Characterization and Antihyperglycemic Activity of a Polysaccharide from Dioscorea opposita Thunb Roots
Abstract
:1. Introduction
2. Results and Discussion
2.1. Polysaccharide Isolation and Purification
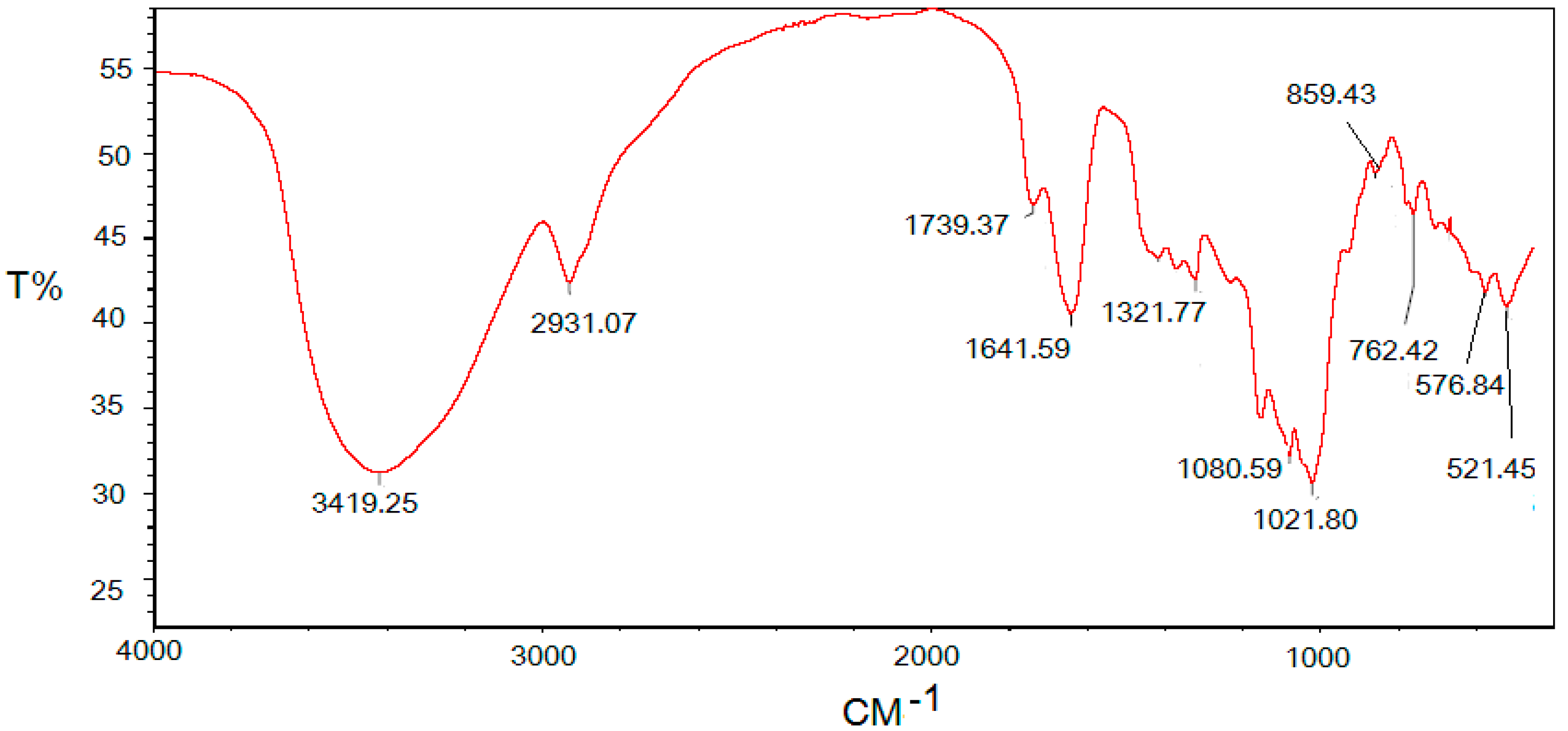
2.2. Infrared Spectrum of the Polysaccharide
2.3. Effect of Polysaccharide on the Blood Glucose Level in Healthy Mice
| Groups | Dose (mg/kg Body wt.) | Plasma Glucose Level (mmol/L) | |||
|---|---|---|---|---|---|
| Day 0 | Day 6 | Day 12 | Day 18 | ||
| Control | - | 5.3 ± 0.9 | 5.5 ± 0.6 | 5.3 ± 0.7 | 5.5 ± 0.7 |
| Glipizide | 200 | 5.5 ± 0.4 | 5.1 ± 0.8 * | 4.8 ± 1.0 * | 4.1 ± 0.3 ** |
| DOTP-80 | 400 | 5.3 ± 0.6 | 5.3 ± 1.0 | 5.4 ± 0.5 | 5.6 ± 0.7 |
| 200 | 5.5 ± 0.7 | 5.5 ± 0.6 | 5.5 ± 0.9 | 5.4 ± 0.8 | |
| 100 | 5.5 ± 0.9 | 5.3 ± 0.7 | 5.2 ± 0.5 | 5. 6 ± 0.4 | |
2.4. Effect of Polysaccharide on the Blood Glucose Level in Alloxan-Induced Diabetic Mice
| Groups | Dose (mg/kg Body wt.) | Plasma Glucose Level (mmol/L) | |||
|---|---|---|---|---|---|
| Day 0 | Day 6 | Day 12 | Day 18 | ||
| Control | - | 20.9 ± 3.1 | 21.8 ± 1.9 | 21.0 ± 2.8 | 22.1 ± 3.3 |
| Metformin hydrochloride | 200 | 21.3 ± 2.5 | 17.6 ± 1.3 | 13.8 ± 1.7 ** | 10.3 ± 2.0 ** |
| DOTP-80 | 400 | 20.1 ± 2.6 | 18.7 ± 2.4 | 15.4 ± 0.5 * | 12.1 ± 1.9 ** |
| 200 | 20.1 ± 2.0 | 19.6 ± 1.9 | 16.7 ± 2.5 * | 13.3 ± 2.2 ** | |
| 100 | 21.1 ± 2.4 | 20.0 ± 2.4 | 18.4 ± 2.7 | 15.5 ± 2.7 * | |
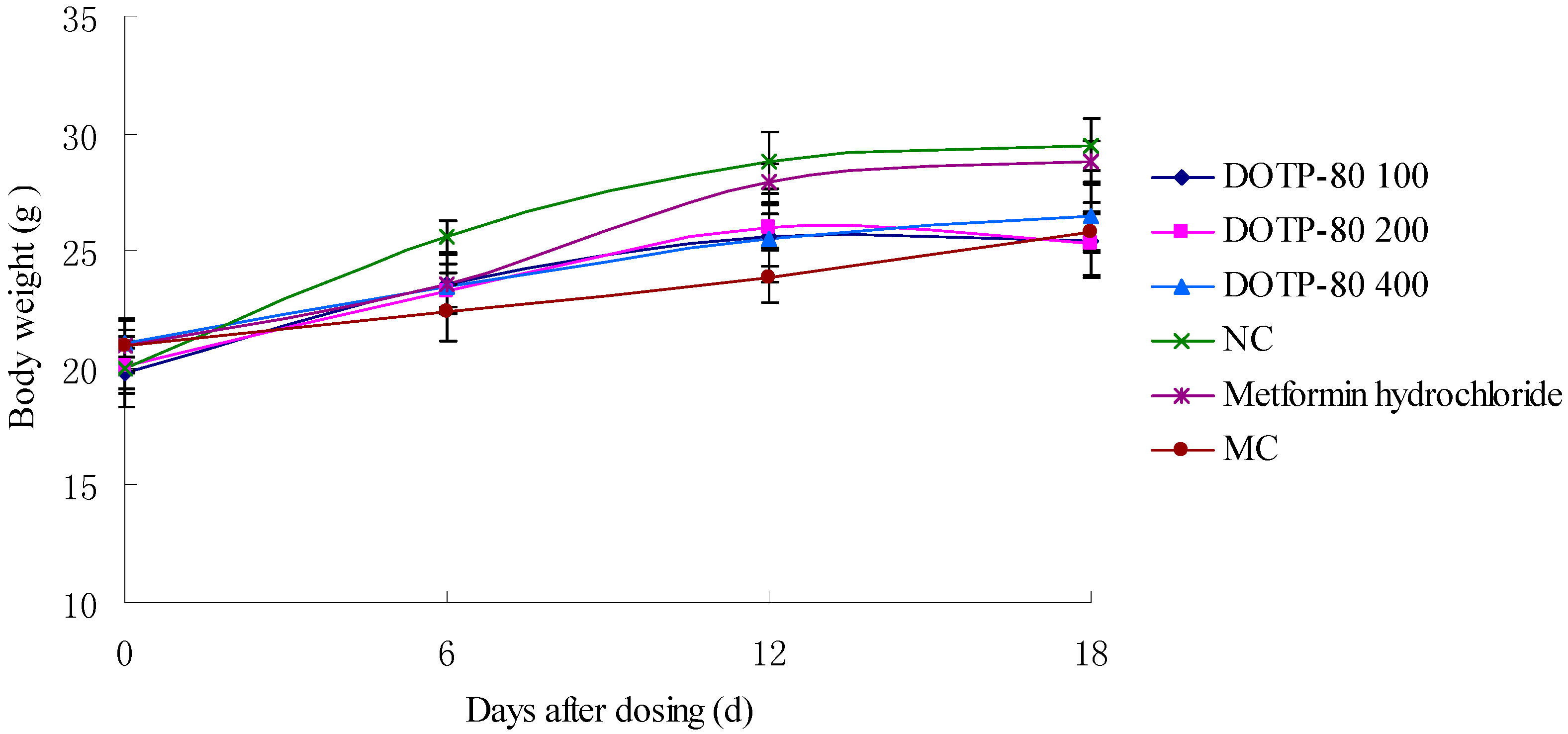
2.5. Effect of DOTP-80 on Body Weight in Mice
2.6. Effect of the Polysaccharide on Blood Glucose Levels in Alloxan-Induced Diabetic Rats
| Groups | Dose (mg/kg Body wt.) | Plasma Glucose Level (mmol/L) | ||
|---|---|---|---|---|
| 0 h | 0.5 h | 2 h | ||
| Control | - | 12.6 ± 1.8 | 25.6 ± 4.2 | 15.0 ± 4.0 |
| Metformin hydrochloride | 200 | 11.8 ± 2.2 | 13.2 ± 2.8 ** | 7.9 ± 1.4 ** |
| DOTP-80 | 400 | 12.4 ± 2.6 | 15.6 ± 2.9 ** | 9.9 ± 2.3 ** |
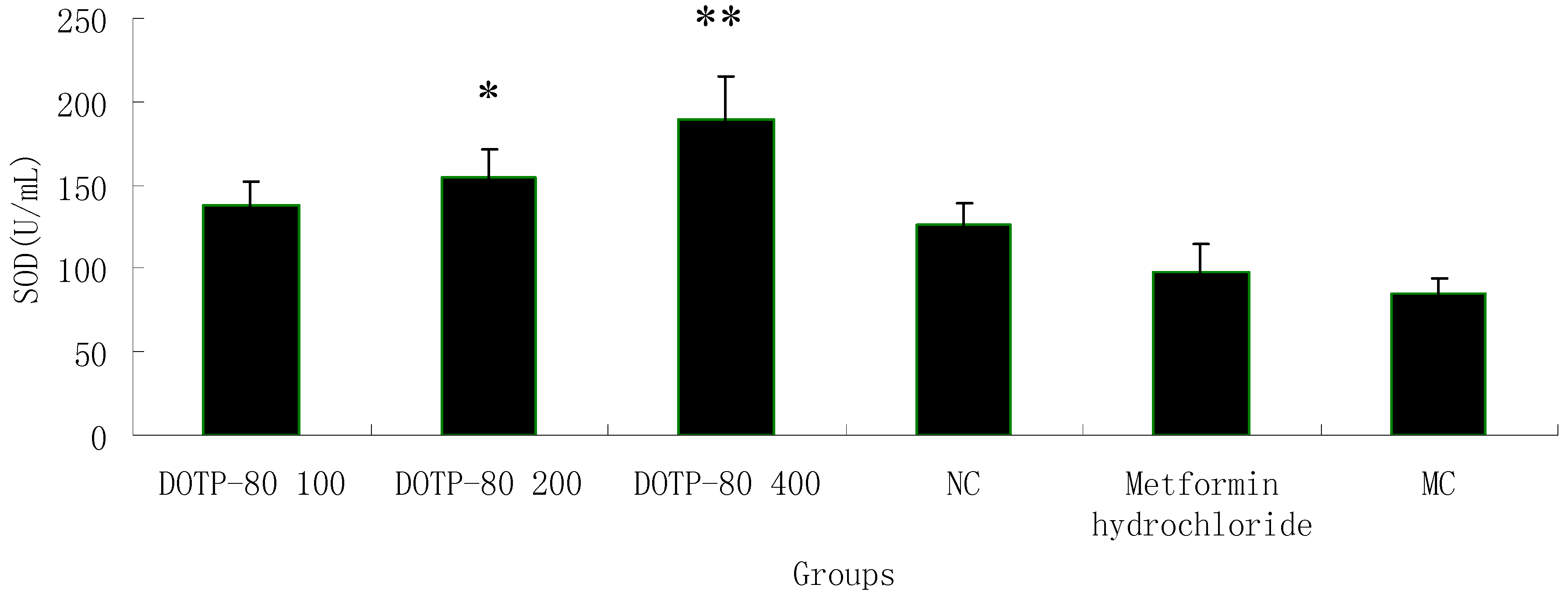
2.7. Effect of the Polysaccharide on SOD Activity in Alloxan-Induced Diabetic Mice
3. Experimental Section
3.1. Materials and Chemicals
3.2. Extraction of Crude Polysaccharide from Dioscorea opposita Thunb
3.3. Purification of the Crude Polysaccharide
3.4. Determination of the Molecular Weight of Purified Polysaccharide
3.5. Infrared Spectrum Analysis
3.6. Analysis of Monosaccharide Composition
3.7. Hypoglycemic Activity Test in Vivo
3.7.1. Effect of Polysaccharide on Fasting Blood Glucose Levels in Mice
3.7.2. Effect of the Polysaccharide on Blood Glucose Levels in Alloxan-Induced Diabetic Rats (OGTT)
3.7.3. Effect of the Polysaccharide on SOD Activity in Alloxan-Induced Diabetic Mice
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alberti, K.; Zimmet, P. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey, S.G.; Stettler, C.; Diem, P. Risk of adverse effects of intensified treatment in insulin-dependent diabetes mellitus: A meta-analysis. Diabet. Med. 1997, 14, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Park, M.; Lee, H.C.; Kang, Y.H.; Kang, E.S.; Kim, S.K. Antidiabetic agents from medicinal plants. Curr. Med. Chem. 2006, 13, 1203–1218. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, P.K.; Doble, M. A target based therapeutic approach towards diabetes mellitus using medicinal plants. Curr. Diabet. Rev. 2008, 4, 291–308. [Google Scholar] [CrossRef]
- Fan, Y.; Lin, M.; Luo, A.; Chun, Z.; Luo, A. Characterization and antitumor activity of a polysaccharide from Sarcodia. Molecules 2014, 19, 10863–10876. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.Y.; Ning, Y.L.; Qi, J.; He, Z.; Jie, J.; Lin, J.J.; Huang, Y.J.; Li, F.S.; Li, X.H. Structure and antitumor and immunomodulatory activities of a water-soluble polysaccharide from Dimocarpus Longan Pulp. Int. J. Mol. Sci. 2014, 15, 5140–5162. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.J.; Luo, A.X. Evaluation of anti-tumor activity of water-soluble polysaccharides from Dendrobium denneanum. Afr. J. Pharm. Pharm. 2011, 5, 415–420. [Google Scholar] [CrossRef]
- Wu, X.; Mao, G.; Fan, Q.; Zhao, T.; Zhao, J.; Li, F.; Yang, L. Isolation, purification, immunological and anti-tumor activities of polysaccharides from Gymnema. sylvestre. Food Res. Int. 2012, 48, 935–939. [Google Scholar] [CrossRef]
- Xu, R.B.; Yang, X.; Wang, J.; Zhao, H.T.; Lu, W.H.; Cui, J.; Cheng, C.L.; Zou, P.; Huang, W.W.; Wang, P.; et al. Chemical composition and antioxidant activities of three polysaccharide fractions from pine cones. Int. J. Mol. Sci. 2012, 13, 14262–14277. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.; Fan, Y. In vitro antioxidant of a water-soluble polysaccharide from Dendrobium fimhriatum Hook.var.oculatum Hook. Int. J. Mol. Sci. 2011, 12, 4068–4079. [Google Scholar] [CrossRef] [PubMed]
- Arita, H.; Tsuzuki, H.; Morihara, K.; Kawanami, J. Structural studies on anti-inflammatory polysaccharides from Streptomyces fradiae. J. Biochem. 1974, 76, 861–869. [Google Scholar] [PubMed]
- Popov, S.V.; Popova, G.Y.; Ovodova, R.G.; Ovodov, Y.S. Antiinflammatory activity of the pectic polysaccharide from Comarum palustre. Fitoterapia 2005, 76, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; Zang, H.Y.; Cui, B.A.; Liu, R.; Xu, D.H. The antivirus activity of isatis root polysaccharide on PRRSV in vitro. Acta Agric. Boreal. Occiden. Sin. 2009, 18, 198–200. [Google Scholar]
- Gong, F.; Li, F.; Zhang, L.; Li, J.; Zhang, Z.; Wang, G. Hypoglycemic effects of crude polysaccharide from Purslane. Int. J. Mol. Sci. 2009, 10, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jin, W.H.; Zhang, W.J.; Hou, Y.; Zhang, H.; Zhang, Q.B. Hypoglycemic property of acidic polysaccharide extracted from Saccharina. japonica and its potential mechanism. Carbohydr. Polym. 2013, 95, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Li, S.P.; Zhang, G.H.; Zeng, Q.; Huang, Z.G.; Wang, Y.T.; Dong, T.T.; Tsim, K.W. Hypoglycemic activity of polysaccharide, with antioxidation, isolated from cultured Cordyceps mycelia. Phytomedicine 2006, 13, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.H.; Kan, J.Q.; Li, Z.X.; Chen, Z.D. Structural features and immunological activity of a polysaccharide from Dioscorea. opposita Thunb roots. Carbohydr. Polym. 2005, 61, 125–131. [Google Scholar] [CrossRef]
- Choi, E.M.; Koo, S.J.; Hwang, J.K. Immune cell stimulating activity of mucopolysaccharide isolated from yam (Dioscorea batatas). J. Ethnopharm. 2004, 91, 1–6. [Google Scholar] [CrossRef]
- Zhao, G.H.; Li, Z.X.; Chen, Z.D. Purification and physicochemical characteristics analysis of Chinese yam polysaccharides fraction RDPS-I. Food Ferment. Ind. 2002, 28, 1–4. [Google Scholar]
- Luo, D.H. Identification of structure and antioxidant activity of a fraction of polysaccharidepurified from Dioscorea nipponica Makino. Carbohydr. Polym. 2008, 71, 544–549. [Google Scholar] [CrossRef]
- Liu, Y.H.; Wang, F.S. Structural characterization of an active polysaccharide from Phellinus ribis. Carbohydr. Polym. 2007, 70, 386–392. [Google Scholar] [CrossRef]
- Zhao, M.M.; Yang, N.; Yang, B. Structural characterization of water-soluble olysaccharides from Opuntia monacanthap cladodes in relation to their anti-glycated activities. Food Chem. 2007, 105, 1480–1486. [Google Scholar] [CrossRef]
- Barker, S.A.; Bourne, E.J.; Stacey, M.; Whiffen, D.H. Infrared spectra of carbohydrates: Part I. Some derivatives of d-glucopyranose. J. Chem. Soc. 1954, 75, 171–176. [Google Scholar] [CrossRef]
- Luo, A.; Ge, Z.; Fan, Y.; Luo, A.; Chun, Z.; He, X. In vitro and in vivo antioxidant activity of a water-soluble polysaccharide from Dendrobium denneanum. Molecules 2011, 16, 1579–1592. [Google Scholar] [CrossRef] [PubMed]
- Navarini, L.; Gilli, R.; Gombac, V.; Abatangelo, A.; Bosco, M.; Toffanin, R. Polysaccharides from hot water extracts of roasted Coffea arabica beans: Isolation and characterization. Carbohydr. Polym. 1999, 40, 71–81. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Nunome, T.; Yamauchi, R.; Kato, K.; Sone, Y. Structure of an exocellular polysaccharide of Lactobacillus helveticus TN-4, a spontaneous mutant strain of Lactobacillus helveticus TY1-2. Carbohydr. Res. 1995, 275, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.G.; Joo, H.S.; Choi, J.W.; Koo, Y.M.; Chang, C.S. Purification and characterization of extracellular polysaccharide from haloalkalophilic Bacillus sp. I-450. Enzym. Microb. Technol. 2004, 34, 673–681. [Google Scholar] [CrossRef]
- Pang, X.B.; Yao, W.B.; Yang, X.B.; Xie, C.; Liu, D.; Zhang, J.; Gao, X.D. Purification, characterization and biological activity on hepatocytes of a polysaccharide from Flammulina velutipes mycelium. Carbohydr. Res. 2007, 70, 291–297. [Google Scholar] [CrossRef]
- Sharma, S.B.; Nasir, A.; Prabhu, K.M.; Murthy, P.S.; Dev, G. Hypoglycaemic and hypolipidemic effect of ethanolic extract of seeds of Eugenia jambolana in alloxan-induced diabetic rabbits. J. Ethnopharmacol. 2003, 85, 201–206. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; He, Q.; Luo, A.; Wang, M.; Luo, A. Characterization and Antihyperglycemic Activity of a Polysaccharide from Dioscorea opposita Thunb Roots. Int. J. Mol. Sci. 2015, 16, 6391-6401. https://doi.org/10.3390/ijms16036391
Fan Y, He Q, Luo A, Wang M, Luo A. Characterization and Antihyperglycemic Activity of a Polysaccharide from Dioscorea opposita Thunb Roots. International Journal of Molecular Sciences. 2015; 16(3):6391-6401. https://doi.org/10.3390/ijms16036391
Chicago/Turabian StyleFan, Yijun, Qinyi He, Aoshuang Luo, Miaoyu Wang, and Aoxue Luo. 2015. "Characterization and Antihyperglycemic Activity of a Polysaccharide from Dioscorea opposita Thunb Roots" International Journal of Molecular Sciences 16, no. 3: 6391-6401. https://doi.org/10.3390/ijms16036391





