.; Potacova, A.; Popelova, O.; Sterba, M.; Mazurova, Y.; Aupperle, H.; Geršl, V. Cardiac remodeling and MMPs on the model of chronic daunorubicin-induced cardiomyopathy in rabbits. Physiol. Res. 2010, 59, 831–836. [Google Scholar] [PubMed]
- Ivanova, M.; Dovinova, I.; Okruhlicová, L.; Tribulova, N.; Simoncíkova, P.; Bartekova, M.; Vlkovicova, J.; Barancik, M. Chronic cardiotoxicity of doxorubicin involves activation of myocardial and circulating matrix metalloproteinases in rats. Acta Pharmacol. Sin. 2012, 33, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Bai, P.; Mabley, J.G.; Liaudet, L.; Virag, L.; Szabo, C.; Pacher, P. Matrix metalloproteinase activation is an early event in doxorubicin-induced cardiotoxicity. Oncol. Rep. 2004, 11, 505–508. [Google Scholar] [PubMed]
- Okamoto, T; Akaike, T.; Sawa, T.; Miyamoto, Y.; van derVliet, A.; Maeda, H. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J. Biol. Chem. 2001, 276, 29596–29602. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R. Intracellular targets of matrix metalloproteinase-2 in cardiac disease: Rationale and therapeutic approaches. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 211–242. [Google Scholar] [CrossRef] [PubMed]
- Viappiani, S.; Nicolescu, A.C.; Holt, A.; Sawicki, G.; Crawford, B.D.; Leon, H.; van Mulligen, T.; Schulz, R. Activation and modulation of 72 kDa matrix metalloproteinase-2 by peroxynitrite and glutathione. Biochem. Pharmacol. 2009, 77, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Kanwar, M. Oxidative stress and the development of diabetic retinopathy: Contributory role of matrix metalloproteinase-2. Free Radic. Biol. Med. 2009, 46, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Mocanu, M.M.; Yellon, D.M. Cross-talk between the survival kinases during early reperfusion: Its contribution to ischemic preconditioning. Cardiovasc. Res. 2004, 63, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Strniskova, M.; Ravingerova, T.; Neckar, J.; Kolar, F.; Pastorekova, S.; Barancik, M. Changes in the expression and/or activation of regulatory proteins in rat hearts adapted to chronic hypoxia. Gen. Physiol. Biophys. 2006, 25, 25–41. [Google Scholar] [PubMed]
- Ravingerova, T.; Matejikova, J.; Neckar, J.; Andelova, E.; Kolar, F. Differential role of PI3K/Akt pathway in the infarct size limitation and antiarrhythmic protection in the rat heart. Mol. Cell. Biochem. 2007, 297, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Tao, J.; del Monte, F.; Lee, K.H.; Li, L.; Picard, M.; Force, T.L.; Franke, T.F.; Hajjar, R.J.; Rosenzweig, A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation 2001, 104, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Tanno, M.; Sato, T. Mitochondrial kinase signaling pathways in myocardial protection from ischaemia/reperfusion-induced necrosis. Cardiovasc. Res. 2010, 88, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Budas, G.R.; Sukhodub, A.; Alessi, D.R.; Jovanovic, A. 3' Phosphoinositidedependent kinase-1 is essential for ischemic preconditioning of the myocardium. FASEB J. 2006, 20, 2556–2558. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Gao, H.; Wang, H.; Li, L.; Di, C.; Luan, R.; Tao, L. Ischemic postconditioning protects both adult and aged Sprague Dawley rat hearts from ischemia/reperfusion injury through the PI3-K/Akt and GSK-3β pathway. Clin. Exp. Pharmacol. Physiol. 2009, 36, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Ding, V.W.; Chen, R.H.; McCormick, F. Differential regulation of glycogen synthase kinase 3β by insulin and Wnt signaling. J. Biol. Chem. 2000, 275, 32475–32481. [Google Scholar] [CrossRef] [PubMed]
- Issan, Y.; Kornowski, R.; Aravot, D.; Shainberg, A.; Laniado-Schwartzman, M.; Sodhi, K.; Abraham, N.G.; Hochhauser, E. Heme oxygenase-1 induction improves cardiac function following myocardial ischemia by reducing oxidative stress. PLoS ONE 2014, 21, e92246. [Google Scholar] [CrossRef]
- Srisakuldee, W.; Jeyaraman, M.M.; Nickel, B.E.; Tanguy, S.; Jiang, Z.S.; Kardami, E. Phosphorylation of connexin-43 at serine 262 promotes a cardiac injury-resistant state. Cardiovasc. Res. 2009, 83, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Dorado, D.; Rodriguez-Sinovas, A.; Ruiz-Meana, M. Gap junction-mediated spread of cell injury and death during myocardial ischemia-reperfusion. Cardiovasc. Res. 2004, 61, 386–401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, A.; Guo, C.; Shi, C.; Zhang, Y.; Liu, Q.; Sparatore, A; Wang, C. S-diclofenac protects against doxorubicin-induced cardiomyopathy in mice via ameliorating cardiac gap junction remodeling. PLoS ONE 2011, 6, e26441. [Google Scholar]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quanties of protein utilizing the principle of protein-dye-binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barteková, M.; Šimončíková, P.; Fogarassyová, M.; Ivanová, M.; Okruhlicová, Ľ.; Tribulová, N.; Dovinová, I.; Barančík, M. Quercetin Improves Postischemic Recovery of Heart Function in Doxorubicin-Treated Rats and Prevents Doxorubicin-Induced Matrix Metalloproteinase-2 Activation and Apoptosis Induction. Int. J. Mol. Sci. 2015, 16, 8168-8185. https://doi.org/10.3390/ijms16048168
Barteková M, Šimončíková P, Fogarassyová M, Ivanová M, Okruhlicová Ľ, Tribulová N, Dovinová I, Barančík M. Quercetin Improves Postischemic Recovery of Heart Function in Doxorubicin-Treated Rats and Prevents Doxorubicin-Induced Matrix Metalloproteinase-2 Activation and Apoptosis Induction. International Journal of Molecular Sciences. 2015; 16(4):8168-8185. https://doi.org/10.3390/ijms16048168
Chicago/Turabian StyleBarteková, Monika, Petra Šimončíková, Mária Fogarassyová, Monika Ivanová, Ľudmila Okruhlicová, Narcisa Tribulová, Ima Dovinová, and Miroslav Barančík. 2015. "Quercetin Improves Postischemic Recovery of Heart Function in Doxorubicin-Treated Rats and Prevents Doxorubicin-Induced Matrix Metalloproteinase-2 Activation and Apoptosis Induction" International Journal of Molecular Sciences 16, no. 4: 8168-8185. https://doi.org/10.3390/ijms16048168