In Silico Analysis of the Genes Encoding Proteins that Are Involved in the Biosynthesis of the RMS/MAX/D Pathway Revealed New Roles of Strigolactones in Plants
Abstract
:1. Introduction
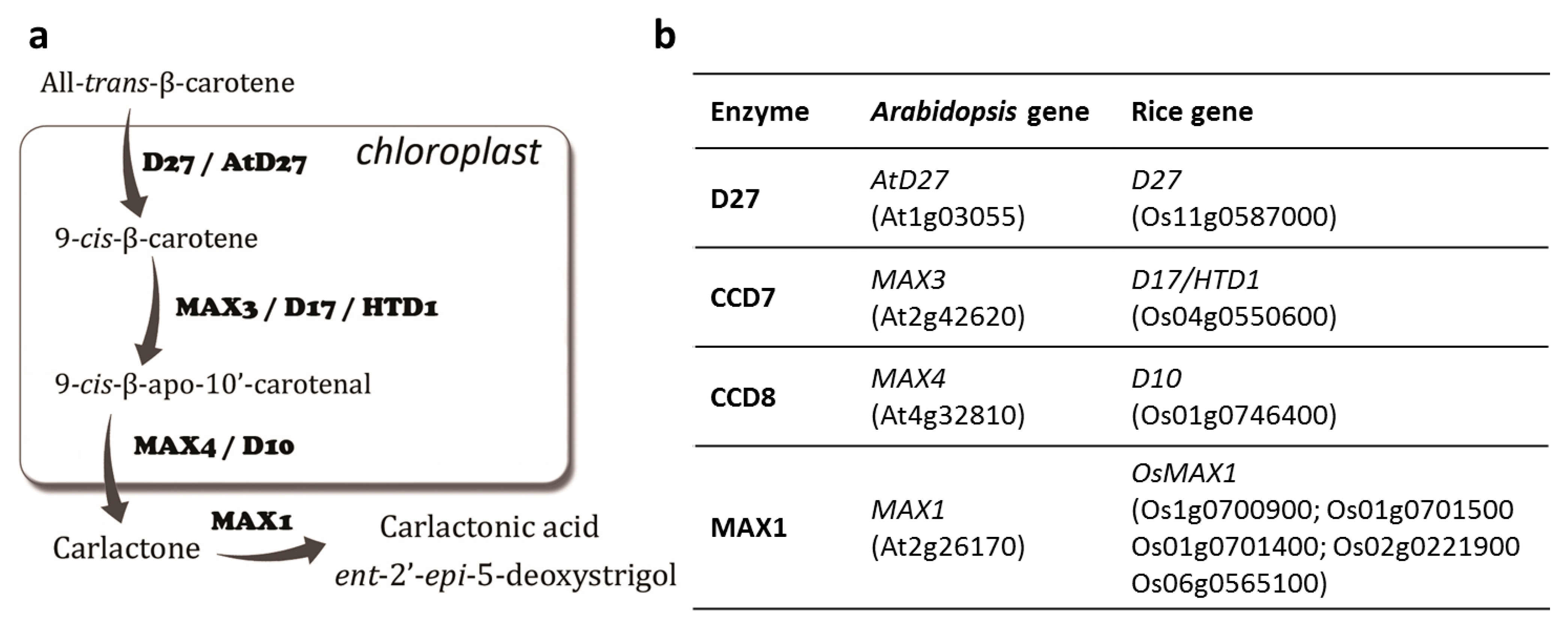
2. Results
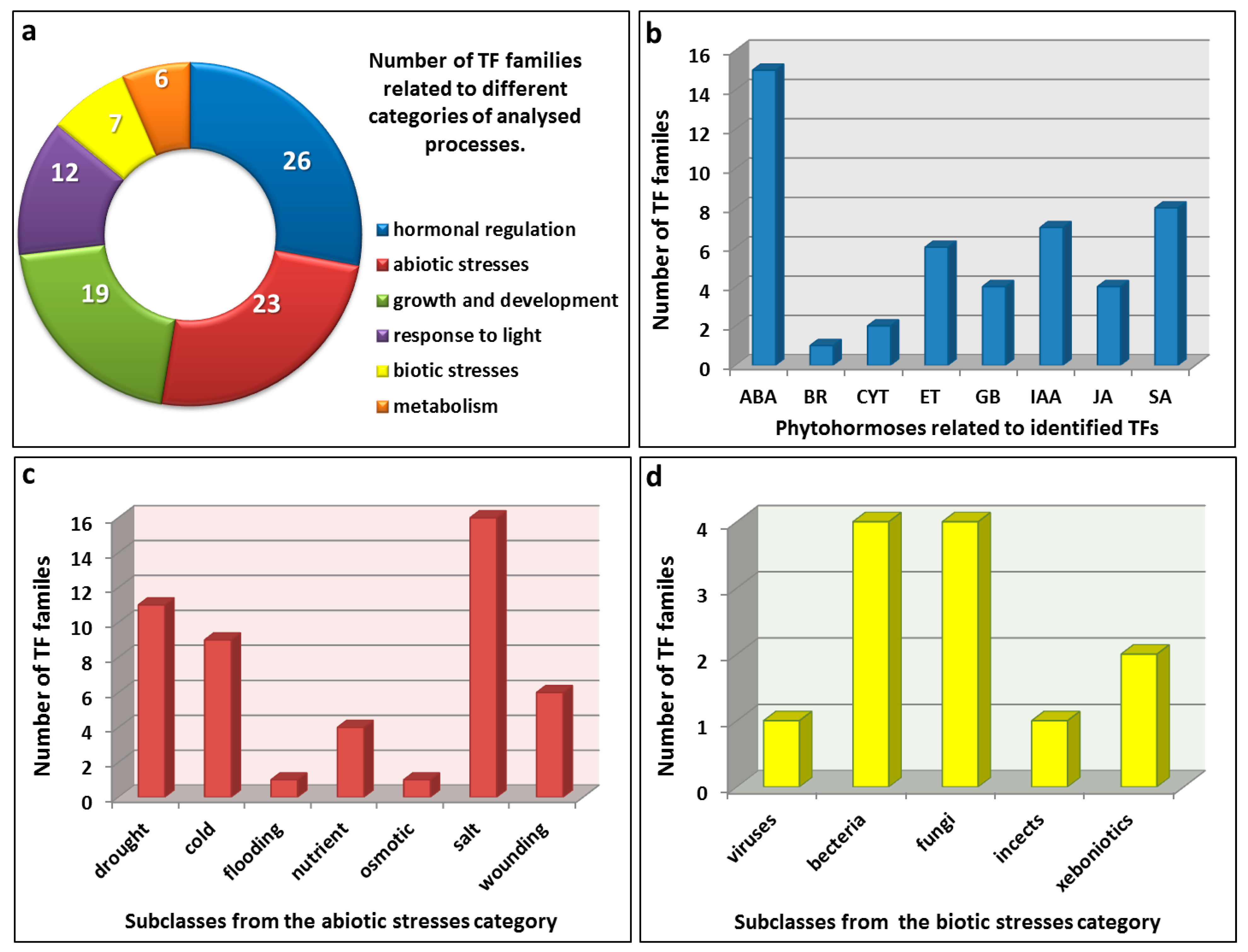
2.1. Analysis of the Promoter Sequences of the Arabidopsis Genes that Are Involved in Strigolactone Biosynthesis
| TFs Motifs | AtD27 | MAX3 | MAX4 | MAX1 | Gene Ontology (GO) Process | |||
|---|---|---|---|---|---|---|---|---|
| AGL3 | 1 | 4 | 4 | 4 | growth and development | |||
| AG | 3 | 4 | 6 | 4 | growth and development | |||
| ATHB-1 | 9 | 11 | 14 | 6 | abiotic stresses (salt, nutrients); growth and development; response to light | |||
| ATHB-5 | 9 | 10 | 8 | 8 | hormonal regulation (ABA) | |||
| ATHB-9 | 5 | 7 | 9 | 7 | growth and development | |||
| RAV1-A/RAV1AAT | 5 | 6 | 1 | 3 | growth and development; hormonal regulation (BR); metabolism | |||
| ACGTATERD1 | 4 | 10 | 6 | 2 | abiotic stresses (drought, salt); response to light | |||
| ANAERO1-3CONSENSUS | 2 | 1 | 3 | 1 | abiotic stresses (flooding) | |||
| ARR10 | 5 | 3 | 5 | 2 | growth and development; hormonal regulation (CYT) | |||
| ARR1AT | 7 | 17 | 10 | 6 | growth and development; hormonal regulation (CYT); metabolism | |||
| ASF1MOTIFCAMV | 1 | 2 | 2 | 1 | biotic stresses ( bacteria, xenobiotics); hormonal regulation (IAA, SA); response to light | |||
| Bellringer | 1 | 1 | 2 | 3 | growth and development | |||
| GATABOX | 10 | 10 | 18 | 5 | abiotic stresses (nutrients); response to light | |||
| GT1CONSENSUS | 9 | 5 | 8 | 9 | response to light | |||
| MYB1AT | 3 | 4 | 1 | 3 | abiotic stresses (drought, salt) | |||
| MYCCONSENSUSAT | 6 | 8 | 6 | 9 | abiotic stresses (drought, salt); hormonal regulation (ABA) | |||
| MYB4 | 2 | 2 | 1 | 5 | abiotic stresses (wounding); hormonal regulation (JA, SA); metabolism | |||
| SURECOREATSULTR11 | 2 | 1 | 1 | 1 | abiotic stress (nutrients) | |||
| WBOXATNPR1 | 3 | 3 | 3 | 1 | biotic stresses (bacteria, fungi, viruses); hormonal regulation (SA) | |||
| CDC5 | 1 | 0 | 1 | 0 | biotic stresses (bacteria, fungi); growth and development | |||
| PIF3 | 0 | 2 | 1 | 0 | response to light; hormonal regulation (GB) | |||
| ABRE-like | 0 | 2 | 0 | 0 | abiotic stresses (cold, drought, salt) | |||
| ABREATCONSENSUS | 0 | 1 | 0 | 0 | abiotic stresses (cold, drought, salt); hormonal regulation (ABA) | |||
| ABRELATERD1 | 0 | 1 | 1 | 0 | abiotic stresses (cold, drought, salt); response to light | |||
| ABRERATCAL | 0 | 0 | 1 | 0 | abiotic and biotic stresses (induced by Ca2+); hormonal regulation (ABA) | |||
| ABRE | 0 | 1 | 0 | 0 | hormonal regulation (ABA) | |||
| ACGTABREMOTIFA2OSEM | 0 | 1 | 0 | 0 | hormonal regulation (ABA) | |||
| AP1 | 0 | 1 | 2 | 1 | growth and development | |||
| ARFAT/ARF | 1 | 1 | 0 | 0 | growth and development; hormonal regulation (IAA) | |||
| Agamous | 0 | 1 | 1 | 3 | growth and development | |||
| AtMYB2 | 0 | 1 | 0 | 0 | abiotic stresses (cold, nutrients, salt, wounding); hormonal regulation (ABA, ET, IAA, JA, SA) | |||
| AtMYC2 | 0 | 1 | 1 | 0 | abiotic stresses (wounding); biotic stresses ( fungi, insects); hormonal regulation (ABA, JA, SA); metabolism | |||
| C8GCARGAT | 0 | 4 | 4 | 0 | growth and development; hormonal regulation (IAA); metabolism | |||
| CGCGBOXAT | 0 | 0 | 4 | 0 | abiotic stresses (low temperatures, salt, wounding); hormonal regulation (ET, IAA) | |||
| CCA1ATLHCB1/CCA1 | 1 | 0 | 0 | 1 | abiotic stresses (low temperatures, salt); hormonal regulation (ABA, ET, GB, IAA, SA) | |||
| DPBFCOREDCDC3 | 0 | 1 | 0 | 0 | abiotic stresses (drought, low temperatures, salt); biotic stresses (fungi); hormonal regulation (ABA, GB) | |||
| GAREAT | 0 | 1 | 1 | 3 | hormonal regulation (GB) | |||
| GBF5 | 0 | 2 | 0 | 0 | biotic stresses (xenobiotics); growth and development | |||
| LEAFYATAG | 0 | 0 | 0 | 1 | growth and development | |||
| LTREATLTI78 | 0 | 1 | 0 | 0 | abiotic stresses (low temperatures) | |||
| LTRECOREATCOR15 | 0 | 1 | 0 | 0 | abiotic stresses (low temperature); response to light | |||
| MYB1LEPR | 0 | 1 | 0 | 1 | biotic stresses ( bacteria); hormonal regulation (ET) | |||
| MYB2CONSENSUSAT | 0 | 1 | 0 | 1 | abiotic stresses (drought, salt); hormonal regulation (ABA) | |||
| MYBATRD22 | 0 | 1 | 0 | 0 | abiotic stresses (salt, wounding); hormonal regulation (ABA, ET, IAA, SA) | |||
| MYBCORE | 0 | 1 | 1 | 1 | abiotic stresses (drought, salt, wounding); hormonal regulation (ABA, ET, IAA, JA, SA) | |||
| MYBPLANT | 1 | 0 | 1 | 2 | abiotic stresses (drought, salt); hormonal regulation (ABA); metabolism | |||
| MYCATERD1/MYCATRD22 | 0 | 1 | 1 | 0 | abiotic stresses (drought, salt); hormonal regulation (ABA) | |||
| PREATPRODH | 0 | 2 | 0 | 0 | abiotic stresses (osmotic) | |||
| SITEIIATCYTC | 0 | 0 | 0 | 1 | growth and development; metabolism | |||
| SORLIP5AT | 1 | 1 | 0 | 0 | response to light | |||
| SREATMSD | 0 | 1 | 0 | 0 | growth and development | |||
| SV40COREENHAN | 1 | 1 | 0 | 1 | response to light | |||
| TBOXATGAPB | 1 | 0 | 2 | 0 | response to light | |||
| ZDNAFORMINGATCAB1 | 0 | 1 | 0 | 0 | growth and development; response to light | |||
| XYLAT | 0 | 0 | 1 | 0 | growth and development | |||
| - | D27 | MAX3 | MAX4 | MAX1 |
|---|---|---|---|---|
| D27 | - | 44% (22/50) | 55.3% (21/38) | 64.7% (22/34) |
| MAX3 | 44% (22/50) | - | 53.8% (28/52) | 52% (26/50) |
| MAX4 | 55.3% (21/38) | 53.8% (28/52) | - | 58.5% (24/41) |
| MAX1 | 64.7% (22/34) | 52% (26/50) | 58.5% (24/41) | - |
2.2. Analysis of the Promoter Region of the Rice Genes that Are Involved in Strigolactone Biosynthesis
| TF Motifs | D27 | D17/HTD1 | D10 | MAX1 | Gene Ontology (GO) Process |
|---|---|---|---|---|---|
| BIHD1OS | 2 | 2 | 1 | 4 | biotic stresses (fungi) |
| GATABOX | 10 | 7 | 3 | 5 | abiotic stresses (nutrients); response to light |
| GT1CONSENSUS | 7 | 12 | 2 | 7 | hormonal regulation (SA); response to light |
| PYRIMIDINEBOXOSRAMY1A | 1 | 2 | 1 | 2 | hormonal regulation (GB); growth and development; sugar repression |
| SITEIIATCYTC | 1 | 2 | 2 | 2 | growth and development; metabolism; Relative to cytochrome, oxidative phosphorylation |
| WRKY71OS | 6 | 6 | 6 | 12 | biotic stresses (pathogens); hormonal regulation (GB) |
| ABREOSRAB21 | 0 | 1 | 1 | 0 | hormonal regulation (ABA); abiotic stresses (osmotic) |
| ACGTABOX | 0 | 2 | 6 | 0 | growth and development; sugar repression |
| ANAERO1-3CONSENSUS | 0 | 1 | 0 | 1 | abiotic stresses (flooding) |
| ARFAT | 0 | 1 | 0 | 0 | growth and development; hormonal regulation (IAA) |
| AMYBOX1 | 0 | 0 | 1 | 0 | Conserved sequence found in 5'-upstream region of alpha-amylase gene |
| E2FCONSENSUS | 0 | 1 | 0 | 0 | growth and development |
| CAREOSREP1 | 0 | 0 | 1 | 0 | hormonal regulation (GB) |
| CGACGOSAMY3 | 0 | 0 | 4 | 0 | Conserved sequence found in 5'-upstream region of alpha-amylase gene |
| GARE1OSREP1 | 0 | 0 | 2 | 0 | hormonal regulation (GB) |
| HEXMOTIFTAH3H4 | 0 | 1 | 0 | 2 | hormonal regulation (IAA, SA); metabolism |
| TATABOXOSPAL | 0 | 1 | 0 | 1 | abiotic stresses (salt); hormonal regulation (ET, GB, IAA, JA, SA) |
| TATCCAOSAMY | 2 | 0 | 0 | 0 | abiotic stresses ( nutrients); hormonal regulation (GB); found in alpha-amylase promoters of rice |
2.3. Identification of miRNA Target Sites in the mRNAs of Genes from the Strigolactone Biosynthesis Pathway
| miRNA | Position of Target Sites | |||
|---|---|---|---|---|
| Arabidopsis Genes | ||||
| AtD27 | MAX3 | MAX4 | MAX1 | |
| ath-miR156g | - | - | - | 302–323 |
| ath-miR165a | - | - | 950–971 | 842–861 |
| ath-miR165b | - | 1469–1493 | 950–971 | 842–861 |
| ath-miR166a-g | - | 1469–1493 | 951–971 | 842–861 |
| 1280–1301 | ||||
| ath-miR395b,c,f | - | - | - | 336–357 |
| ath-miR401 | - | 1701–1725 | - | - |
| - | Rice Genes | |||
| D27 | D17/HTD1 | D10 | OsMAX1 | |
| osa-miR444 | 47–70 | 557–581 | 172–154 | 458–478 |
| 1055–1076 | ||||
| 363–380 | 221–239 | |||
| 1292–1318 | ||||
| osa-miR528 | 14–33 | 363–380 | 1192–1214 | 331–351 |
| 864–885 | 1113–1138 | |||
2.4. Gene Expression after Hormone Treatment and during Responses to Abiotic Stresses
| Treatment | Time Point | log2 Ratio (Sample Signal/Control Signal) | |||
|---|---|---|---|---|---|
| AtD27 | MAX3 | MAX4 | MAX1 | ||
| Abscisic acid (10 µM) | 0.5 h | 0.01 | −0.27 | 0.23 | −0.15 |
| 1 h | 0.44 | 0.04 | 0.23 | −0.39 | |
| 3 h | −0.56 | 0.83 | 0.23 | 0.05 | |
| Auxin (IAA 1 µM) | 0.5 h | 0.15 | −0.19 | 0.3 | −0.04 |
| 1 h | −0.22 | −0.54 | 1.34 | −0.38 | |
| 3 h | −0.24 | 0.24 | 0.58 | −0.52 | |
| Brassinolide (10 nM) | 0.5 h | 0.56 | −0.35 | 1.05 | 0.07 |
| 1 h | −0.05 | −0.7 | 1.07 | 0.04 | |
| 3 h | −0.3 | 0.1 | 0.12 | 0.15 | |
| Cytokinin (zeatin 1 µM) | 0.5 h | −0.1 | −0.05 | −0.04 | −0.43 |
| 1 h | 0 | −0.03 | 0.72 | −0.76 | |
| 3 h | −0.33 | 0.37 | −0.39 | −0.45 | |
| Ethylene (ACC 10 µM) | 0.5 h | 0.45 | 0.06 | 0.8 | −0.04 |
| 1 h | 0.34 | 0.04 | 0.67 | −0.07 | |
| 3 h | −0.63 | 0.55 | −0.26 | 0.09 | |
| Gibberellin acid (1 µM) | 0.5 h | 0.13 | −0.29 | −0.08 | −0.11 |
| 1 h | 0.28 | −0.15 | 0.01 | −0.25 | |
| 3 h | −0.17 | 0.44 | −0.34 | −0.19 | |
| Methyl jasmonate (10 µM) | 0.5 h | 0.53 | 0.1 | 0.48 | −0.13 |
| 1 h | −0.37 | −0.2 | 0.37 | −0.21 | |
| 3 h | −1.2 | 0.22 | −0.2 | −0.05 | |
| Treatment | Time Point | log2 Ratio | Treatment | Time Point | log2 Ratio | ||||
|---|---|---|---|---|---|---|---|---|---|
| D17/HTD1 | D10 | MAX1 | D17/HTD1 | D10 | MAX1 | ||||
| Abscisic acid (50 µM) | 0.25 h | 0.10 | −0.07 | 0.07 | Cytokinin (zeatin 1 µM) | 0.25 h | −0.31 | −0.37 | 0.03 |
| 0.5 h | −0.06 | −0.22 | 0.30 | 0.5 h | 0.26 | −0.39 | 0.71 | ||
| 1 h | −0.31 | −0.15 | 0.89 | 1 h | −0.12 | −0.28 | 0.92 | ||
| 3 h | 0.26 | 0.06 | 1.43 | 3 h | −0.20 | −0.24 | 1.06 | ||
| 6 h | 0.57 | 0.24 | 2.32 | 6 h | −0.17 | −0.11 | 0.79 | ||
| Auxin (IAA 10 µM) | 0.25 h | 0.21 | 0.05 | 0.21 | Gibberellin acid (10 µM) | 0.25 h | −0.02 | −0.44 | −0.34 |
| 0.5 h | −0.35 | −0.17 | 0.09 | 0.5 h | −0.27 | −0.06 | −0.66 | ||
| 1 h | −0.26 | −0.38 | 0.33 | 1 h | −0.01 | −0.12 | −0.41 | ||
| 3 h | 1.11 | −0.06 | 0.66 | 3 h | 0.23 | −0.13 | −0.63 | ||
| 6 h | 0.29 | −0.09 | −0.02 | 6 h | −0.12 | 0.38 | 0.07 | ||
| Brassinolide (1 µM) | 0.25 h | −0.43 | 0.02 | 0.15 | Jasmonic acid (100 µM) | 0.25 h | −0.26 | −1.65 | −0.17 |
| 0.5 h | −0.13 | −0.13 | 0.22 | 0.5 h | 0.90 | −1.01 | −0.30 | ||
| 1 h | −0.44 | −0.25 | 0.13 | 1 h | 0.79 | −0.69 | −0.59 | ||
| 3 h | −0.19 | −0.02 | 0.25 | 3 h | 2.46 | −0.82 | −0.70 | ||
| 6 h | −0.15 | 0.02 | 0.35 | 6 h | 2.77 | −0.85 | −0.87 | ||
| Stress | Time | AtD27 | MAX3 | MAX4 | MAX1 | Stress | Time | AtD27 | MAX3 | MAX4 | MAX1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cold | 0.5 h | 0.25 | −0.3 | −0.47 | 0.08 | Drought | 0.25 h | −1.01 | 0.33 | −0.61 | 0.26 |
| 1 h | −0.08 | 0.29 | −0.1 | 0.37 | 0.5 h | −0.15 | −0.44 | −0.28 | −0.25 | ||
| 3 h | 0.24 | 0.14 | 0.05 | 0.04 | 1 h | −0.7 | 0.76 | −0.08 | 0.62 | ||
| 6 h | 0.15 | −0.93 | 0.33 | 0.35 | 3 h | 0.03 | 0.9 | 0.08 | 0.01 | ||
| 12 h | −0.22 | −0.82 | 0.31 | −0.87 | 6 h | 0.21 | 0.57 | −0.09 | 0.49 | ||
| 24 h | −0.04 | −0.13 | −0.28 | −2.27 | 12 h | 0 | 0.36 | 0.14 | −0.03 | ||
| Osmotic | 0.5 h | 0.23 | −0.24 | −0.03 | −0.28 | 24 h | −0.55 | 0.19 | 0.16 | −0.09 | |
| 1 h | −0.05 | 0.62 | −0.13 | −0.04 | Wounding | 0.25 h | −1.36 | 0.8 | −0.09 | −0.06 | |
| 3 h | −0.11 | 1.06 | −0.01 | −0.15 | 0.5 h | 0.13 | 0.19 | −0.14 | −0.02 | ||
| 6 h | 0.33 | 0.72 | 0.73 | 0.53 | 1 h | −0.31 | 0.52 | −0.33 | 0.39 | ||
| 12 h | −0.82 | 1.35 | 1.6 | 0.39 | 3 h | 0.38 | 0.42 | −0.24 | 0.08 | ||
| 24 h | −0.67 | 0.97 | 1.4 | 0.71 | 6 h | −0.49 | 0.46 | −0.11 | 0.51 | ||
| Salt | 0.5 h | −0.02 | 0.24 | −0.14 | 0.21 | 12 h | −1.44 | 0.75 | −0.34 | −0.14 | |
| 1 h | −0.54 | 0.66 | −0.34 | 0.13 | 24 h | −0.51 | 0.38 | 0.24 | −0.05 | ||
| 3 h | −0.35 | 0.43 | −0.03 | −0.46 | Heat | 0.25 h | −0.09 | 0.45 | 0.23 | 0.08 | |
| 6 h | −0.36 | −0.6 | 0.38 | −0.61 | 0.5 h | 0.23 | 0.21 | 0.03 | 0.49 | ||
| 12 h | −0.94 | 0.18 | 1.24 | 0.27 | 1 h | −0.2 | 1.17 | −0.86 | 0.09 | ||
| 24 h | −0.61 | 0.13 | 0.42 | −0.15 | 3 h | −0.02 | 1.65 | −0.49 | −0.55 | ||
| Genotoxic | 0.5 h | −0.23 | 0.15 | −0.15 | 0.01 | 4 h | 0.05 | 0.2 | 0.53 | 0.46 | |
| 1 h | −1.16 | 0.64 | −0.19 | 0.14 | 6 h | −0.55 | 0.47 | −0.18 | −0.15 | ||
| 3 h | −0.59 | 0.18 | −0.45 | −0.1 | 12 h | −0.96 | 0.95 | 0.61 | 0.75 | ||
| 6 h | −0.51 | 0.01 | 0.18 | 0.12 | 24 h | 0.2 | 0.18 | 0.12 | 0.19 | ||
| 12 h | −0.95 | 0.16 | 0.46 | 0.22 | UV-B | 0.25 h | −0.34 | 0.24 | −0.25 | −0.22 | |
| 24 h | −0.11 | 0.1 | 0.44 | 0.09 | 0.5 h | −0.11 | 0.64 | −0.11 | 0.34 | ||
| Oxidative | 0.5 h | −0.19 | 0.18 | −0.25 | 0.01 | 1 h | −0.68 | 1.15 | −0.96 | 0.49 | |
| 1 h | −0.47 | 0.42 | −0.23 | 0.22 | 3 h | −0.26 | 0.56 | −1.01 | −0.32 | ||
| 3 h | 0.5 | 0.68 | −0.03 | −0.24 | 6 h | −0.36 | 0.16 | −0.55 | 0.13 | ||
| 6 h | −0.52 | 0.41 | −0.34 | 0.04 | 12 h | −0.57 | 0.29 | −0.22 | 0.22 | ||
| 12 h | −0.4 | −0.1 | 0.48 | 0.06 | 24 h | −0.53 | 0.2 | 0.07 | −0.12 | ||
| 24 h | −0.27 | 0.42 | 0.55 | 0.51 | - | - | - | - | - |
3. Discussion
3.1. Regulation of the Expression of Genes that Are Responsible for Strigolactone Biosynthesis via TFs and miRNAs
3.2. Expression of Strigolactone Biosynthesis Genes after Hormone Treatment and under Stress Conditions
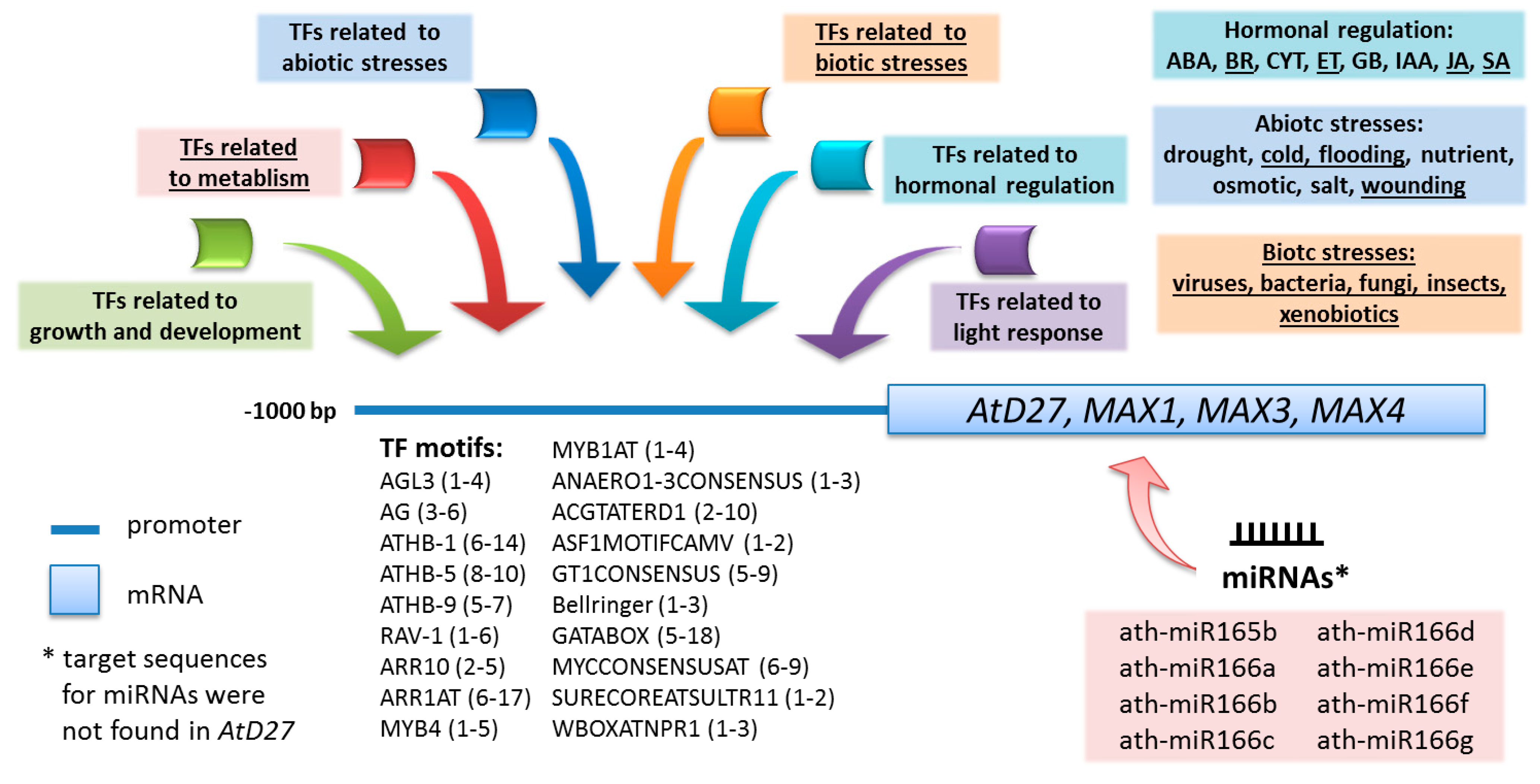
3.3. In Silico Analysis for the Prediction of New Roles of Strigolactones in Plants
4. Experimental Section
4.1. Promoter Analysis
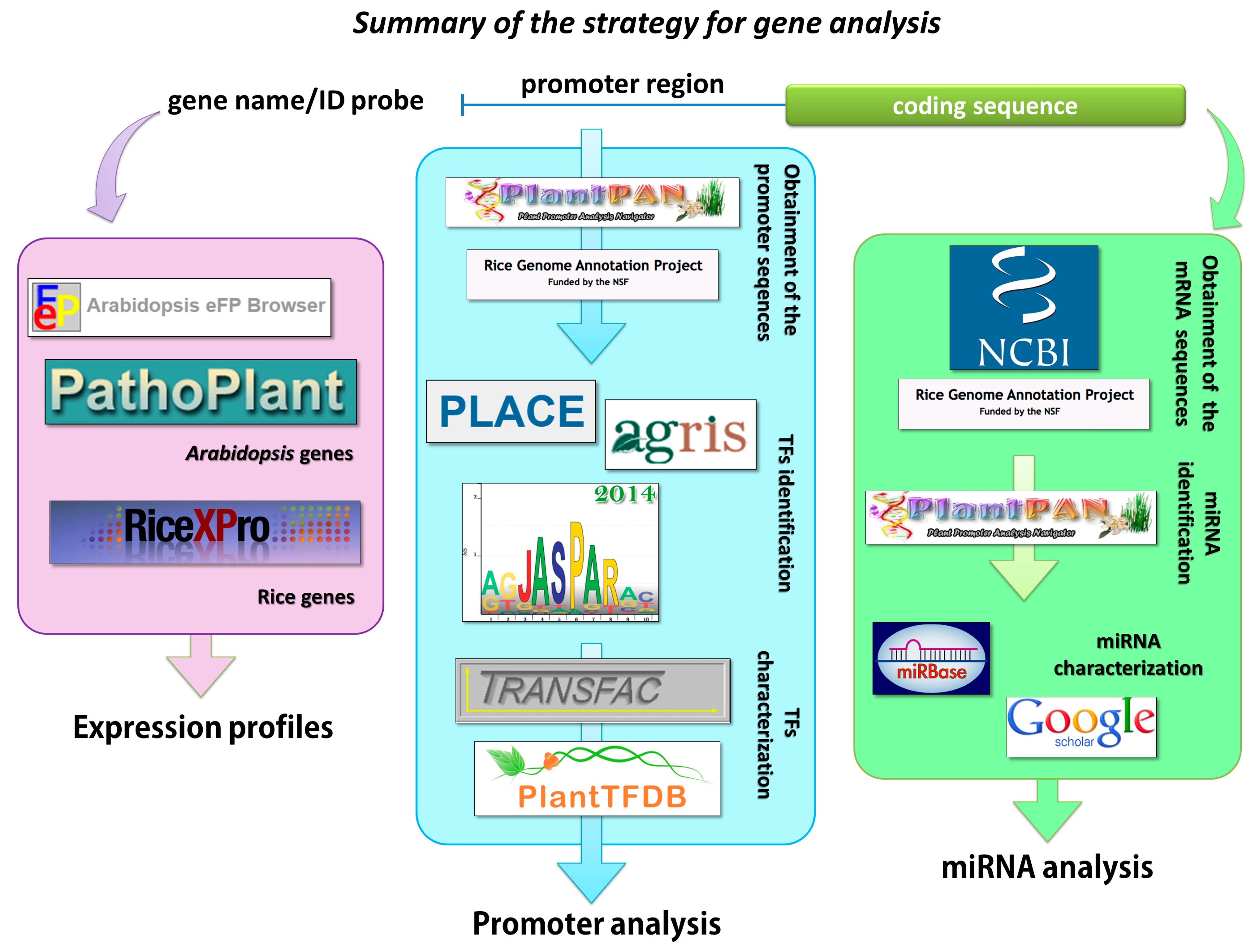
4.2. Identification of the Sequences that Were Recognized by miRNA
4.3. Expression Profiles
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cook, C.E.; Whichard, L.P.; Turner, B.; Wall, M.E.; Egley, G.H. Germination of witchweed (Striga lutea Lour.): Isolation and properties of a potent stimulant. Science 1966, 154, 1189–1190. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Matsuzaki, K.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005, 7043, 824–827. [Google Scholar] [CrossRef]
- Soto, M.J.; Fernandez-Aparicio, M.; Castellanos-Morales, V.; Garcia-Garrido, J.A.; Delgado, M.J.; Vierheilig, H. First indications for the involvement of strigolactones on nodule formation in alfalfa (Medicago sativa). Soil Biol. Biochem. 2010, 42, 383–385. [Google Scholar] [CrossRef]
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pagès, V.; Dun, E.A.; Pillot, J.P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.C.; et al. Strigolactone inhibition of shoot branching. Nature 2008, 455, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Takeda-Kamiya, N.; Magome, H.; Kamiya, Y.; Shirasu, K.; Yoneyama, K.; et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature 2008, 455, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Kapulnik, Y.; Delaux, P.M.; Resnick, N.; Mayzlish-Gati, E.; Wininger, S.; Bhattacharya, C.; Séjalon-Delmas, N.; Combier, J.P.; Bécard, G.; Belausov, E.; et al. Strigolactones affect lateral root formation and root-hair elongation in Arabidopsis. Planta 2011, 233, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Koltai, H. Strigolactones are regulators of root development. New Phytol. 2011, 190, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yan, H.; Yang, J.; Yamaguchi, S.; Maekawa, M.; Takamure, I.; Tsutsumi, N.; Kyozuka, J.; Nakazono, M. Strigolactones negatively regulate mesocotyl elongation in rice during germination and growth in darkness. Plant Cell Physiol. 2010, 51, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- De Saint Germain, A.; Ligerot, Y.; Dun, E.A.; Pillot, J.P.; Ross, J.J.; Beveridge, C.A.; Rameau, C. Strigolactones stimulate internode elongation independently of gibberellins. Plant Physiol. 2013, 163, 1012–1025. [Google Scholar] [CrossRef] [PubMed]
- Agusti, J.; Herold, S.; Schwarz, M.; Sanchez, P.; Ljung, K.; Dun, E.A.; Brewer, P.B.; Beveridge, C.A.; Sieberer, T.; Sehr, E.M.; et al. Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants. Proc. Natl. Acad. Sci. USA 2011, 108, 20242–20247. [Google Scholar] [CrossRef] [PubMed]
- Sang, D.; Chen, D.; Liu, G.; Liang, Y.; Huang, L.; Meng, X.; Chu, J.; Sun, X.; Dong, G.; Yuan, Y.; et al. Strigolactones regulate rice tiller angle by attenuating shoot gravitropism through inhibiting auxin biosynthesis. Proc. Natl. Acad. Sci. USA 2014, 111, 11199–11204. [Google Scholar] [CrossRef] [PubMed]
- Brewer, P.B.; Koltai, H.; Beveridge, C.A. Diverse roles of strigolactones in plant development. Mol. Plant 2013, 6, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Kapulnik, Y.; Koltai, H. Strigolactone involvement in root development, response to abiotic stress and interactions with the biotic soil environment. Plant Physiol. 2014, 166, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Waldie, T.; McCulloch, H.; Leyser, O. Strigolactones and the control of plant development: Lessons from shoot branching. Plant J. 2014, 79, 607–622. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, K.; Yoneyama, K.; Takeuchi, Y.; Sekimoto, H. Phosphorus deficiency in red clover promotes exudation of orobanchol, the signal for mycorrhizal symbionts and germination stimulant for root parasites. Planta 2007, 225, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, K.; Xie, X.; Kisugi, T.; Nomura, T.; Yoneyama, K. Nitrogen and phosphorus fertilization negatively affects strigolactone production and exudation in sorghum. Planta 2013, 238, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, K.; Xie, X.; Kusumoto, D.; Sekimoto, H.; Sugimoto, Y.; Takeuchi, Y.; Yoneyama, K. Nitrogen deficiency as well as phosphorus deficiency in sorghum promotes the production and exudation of 5-deoxystrigol, the host recognition signal for arbuscular mycorrhizal fungi and root parasites. Planta 2007, 227, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, K.; Xie, X.; Kim, H.I.; Kisugi, T.; Nomura, T.; Sekimoto, H.; Yokota, T.; Yoneyama, K. How do nitrogen and phosphorus deficiencies affect strigolactone production and exudation? Planta 2012, 235, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- López-Ráez, J.A.; Charnikhova, T.; Gómez-Roldán, V.; Matusova, R.; Kohlen, W.; de Vos, R.; Verstappen, F.; Puech-Pages, V.; Bécard, G.; Mulder, P.; et al. Tomato strigolactones are derived from carotenoids and their biosynthesis is promoted by phosphate starvation. New Phytol. 2008, 178, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Foo, E.; Yoneyama, K.; Hugill, C.J.; Quittenden, L.J.; Reid, J.B. Strigolactones and the regulation of pea symbioses in response to nitrate and phosphate deficiency. Mol. Plant 2013, 6, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Marzec, M.; Muszynska, A.; Gruszka, D. The role of strigolactones in nutrient-stress responses in plants. Int. J. Mol. Sci. 2014, 14, 9286–9304. [Google Scholar] [CrossRef]
- Aroca, R.; Ruiz-Lozano, J.M.; Zamarreño, A.M.; Paz, J.A.; García-Mina, J.M.; Pozo, M.J.; López-Ráez, J.A. Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J. Plant Physiol. 2013, 170, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.; Lv, T.; Shen, H.; Luong, P.; Wang, J.; Wang, Z.; Huang, Z.; Xiao, L.; Engineer, C.; Kim, T.H.; et al. Regulation of drought tolerance by the F-box protein MAX2 in Arabidopsis. Plant Physiol. 2014, 164, 424–439. [Google Scholar] [CrossRef] [PubMed]
- Ha, C.V.; Leyva-González, M.A.; Osakabe, Y.; Tran, U.T.; Nishiyama, R.; Watanabe, Y.; Tanaka, M.; Seki, M.; Yamaguchi, S.; Dong, N.V.; et al. Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc. Natl. Acad. Sci. USA 2014, 111, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Matusova, R.; Rani, K.; Verstappen, F.W.; Franssen, M.C.; Beale, M.H.; Bouwmeester, H.J. The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiol. 2005, 139, 920–934. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, B.; Mwakaboko, A.S.; Reizelman, A.; Anilkumar, G.; Sethumadhavan, D. Structure and function of natural and synthetic signalling molecules in parasitic weed germination. Pest Manag. Sci. 2009, 65, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Yoneyama, K.; Yoneyama, K. The strigolactone story. Ann. Rev. Phytopathol. 2010, 48, 93–117. [Google Scholar] [CrossRef]
- Xie, X.; Yoneyama, K.; Kisugi, T.; Uchida, K.; Ito, S.; Akiyama, K.; Hayashi, H.; Yokota, T.; Nomura, T.; Yoneyama, K. Confirming stereochemical structures of strigolactones produced by rice and tobacco. Mol. Plant 2013, 6, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Alder, A.; Jamil, M.; Marzorati, M.; Bruno, M.; Vermathen, M.; Bigler, P.; Ghisla, S.; Bouwmeester, H.; Beyer, P.; Al-Babili, S. The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science 2012, 335, 1348–1351. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Wang, R.; Qian, Q.; Yan, M.; Meng, X.; Fu, Z.; Yan, C.; Jiang, B.; Su, Z.; Li, J.; et al. DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell 2009, 21, 1512–1525. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.T.; Brewer, P.B.; Bussell, J.D.; Smith, S.M.; Beveridge, C.A. The Arabidopsis ortholog of rice DWARF27 acts upstream of MAX1 in the control of plant development by strigolactones. Plant Physiol. 2012, 159, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Proust, H.; Hoffmann, B.; Xie, X.; Yoneyama, K.; Schaefer, D.G.; Yoneyama, K.; Nogué, F.; Rameau, C. Strigolactones regulate protonema branching and act as a quorum sensing-like signal in the moss Physcomitrella patens. Development 2011, 138, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.K.; Lu, J.J.; Xing, G.N.; Gai, J.Y.; Zhao, T.J. Molecular evolution of two consecutive carotenoid cleavage dioxygenase genes in strigolactone biosynthesis in plants. Genet. Mol. Res. 2011, 10, 3664–3673. [Google Scholar] [CrossRef] [PubMed]
- Booker, J.; Auldridge, M.; Wills, S.; McCarty, D.; Klee, H.; Leyser, O. MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signaling molecule. Curr. Biol. 2004, 14, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Maekawa, M.; Arite, T.; Onishi, K.; Takamure, I.; Kyozuka, J. Suppression of tiller bud activity in tillering dwarf mutants of rice. Plant Cell Physiol. 2005, 46, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Zhang, S.; Zhang, W.; Li, G.; Chen, Z.; Zhai, W.; Zhao, X.; Pan, X.; Xie, Q.; Zhu, L. The rice HIGH-TILLERING DWARF1 encoding an ortholog of Arabidopsis MAX3 is required for negative regulation of the outgrowth of axillary buds. Plant J. 2006, 48, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, C.A.; Kyozuka, J. New genes in the strigolactone-related shoot branching pathway. Curr. Opin. Plant Biol. 2010, 13, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Scaffidi, A.; Waters, M.T.; Ghisalberti, E.L.; Dixon, K.W.; Flematti, G.R.; Smith, S.M. Carlactone-independent seedling morphogenesis in Arabidopsis. Plant J. 2013, 76, 1–9. [Google Scholar] [PubMed]
- Seto, Y.; Sado, A.; Asami, K.; Hanada, A.; Umehara, M.; Akiyama, K.; Yamaguchi, S. Carlactone is an endogenous biosynthetic precursor for strigolactones. Proc. Natl. Acad. Sci. USA 2014, 111, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Booker, J.; Sieberer, T.; Wright, W.; Williamson, L.; Willett, B.; Stirnberg, P.; Turnbull, C.; Srinivasan, M.; Goddard, P.; Leyser, O. MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev. Cell 2005, 8, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.; Zhang, Y.; Jamil, M.; Hepworth, J.; Charnikhova, T.; Dimkpa, S.O.; Meharg, C.; Wright, M.H.; Liu, J.; Meng, X. Natural variation of rice strigolactone biosynthesis is associated with the deletion of two MAX1 orthologs. Proc. Natl. Acad. Sci. USA 2014, 111, 2379–2384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; van Dijk, A.D.; Scaffidi, A.; Flematti, G.R.; Hofmann, M.; Charnikhova, T.; Verstappen, F.; Hepworth, J.; van der Krol, S.; Leyser, O.; et al. Rice cytochrome P450 MAX1 homologs catalyze distinct steps in strigolactone biosynthesis. Nat. Chem. Biol. 2014, 10, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Sado, A.; Tanaka, K.; Kisugi, T.; Asami, K.; Ota, S.; Kim, H.I.; Yoneyama, K.; Xie, X.; Ohnishi, T.; et al. Carlactone is converted to carlactonoic acid by MAX1 in Arabidopsis and its methyl ester can directly interact with AtD14 in vitro. Proc. Natl. Acad. Sci. USA 2014, 111, 18084–18089. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.J.; Zhang, J.J.; Xue, H.W. Characterization and expression profiles of miRNAs in rice seeds. Nucleic Acids Res. 2009, 37, 916–930. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.; Vinegar, B.; Nahal, H.; Ammar, R.; Wilson, G.V.; Provart, N.J. An “Electronic Fluorescent Pictograph” Browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2007, 2, e718. [Google Scholar] [CrossRef] [PubMed]
- Arabidopsis eFP Browser. Available online: http://bar.utoronto.ca/efp (accessed on 11–30 November 2014).
- Bolivar, J.C.; Machens, F.; Brill, Y.; Romanov, A.; Bülow, L.; Hehl, R. “In silico expression analysis”, a novel PathoPlant web tool to identify abiotic and biotic stress conditions associated with specific cis-regulatory sequences. Database 2014. [CrossRef]
- PathoPlant. Available online: http://www.pathoplant.de (accessed on 11–30 November 2014).
- Sato, Y.; Takehisa, H.; Kamatsuki, K.; Minami, H.; Namiki, N.; Ikawa, H.; Ohyanagi, H.; Sugimoto, K.; Antonio, B.A.; Nagamura, Y. RiceXPro version 3.0: Expanding the informatics resource for rice transcriptome. Nucleic Acids Res. 2013, 41, D1206–D1213. [Google Scholar] [CrossRef] [PubMed]
- RiceXPro. Available online: http://ricexpro.dna.affrc.go.jp (accessed on 15–30 November 2014).
- Kilian, J.; Whitehead, D.; Horak, J.; Wanke, D.; Weinl, S.; Batistic, O.; D’Angelo, C.; Bornberg-Bauer, E.; Kudla, J.; Harter, K. The AtGenExpress global stress expression data set: Protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J. 2007, 50, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Ruyter-Spira, C.; Al-Babili, S.; van der Krol, S.; Bouwmeester, H. The biology of strigolactones. Trends Plant Sci. 2013, 18, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Meshi, T.; Iwabuchi, M. Plant transcription factors. Plant Cell Physiol. 1995, 36, 1405–1420. [Google Scholar] [PubMed]
- Lan, A.; Huang, J.; Zhao, W.; Peng, Y.; Chen, Z.; Kang, D. A salicylic acid-induced rice (Oryza sativa L.) transcription factor OsWRKY77 is involved in disease resistance of Arabidopsis thaliana. Plant Biol. 2013, 15, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Negi, J.; Moriwaki, K.; Konishi, M.; Yokoyama, R.; Nakano, T.; Kusumi, K.; Hashimoto-Sugimoto, M.; Schroeder, J.I.; Nishitani, K.; Yanagisawa, S.; et al. A Dof transcription factor, SCAP1, is essential for the development of functional stomata in Arabidopsis. Curr. Biol. 2013, 23, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Herrero, J.; Esteban Carrasco, A.; Zapata, J.M. Arabidopsis thaliana peroxidases involved in lignin biosynthesis: In silico promoter analysis and hormonal regulation. Plant Physiol. Biochem. 2013, 80, 192–202. [Google Scholar] [CrossRef]
- Yadav, D.K.; Shukla, D.; Tuteja, N. Rice heterotrimeric G-protein alpha subunit (RGA1): In silico analysis of the gene and promoter and its upregulation under abiotic stress. Plant Physiol. Biochem. 2013, 63, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.L.; Xie, X.R.; Zhang, J.; Xiang, G.; Li, Y.; Wu, H.B. In silico analysis of a MRP transporter gene reveals its possible role in anthocyanins or flavonoids transport in Oryza sativa. Am. J. Plant Sci. 2013, 4, 555. [Google Scholar] [CrossRef]
- Jensen, M.K.; Lindemose, S.; Masi, F.D.; Reimer, J.J.; Nielsen, M.; Perera, V.; Workman, C.T.; Turck, F.; Grant, M.R.; Mundy, J.; et al. ATAF1 transcription factor directly regulates abscisic acid biosynthetic gene NCED3 in Arabidopsis thaliana. FEBS Open Biol. 2013, 3, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, T.; Morinaka, Y.; Inukai, Y.; Kitano, H.; Fujioka, S. Auxin signal transcription factor regulates expression of the brassinosteroid receptor gene in rice. Plant J. 2013, 73, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Tao, J.; Liu, S.; Huang, S.; Chen, S.; Xie, X.; Yoneyama, K.; Zhang, Y.; Xu, G. Strigolactones are involved in phosphate-and nitrate-deficiency-induced root development and auxin transport in rice. J. Exp. Bot. 2014, 65, 6735–6746. [Google Scholar] [CrossRef] [PubMed]
- Djennane, S.; Hibrand-Saint Oyant, L.; Kawamura, K.; Lalanne, D.; Laffaire, M.; Thouroude, T.; Chalain, S.; Sakr, S.; Boumaza, R.; Foucher, F.; et al. Impacts of light and temperature on shoot branching gradient and expression of strigolactone synthesis and signalling genes in rose. Plant Cell Environ. 2014, 37, 742–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, X. Strigolactones, karrikins and more: Newly discovered molecules light up plant signaling. Mol. Plant 2014, 7, 579–581. [Google Scholar] [CrossRef]
- Yu, D.; Chen, C.; Chen, Z. Evidence for an important role of WRKY DNA binding proteins in the regulation of NPR1 gene expression. Plant Cell 2001, 13, 1527–1540. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Chen, C.; Chen, Z. Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol. Biol. 2003, 51, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Kalde, M.; Barth, M.; Somssich, I.E.; Lippok, B. Members of the Arabidopsis WRKY group III transcription factors are part of different plant defense signaling pathways. Mol. Plant Microbe Interact. 2003, 16, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bai, X.; Wang, X.; Chu, C. OsWRKY71, a rice transcription factor, is involved in rice defense response. J. Plant Physiol. 2007, 164, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Gutha, L.R.; Reddy, A.R. Rice DREB1B promoter shows distinct stress-specific responses and the overexpression of cDNA in tobacco confers improved abiotic and biotic stress tolerance. Plant Mol. Biol. 2008, 68, 533–555. [Google Scholar] [CrossRef] [PubMed]
- Pozo, M.J.; Jung, S.C.; López-Ráez, J.A.; Azcón-Aguilar, C. Impact of arbuscular mycorrhizal symbiosis on plant response to biotic stress: The role of plant defence mechanisms. In Arbuscular Mycorrhizas: Physiology and Function; Koltai, H., Kapulnik, Y., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 193–207. [Google Scholar]
- Mohanty, B.; Krishnan, S.P.T.; Swarup, S.; Bajic, V.B. Detection and preliminary analysis of motifs in promoters of anaerobically induced genes of different plant species. Ann. Bot. 2005, 96, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; van Toai, T.; Huynh, L.; Preiszner, J. Development of flooding-tolerant Arabidopsis thaliana by autoregulated cytokinin production. Mol. Breed. 2000, 6, 135–144. [Google Scholar] [CrossRef]
- Zhai, L.; Liu, Z.; Zou, X.; Jiang, Y.; Qiu, F.; Zheng, Y.; Zhang, Z. Genome-wide identification and analysis of microRNA responding to long-term waterlogging in crown roots of maize seedlings. Physiol. Plant. 2013, 147, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Seo, P.J.; Park, C.M. MicroRNA biogenesis and function in higher plants. Plant Biotechnol. Rep. 2009, 3, 111–126. [Google Scholar] [CrossRef]
- Voinnet, O. Origin, biogenesis and activity of plant microRNAs. Cell 2009, 136, 669–687. [Google Scholar] [CrossRef] [PubMed]
- Si-Ammour, A.; Windels, D.; Arn-Bouldoires, E.; Kutter, C.; Ailhas, J.; Meins, F.; Vazquez, F. miR393 and secondary siRNAs regulate expression of the TIR1/AFB2 auxin receptor clade and auxin-related development of Arabidopsis leaves. Plant Physiol. 2009, 157, 683–691. [Google Scholar] [CrossRef]
- Ding, Y.; Tao, Y.; Zhu, C. Emerging roles of microRNAs in the mediation of drought stress response in plants. J. Exp. Bot. 2013, 64, 3077–3086. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Wang, Y.; Li, Z.; Gui, Y.; Xiao, B.; Xie, J.; Zhu, Q.H.; Fan, L. Identification of wounding and topping responsive small RNAs in tobacco (Nicotiana tabacum). BMC Plant Biol. 2012, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Raghuram, B.; Sheikh, A.H.; Rustagi, Y.; Sinha, A.K. MicroRNA biogenesis factor DRB1 is a phosphorylation target of mitogen activated protein kinase, MPK3 in both rice and Arabidopsis. FEBS J. 2014, 282, 521–536. [Google Scholar] [CrossRef] [PubMed]
- Rhoades, M.W.; Reinhart, B.J.; Lim, L.P.; Burge, C.B.; Bartel, B.; Bartel, D.P. Prediction of plant microRNA targets. Cell 2002, 110, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Ruyter-Spira, C.; Bouwmeester, H. The interaction between strigolactones and other plant hormones in the regulation of plant development. Front. Plant Sci. 2013, 4, 199. [Google Scholar] [CrossRef] [PubMed]
- Crawford, S.; Shinohara, N.; Sieberer, T.; Williamson, L.; George, G.; Hepworth, J.; Müller, D.; Domagalska, M.A.; Leyser, O. Strigolactones enhance competition between shoot branches by dampening auxin transport. Development 2010, 137, 2905–2913. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, N.; Taylor, C.; Leyser, O. Strigolactone can promote or inhibit shoot branching by triggering rapid depletion of the auxin efflux protein PIN1 from the plasma membrane. PLoS Biol. 2013, 11, e1001474. [Google Scholar] [CrossRef] [PubMed]
- Dun, E.A.; de Saint Germain, A.; Rameau, C.; Beveridge, C.A. Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiol. 2012, 158, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Hayward, A.; Stirnberg, P.; Beveridge, C.; Leyser, O. Interactions between auxin and strigolactone in shoot branching control. Plant Physiol. 2009, 151, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Foo, E.; Bullier, E.; Goussot, M.; Foucher, F.; Rameau, C.; Beveridge, C.A. The branching gene RAMOSUS1 mediates interactions among two novel signals and auxin in pea. Plant Cell 2005, 17, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Arite, T.; Iwata, H.; Ohshima, K.; Maekawa, M.; Nakajima, M.; Kojima, M.; Sakakibara, H.; Kyozuka, J. DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J. 2007, 51, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Pandya-Kumar, N.; Shema, R.; Kumar, M.; Mayzlish-Gati, E.; Levy, D.; Zemach, H.; Belausov, E.; Wininger, S.; Abu-Abied, M.; Kapulnik, T.; et al. Strigolactone analog GR24 triggers changes in PIN2 polarity, vesicle trafficking and actin filament architecture. New Phytol. 2014, 202, 1184–1196. [Google Scholar] [CrossRef] [PubMed]
- Umehara, M.; Hanada, A.; Magome, H.; Takeda-Kamiya, N.; Yamaguchi, S. Contribution of strigolactones to the inhibition of tiller bud outgrowth under phosphate deficiency in rice. Plant Cell Physiol. 2010, 51, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.C.; Lee, T.Y.; Huang, H.D.; Huang, H.Y.; Pan, R.L. PlantPAN: Plant promoter analysis navigator, for identifying combinatorial cis-regulatory elements with distance constraint in plant gene groups. BMC Genomics 2008, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Plant PAN. Available online: http://plantpan.mbc.nctu.edu.tw (accessed on 12–30 November 2014).
- Rice Annotation Project Database. Available online: http://rapdblegacy.dna.affrc.go.jp (accessed on 12–30 November 2014).
- Yilmaz, A.; Mejia-Guerra, M.K.; Kurz, K.; Liang, X.; Welch, L.; Grotewold, E. AGRIS: The arabidopsis gene regulatory information server, an update. Nucleic Acids Res. 2011, 39, D1118–D1122. [Google Scholar] [CrossRef] [PubMed]
- AGRIS. Available online: http://arabidopsis.med.ohio-state.edu (accessed on 15–30 November 2014).
- Mathelier, A.; Zhao, X.; Zhang, A.W.; Parcy, F.; Worsley-Hunt, R.; Arenillas, D.J.; Buchman, S.; Chen, C.Y.; Chou, A.; Ienasescu, H.; et al. JASPAR 2014: An extensively expanded and updated open-access database of transcription factor binding profiles. Nucleic Acids Res. 2014, 42, D142–D147. [Google Scholar] [CrossRef] [PubMed]
- YASPAR. Available online: http://jaspar.genereg.net (accessed on 15–30 November 2014).
- Higo, K.; Ugawa, Y.; Iwamoto, M.; Korenaga, T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999, 27, 297–300. [Google Scholar] [CrossRef] [PubMed]
- PLACE. Available online: http://www.dna.affrc.go.jp/PLACE/ (accessed on 15–30 November 2014).
- Matys, V.; Fricke, E.; Geffers, R.; Gössling, E.; Haubrock, M.; Hehl, R.; Hornischer, K.; Karas, D.; Kel, A.E.; Kel-Margoulis, O.V.; et al. TRANSFAC: Transcriptional regulation, from patterns to profiles. Nucleic Acids Res. 2003, 31, 374–378. [Google Scholar] [CrossRef] [PubMed]
- TRANSFAC Database. Available online: http://www.gene-regulation.com/cgi-bin/pub/databases/transfac (accessed on 15–30 November 2014).
- Jin, J.P.; Zhang, H.; Kong, L.; Gao, G.; Luo, J.C. PlantTFDB 3.0: A portal for the functional and evolutionary study of plant transcription factors. Nucleic Acids Res. 2014, 42, D1182–D1187. [Google Scholar] [CrossRef] [PubMed]
- PlantTF Database. Available online: http://planttfdb.cbi.pku.edu.cn (accessed on 15–30 November 2014).
- Kozomara, A.; Griffiths-Jones, S. miRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014, 42, D68–D73. [Google Scholar] [CrossRef] [PubMed]
- miRBase. Available online: http://www.mirbase.org (accessed on 20–30 November 2014).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzec, M.; Muszynska, A. In Silico Analysis of the Genes Encoding Proteins that Are Involved in the Biosynthesis of the RMS/MAX/D Pathway Revealed New Roles of Strigolactones in Plants. Int. J. Mol. Sci. 2015, 16, 6757-6782. https://doi.org/10.3390/ijms16046757
Marzec M, Muszynska A. In Silico Analysis of the Genes Encoding Proteins that Are Involved in the Biosynthesis of the RMS/MAX/D Pathway Revealed New Roles of Strigolactones in Plants. International Journal of Molecular Sciences. 2015; 16(4):6757-6782. https://doi.org/10.3390/ijms16046757
Chicago/Turabian StyleMarzec, Marek, and Aleksandra Muszynska. 2015. "In Silico Analysis of the Genes Encoding Proteins that Are Involved in the Biosynthesis of the RMS/MAX/D Pathway Revealed New Roles of Strigolactones in Plants" International Journal of Molecular Sciences 16, no. 4: 6757-6782. https://doi.org/10.3390/ijms16046757






