Design of an Osteoinductive Extracellular Fibronectin Matrix Protein for Bone Tissue Engineering
Abstract
:1. Introduction
2. Results and Discussion
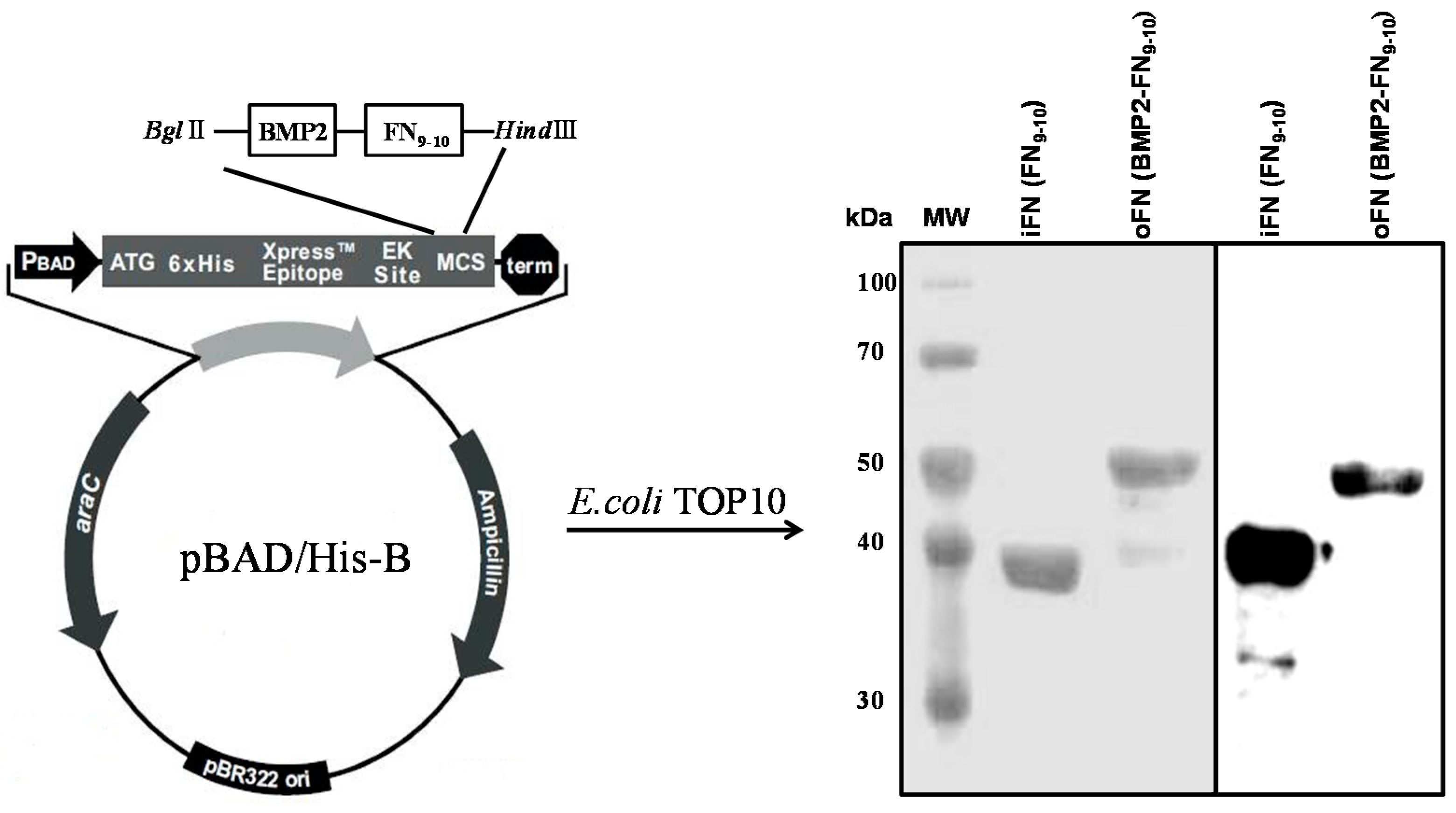
2.1. Expression and Purification of oFN Matrix Protein
2.2. Cell Adhesion Activity of oFN Matrix Protein
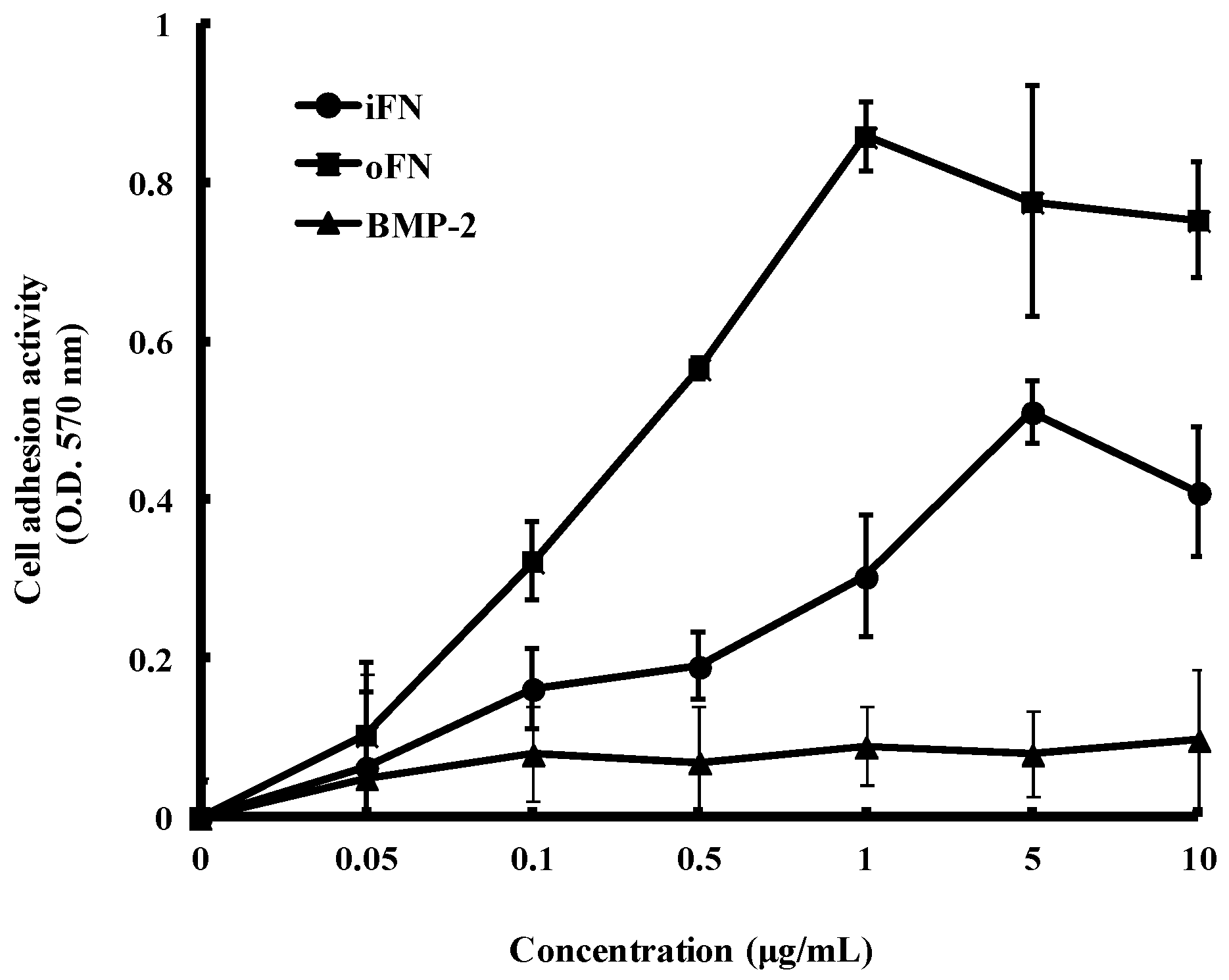
2.3. Osteogenic Differentiation Activity of oFN Matrix Protein
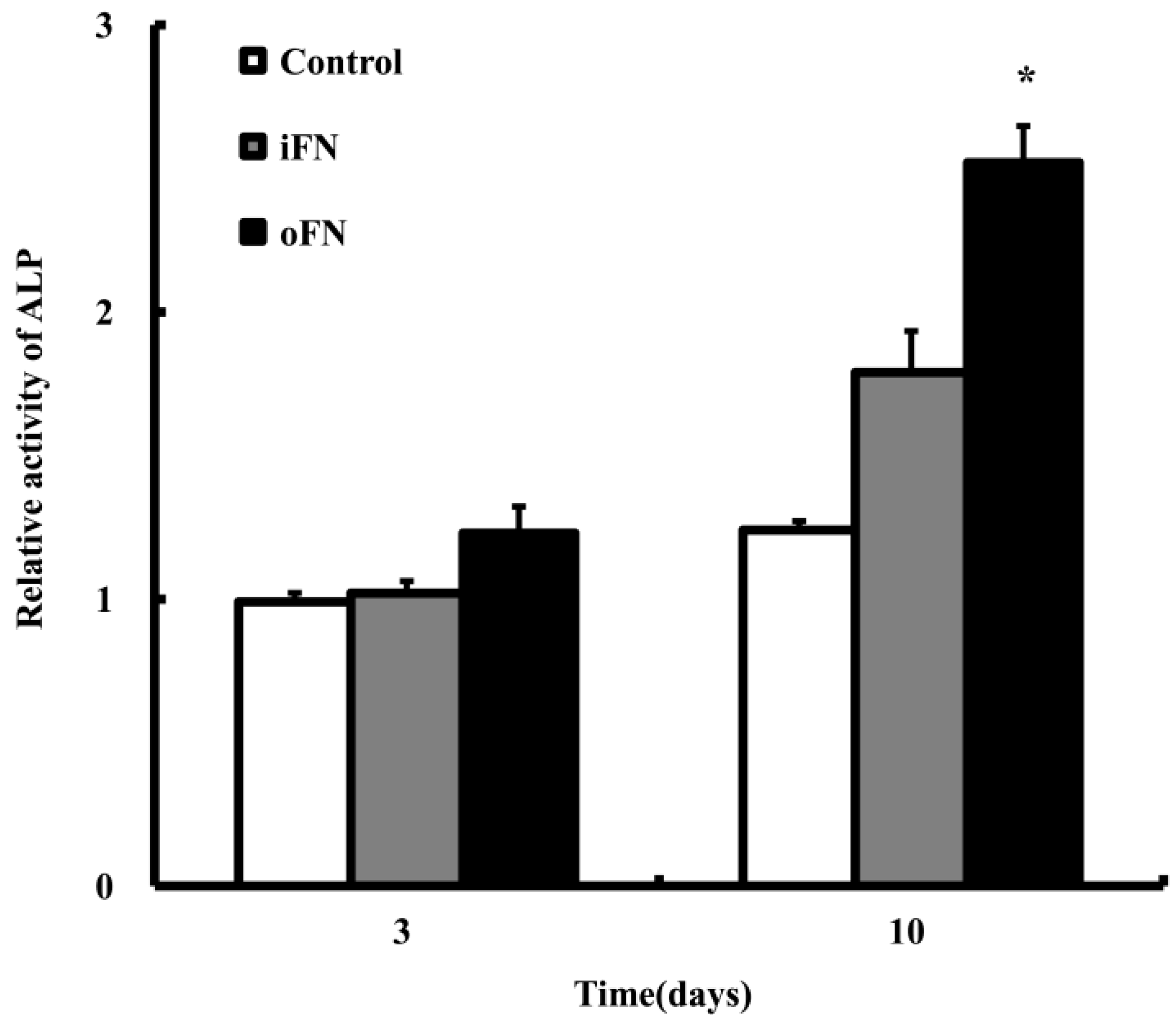
2.4. Effect of oFN-Loaded Collagen Matrix on MC3T3-E1 Cells Differentiation

2.5. Osteogenic Differentiation Activity of oFN Matrix Protein
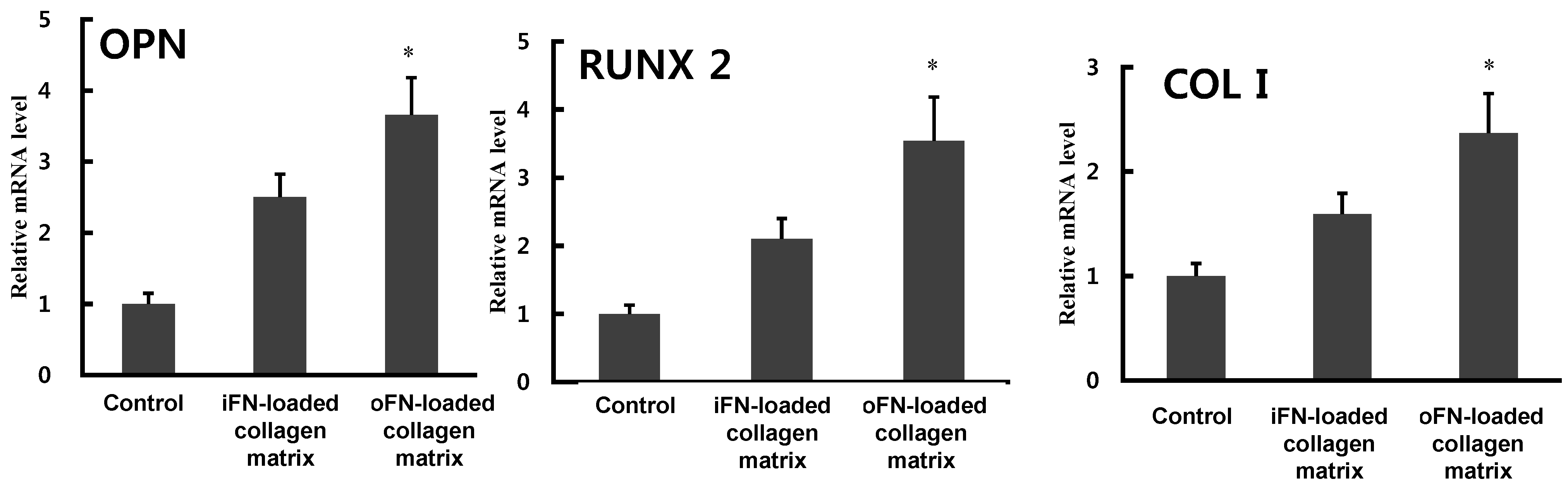
3. Experimental Section
3.1. Construction of the Osteoinductive FN Matrix Protein
3.2. Protein Expression and Purification
3.3. Cell Culture
3.4. Cell Adhesion Assay
3.5. Cell Differentiation Assay
3.6. RNA Extraction and cDNA Synthesis
3.7. Quantitative Real-Time PCR Analysis
| Genes | Forward Primer | Reverse Primer |
|---|---|---|
| Runx2 | GGCCGGGAATGATGAGAACTA | GGCCCACAAATCTCAGATCGT |
| OPN | CCAATGAAAGCCATGACCAC | CGACTGTAGGGACGATTGGA |
| Col І | GAAAGGATCTCCTGGTGCTG | ACCTTGTTTGCCAGGTTCAC |
| GAPDH | TCCACTCACGGCAAATTCAAC | AGCCCAAGATGCCCTTCAGT |
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moursi, A.M.; Damsky, C.H.; Lull, J.; Zimmerman, D.; Doty, S.B.; Aota, S.; Globus, R.K. Fibronectin regulates calvarial osteoblast differentiation. J. Cell Sci. 1996, 109, 1369–1380. [Google Scholar] [PubMed]
- Ruoslahti, E. Fibronectin and its receptors. Annu. Rev. Biochem. 1988, 57, 375–413. [Google Scholar] [CrossRef] [PubMed]
- Oldberg, A.; Linney, E.; Ruoslahti, E. Molecular cloning and nucleotide sequence of a cDNA clone coding for the cell attachment domain in human fibronectin. J. Biol. Chem. 1983, 258, 10193–10196. [Google Scholar] [PubMed]
- Assoian, R.K.; Schwartz, M.A. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr. Opin. Genet. Dev. 2001, 11, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Kang, W.; Jeon, E.; Jang, J.H. Construction and expression of a recombinant fibronectinIII10 protein for integrin-mediated cell adhesion. Biotechnol. Lett. 2010, 32, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Lecanda, F.; Avioli, L.V.; Cheng, S.L. Regulation of bone matrix protein expression and induction of differentiation of human osteoblasts and human bone marrow stromal cells by bone morphogenetic protein-2. J. Cell. Biochem. 1997, 67, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Wozney, J.M.; Rosen, V. Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin. Orthop. Relat. Res. 1998, 346, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Govender, S.; Csimma, C.; Genant, H.K.; Valentin-Opran, A.; Amit, Y.; Arbel, R.; Aro, H.; Atar, D.; Bishay, M.; Borner, M.G.; et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: A prospective, controlled, randomized study of four hundred and fifty patients. J. Bone. Jt. Surg. Am. 2002, 84, 2123–2134. [Google Scholar]
- Sakou, T. Bone morphogenetic proteins: From basic studies to clinical approaches. Bone 1998, 22, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Choung, P.H.; Seo, B.M.; Chung, C.P.; Yamada, K.M.; Jang, J.H. Synergistic activity of fibronectin and fibroblast growth factor receptors on neuronal adhesion and neurite extension through extracellular signal-regulated kinase pathway. Biochem. Biophys. Res. Commun. 2002, 295, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Ku, Y.; Chung, C.P.; Heo, S.J. Enhanced fibronectin-mediated cell adhesion of human osteoblast by fibroblast groth factor, FGF-2. Biotechnol. Lett. 2002, 24, 1659–1663. [Google Scholar] [CrossRef]
- Jeon, E.; Yun, Y.R.; Kim, H.W.; Jang, J.H. Engineering and application of collagen-binding fibroblast growth factor 2 for sustained release. J. Biomed. Mater. Res. A 2014, 102, 1–7. [Google Scholar] [CrossRef]
- Chrzanowski, W.; Lee, J.H.; Kondyurin, A.; Lord, M.S.; Jang, J.H.; Kim, H.W.; Bilek, M.M.M. Nano-Bio-Chemical Braille for Cells: The regulation of stem cell responses using bi-functional surfaces. Adv. Funct. Mater. 2015, 25, 193–205. [Google Scholar] [CrossRef]
- Jang, J.H.; Chung, C.P. Engineering and expression of a recombinant fusion protein possessing fibroblast growth factor-2 and fibronectin fragment. Biotechnol. Lett. 2004, 26, 1837–1840. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Teramoto, H.; Gutkind, J.S.; Yamada, K.M. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: Roles of integrin aggregation and occupancy of receptors. J. Cell Biol. 1996, 135, 1633–1642. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.; Chung, C.P.; Jang, J.H. The effect of the surface modification of titanium using a recombinant fragment of fibronectin and vitronectin on cell behavior. Biomaterials 2005, 26, 5153–5157. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Lee, D.-S.; Choi, I.; Pham, L.B.H.; Jang, J.-H. Design of an Osteoinductive Extracellular Fibronectin Matrix Protein for Bone Tissue Engineering. Int. J. Mol. Sci. 2015, 16, 7672-7681. https://doi.org/10.3390/ijms16047672
Lee S, Lee D-S, Choi I, Pham LBH, Jang J-H. Design of an Osteoinductive Extracellular Fibronectin Matrix Protein for Bone Tissue Engineering. International Journal of Molecular Sciences. 2015; 16(4):7672-7681. https://doi.org/10.3390/ijms16047672
Chicago/Turabian StyleLee, Sujin, Dong-Sung Lee, Ilsan Choi, Le B. Hang Pham, and Jun-Hyeog Jang. 2015. "Design of an Osteoinductive Extracellular Fibronectin Matrix Protein for Bone Tissue Engineering" International Journal of Molecular Sciences 16, no. 4: 7672-7681. https://doi.org/10.3390/ijms16047672






