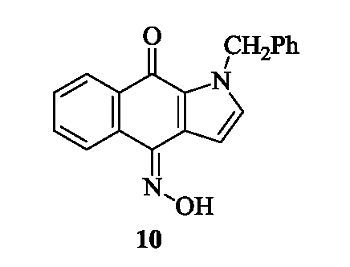
Discovery of Benzo[f]indole-4,9-dione Derivatives as New Types of Anti-Inflammatory Agents
Abstract
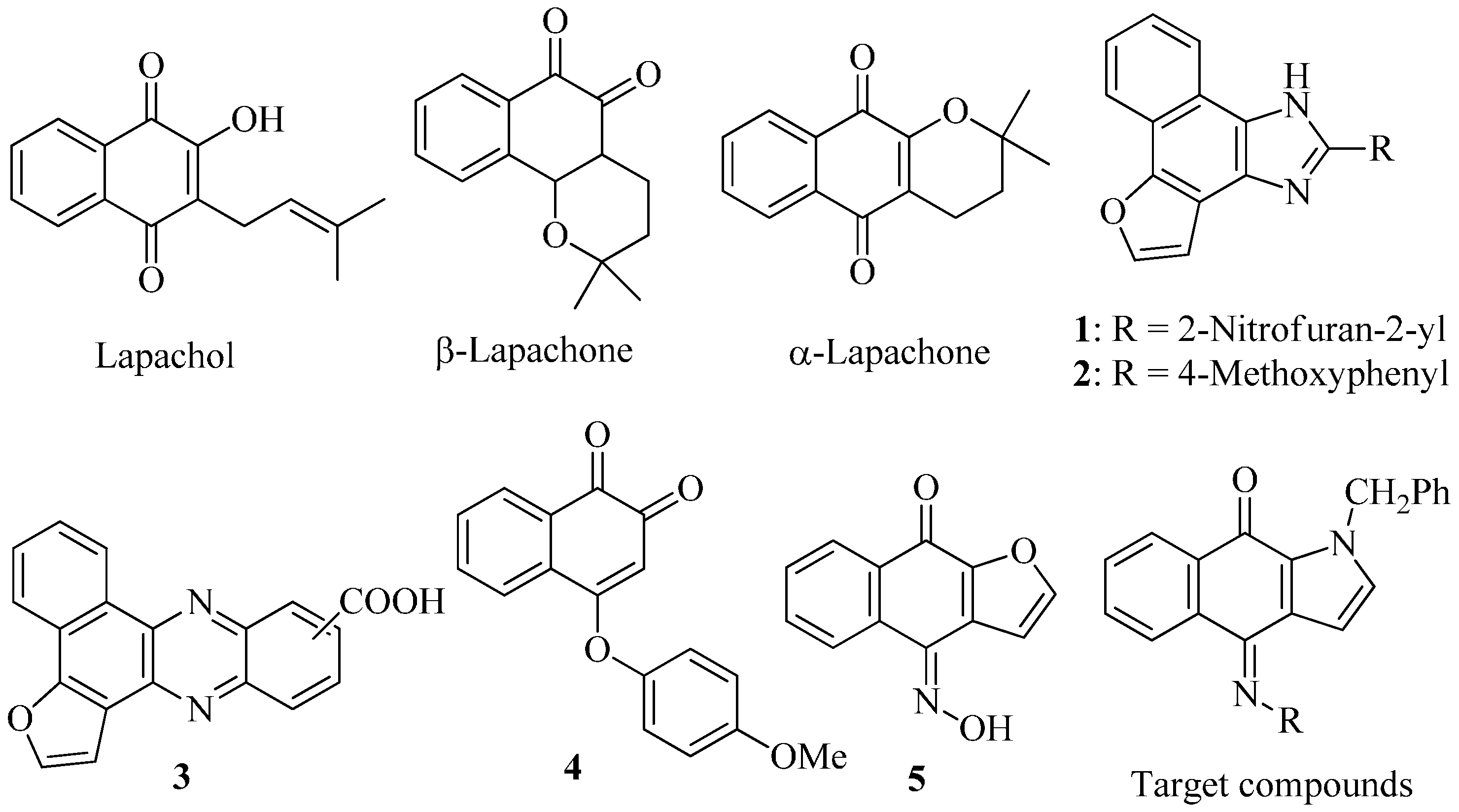
:1. Introduction
2. Results and Discussion
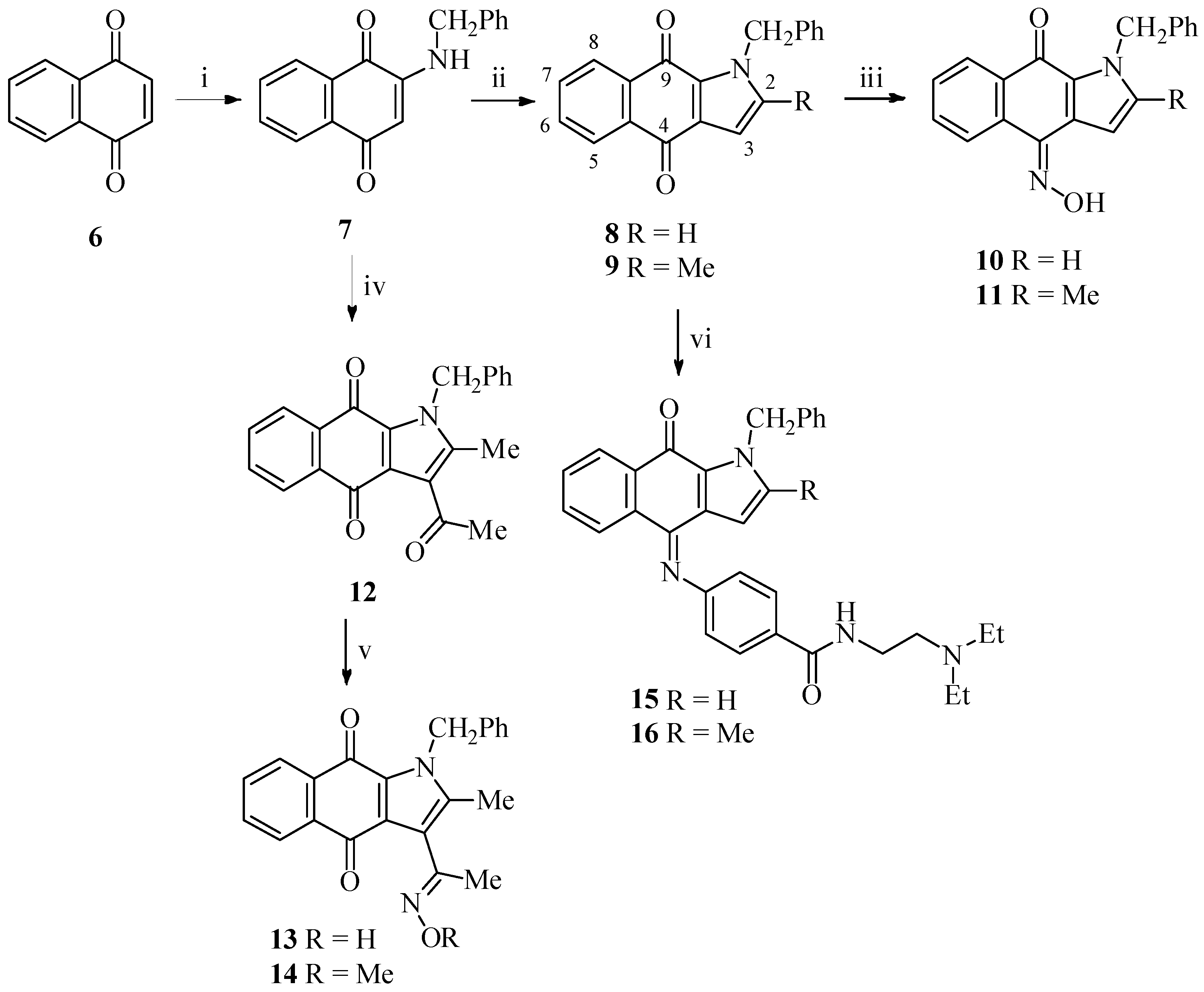
2.1. Chemistry
2.2. Biological Results and Discussion
| Compound | Superoxide Anion | Elastase Release |
|---|---|---|
| 8 | 16.10 ± 1.87 | >30 |
| 9 | 14.04 ± 4.40 | >30 |
| 10 | 2.78 ± 0.89 | 2.74 ± 1.20 |
| 11 | 7.47 ± 1.39 | >30 |
| 12 | >30 | >30 |
| 13 | 10.54 ± 0.52 | 14.65 ± 2.44 |
| 14 | >30 | >30 |
| 15 | 0.51 ± 0.12 | 2.05 ± 0.21 |
| 16 | 0.52 ± 0.11 | – b |
| LY294002 c | 1.36 ± 0.33 | 2.21±0.45 |
| Cells\Compd | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | CPT a |
|---|---|---|---|---|---|---|---|---|---|---|
| MCF7 | 99 | 105 | 110 | 104 | 78 | 106 | 104 | 82 | 57 | 33 |
| NCI-H460 | 96 | 104 | 110 | 110 | 88 | 111 | 108 | 31 | 18 | 1 |
| SF-268 | 103 | 106 | 106 | 102 | 115 | 109 | 112 | 120 | 106 | 25 |
| Compd\Cells | MCF7 | NCI-H460 | SF-268 | Detroit 551 |
|---|---|---|---|---|
| 15 | 4.98 ± 0.15 | 3.16 ± 0.13 | 27.41 ± 0.39 | 7.68 ± 0.58 |
| 16 | 4.95 ± 0.21 | 3.23 ± 0.34 | 25.76 ± 1.66 | 4.78 ± 0.57 |
| CPT b | 0.57 ± 0.03 | 0.03 ± 0.003 | 0.19 ± 0.006 | 0.99 ± 0.09 |


3. Experimental Section
3.1. General
3.1.1. 2-(Benzylamino)naphthalene-1,4-dione (7)
3.1.2. 1-Benzyl-1H-benzo[f]indole-4,9-dione (8)
3.1.3. 1-Benzyl-2-methyl-1H-benzo[f]indole-4,9-dione (9)
3.1.4. 1-Benzyl-4-(hydroxyimino)-1H-benzo[f]indol-9(4H)-one (10)
3.1.5. 1-Benzyl-4-(hydroxyimino)-2-methyl-1H-benzo[f]indol-9(4H)-one (11)
3.1.6. 3-Acetyl-1-benzyl-2-methyl-1H-benzo[f]indole-4,9-dione (12)
3.1.7. 1-Benzyl-3-[1-(hydroxyimino)ethyl]-2-methyl-1H-benzo[f]indole-4,9-dione (13)
3.1.8. 1-Benzyl-3-[1-(methoxyimino)ethyl]-2-methyl-1H-benzo[f]indole-4,9-dione (14)
3.1.9. 4-(1-Benzyl-9-oxo-1H-benzo[f]indol-4(9H)-ylideneamino)-N-[2-(diethylamino)ethyl]benzamide (15)
3.1.10. 4-(1-Benzyl-2-methyl-9-oxo-1H-benzo[f]indol-4(9H)-ylideneamino)-N-[2-(diethylamino)-ethyl]benzamide (16)
3.2. Biological Evaluation
3.2.1. Preparation of Human Neutrophils
3.2.2. Superoxide Generation and Elastase Release
3.2.3. Western Analysis
3.2.4. Measurement of Intracellular Calcium Concentration ([Ca2+]i)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Malech, H.L.; Gallin, J.I. Current concepts: Immunology. Neutrophils in human diseases. N. Eng. J. Med. 1987, 317, 687–694. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Rieu, P.; Descamps-Latscha, B.; Lesavre, P.; Halbwachs-Mecarelli, L. Neutrophils: Molecules, functions and pathophysiological aspects. Lab. Investig. 2000, 80, 617–653. [Google Scholar] [CrossRef] [PubMed]
- Okajima, K.; Harada, N.; Uchiba, M. Ranitidine reduces ischemia/reperfusion-induced liver injury in rats by inhibiting neutrophil activation. J. Pharmacol. Exp. Ther. 2002, 301, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Ennis, M. Neutrophils in asthma pathophysiology. Curr. Allergy Asthma Rep. 2003, 3, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Vinten-Johansen, J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc. Res. 2004, 61, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Borregaard, N. The human neutrophil. Function and dysfunction. Eur. J. Haematol. 1998, 41, 401–413. [Google Scholar] [CrossRef]
- Roos, D.; van Bruggen, R.; Meischl, C. Oxidative killing of microbes by neutrophils. Microbes Infect. 2003, 5, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Burgos, R.A.; Hidalgo, M.A.; Figueroa, C.D.; Conejeros, I.; Hancke, J.L. New potential targets to modulate neutrophil function in inflammation. Mini Rev. Med. Chem. 2009, 9, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.W.; Hwang, T.L.; Wu, C.C.; Chang, F.R.; Wang, T.W.; Wu, Y.C. The evaluation of 2,8-disubstituted benzoxazinone derivatives as anti-inflammatory and anti-platelet aggregation agents. Bioorg. Med. Chem. Lett. 2005, 15, 2786–2789. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Kleeberger, S.R. Genetic mechanisms of susceptibility to oxidative lung injury in mice. Free Radic. Biol. Med. 2007, 42, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.L.; Yang, J.Y.; Chen, G.L.; Zhang, L.J.; Wang, L.H.; Li, J.; Wu, C.F. RV09, a novel resveratrol analogue, inhibits NO and TNF-alpha production by LPS-activated microglia. Int. Immunopharmacol. 2008, 8, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.M.; Lee, Y.R.; Chang, Y.S.; Lee, J.Y.; Kim, B.H.; Min, K.R.; Kim, Y. Suppression of interleukin-6 production in macrophages by furonaphthoquinone NFD-37. Int. Immunopharmacol. 2006, 6, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.L.; Yang, J.Y.; Chen, G.L.; Wang, L.H.; Zhang, L.J.; Wang, S.; Li, J.; Wu, C.F. Effects of resveratrol and its derivatives on lipopolysaccharide-induced microglial activation and their structure-activity relationships. Chem. Biol. Interact. 2008, 174, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.M.; Zhou, H.Y.; Guo, L.Y.; Kim, J.A.; Lee, S.H.; Merfort, I.; Kang, S.S.; Kim, H.S.; Kim, S.; Kim, Y.S. Anti-inflammatory effects of glycyrol isolated from Glycyrrhiza uralensis in LPS-stimulated RAW264.7 macrophages. Int. Immunopharmacol. 2008, 8, 1524–1532. [Google Scholar] [CrossRef]
- Cote, B.; Boulet, L.; Brideau, C.; Claveau, D.; Ethier, D.; Frenette, R.; Gagnon, M.; Giroux, A.; Guay, J.; Guiral, S.; et al. Substituted phenanthrene imidazoles as potent, selective, and orally active mPGES-1 inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 6816–6820. [Google Scholar]
- Liu, F.Z.; Fang, H.; Zhu, H.W.; Wang, Q.; Yang, Y.; Xu, W.F. Design, synthesis, and preliminary evaluation of 4-[6-(3-nitroguanidino)hexanamido]pyrrolidine derivatives as potential iNOS inhibitors. Bioorg. Med. Chem. 2008, 16, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Tzeng, H.P.; Kuo, M.L.; Lin-Shiau, S.Y. Inhibition of inducible nitric oxide synthase by β-lapachone in rat alveolar macrophages and aorta. Br. J. Pharmacol. 1999, 126, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H.; Lin, C.S.; Shih, P.K.; Tsao, L.T.; Wang, J.P.; Cheng, C.M.; Tzeng, C.C.; Chen, Y.L. Furo[3',2':3,4]naphtho[1,2-d]imidazole derivatives as potential inhibitors of inflammatory factors in sepsis. Bioorg. Med. Chem. 2009, 17, 6773–6779. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H.; Tzeng, C.C.; Shih, P.K.; Yang, C.N.; Chuang, Y.C.; Peng, S.I.; Lin, C.S.; Wang, J.P.; Cheng, C.M.; Chen, Y.L. Identification of furo[3',2':3,4]naphtho[1,2-d]imidazole derivatives as orally active and selective inhibitors of microsomal prostaglandin E(2) synthase-1 (mPGES-1). Mol. Divers. 2012, 16, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.R.; Huang, L.J.; Lee, M.R.; Chen, Y.L.; Kuo, S.C.; Tzeng, C.C.; Hsu, M.F.; Wang, J.P. The signaling mechanisms mediating the inhibitory effect of TCH-1116 on formyl peptide-stimulated superoxide anion generation in neutrophils. Eur. J. Pharm. 2012, 682, 171–180. [Google Scholar] [CrossRef]
- Tseng, C.H.; Cheng, C.M.; Tzeng, C.C.; Peng, S.I.; Yang, C.L.; Chen, Y.L. Synthesis and anti-inflammatory evaluations of β-lapachone derivatives. Bioorg. Med. Chem. 2013, 21, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H.; Chen, Y.L.; Yang, S.H.; Peng, S.I.; Cheng, C.M.; Han, C.H.; Lin, S.R.; Tzeng, C.C. Synthesis and antiproliferative evaluation of certain iminonaphtho[2,3-b]furan derivatives. Bioorg. Med. Chem. 2010, 18, 5172–5182. [Google Scholar] [CrossRef] [PubMed]
- Aristoff, P.A.; Johnson, P.D. Synthesis of CBI-PDE-I-dimer, the benzannelated analog of CC-1065. J. Org. Chem. 1992, 57, 6234–6239. [Google Scholar] [CrossRef]
- Urbanek, R.A.; Suchard, S.J.; Steelman, G.B.; Knappenberger, K.S.; Sygowski, L.A.; Veale, C.A.; Chapdelaine, M.J. Potent reversible inhibitors of the protein tyrosine phosphatase CD45. J. Med. Chem. 2001, 44, 1777–1793. [Google Scholar] [CrossRef] [PubMed]
- Ito, C.; Katsuno, S.; Kondo, Y.; Tan, H.T.; Furukawa, H. Chemical constituents of Avicennia alba. Isolation and structural elucidation of new naphthoquinones and their analogues. Chem. Pharm. Bull. 2000, 48, 339–343. [Google Scholar]
- Tseng, C.C.; Wu, Y.L.; Chuang, C.P. Cerium salts in the oxidative free radical reactions between 2-amino-1,4-naphthoquinones and β-dicarbonyl compounds. Tetrahedron 2002, 58, 7625–7633. [Google Scholar] [CrossRef]
- Chen, Y.C.; Cheng, M.J.; Lee, S.J.; Dixit, A.K.; Ishikawa, T.; Tsai, I.L.; Chen, I.S. Coumarinolignans from the Root of Formosan Antidesma pentandrum var.barbatum. Helv. Chim. Acta 2004, 87, 2805–2811. [Google Scholar] [CrossRef]
- Hwang, T.L.; Su, Y.C.; Chang, H.L.; Leu, Y.L.; Chung, P.J.; Kuo, L.M.; Chang, Y.J. Suppression of superoxide anion and elastase release by C18 unsaturated fatty acids in human neutrophils. J. Lipid Res. 2009, 50, 1395–1408. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.L.; Wang, C.C.; Kuo, Y.H.; Huang, H.C.; Wu, Y.C.; Kuo, L.M.; Wu, Y.H. The hederagenin saponin SMG-1 is a natural FMLP receptor inhibitor that suppresses human neutrophil activation. Biochem. Pharmacol. 2010, 80, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Boyum, A.; Lovhaug, D.; Tresland, L.; Nordlie, E.M. Separation of leucocytes: Improved cell purity by fine adjustments of gradient medium density and osmolality. Scand. J. Immunol. 1991, 34, 697–712. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.L.; Li, G.L.; Lan, Y.H.; Chia, Y.C.; Hsieh, P.W.; Wu, Y.H.; Wu, Y.C. Potent inhibition of superoxide anion production in activated human neutrophils by isopedicin, a bioactive component of the Chinese medicinal herb Fissistigma oldhamii. Free Radic. Biol. Med. 2009, 46, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, C.; Rollet-Labelle, E.; Caon, A.C.; Naccache, P.H. Immunoblotting and sequential lysis protocols for the analysis of tyrosine phosphorylation-dependent signaling. J. Immunol. Methods 2002, 271, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.H.; Hwang, T.L.; Thang, T.D.; Leu, Y.L.; Kuo, P.C.; Nguyet, B.; Dai, D.N.; Wu, T.S. Isolation and synthesis of melodamide A, a new anti-inflammatory phenolic amide from the leaves of Melodorum fruticosum. Planta Med. 2013, 79, 288–294. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-R.; Tseng, C.-H.; Chen, Y.-L.; Hwang, T.-L.; Tzeng, C.-C. Discovery of Benzo[f]indole-4,9-dione Derivatives as New Types of Anti-Inflammatory Agents. Int. J. Mol. Sci. 2015, 16, 6532-6544. https://doi.org/10.3390/ijms16036532
Chen Y-R, Tseng C-H, Chen Y-L, Hwang T-L, Tzeng C-C. Discovery of Benzo[f]indole-4,9-dione Derivatives as New Types of Anti-Inflammatory Agents. International Journal of Molecular Sciences. 2015; 16(3):6532-6544. https://doi.org/10.3390/ijms16036532
Chicago/Turabian StyleChen, You-Ren, Chih-Hua Tseng, Yeh-Long Chen, Tsong-Long Hwang, and Cherng-Chyi Tzeng. 2015. "Discovery of Benzo[f]indole-4,9-dione Derivatives as New Types of Anti-Inflammatory Agents" International Journal of Molecular Sciences 16, no. 3: 6532-6544. https://doi.org/10.3390/ijms16036532