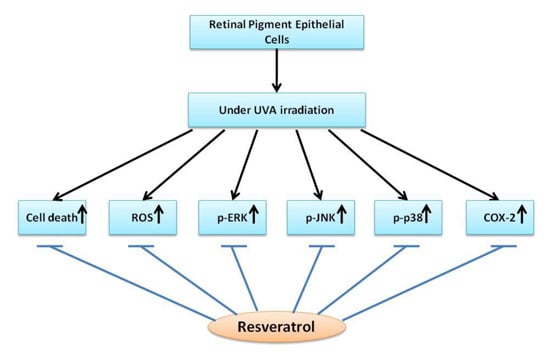
Protective Effects of Resveratrol against UVA-Induced Damage in ARPE19 Cells
Abstract
:1. Introduction
2. Results
2.1. Resveratrol Has no Cytotoxicity on ARPE19 Cells
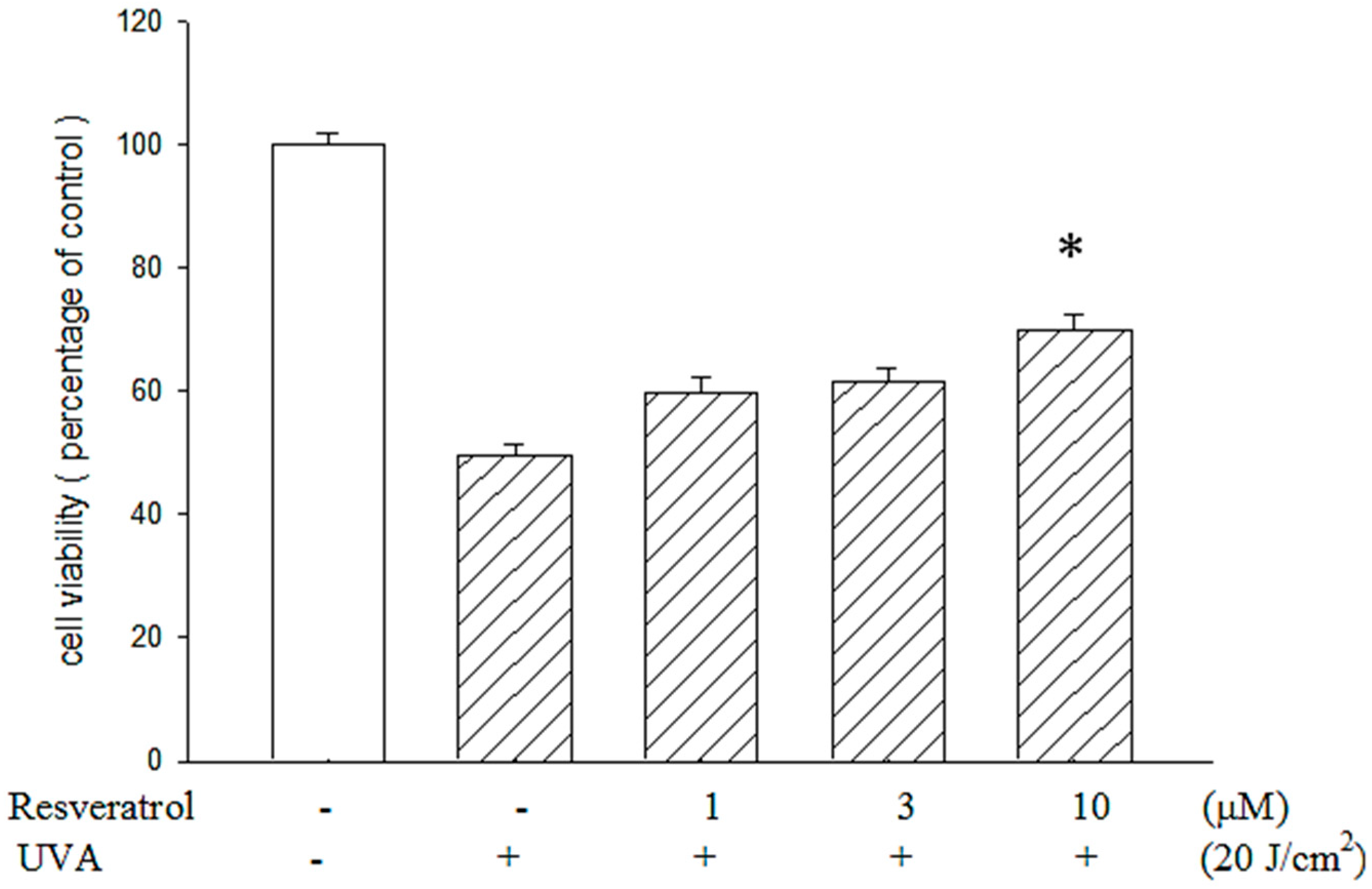
2.2. Resveratrol Reduced UVA-Induced Decrease in Cell Viability
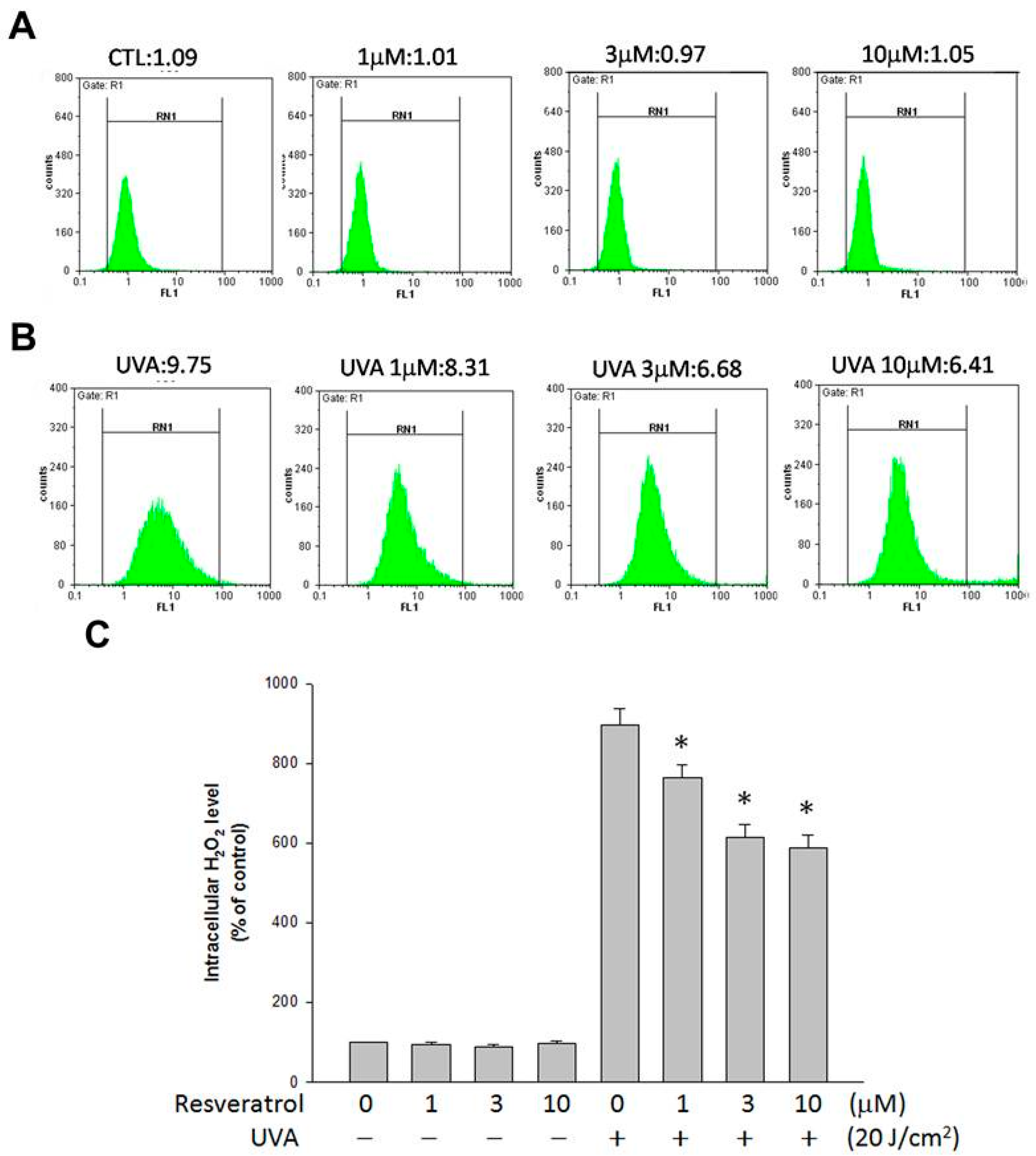
2.3. Resveratrol Lessened UVA-Induced H2O2 Production
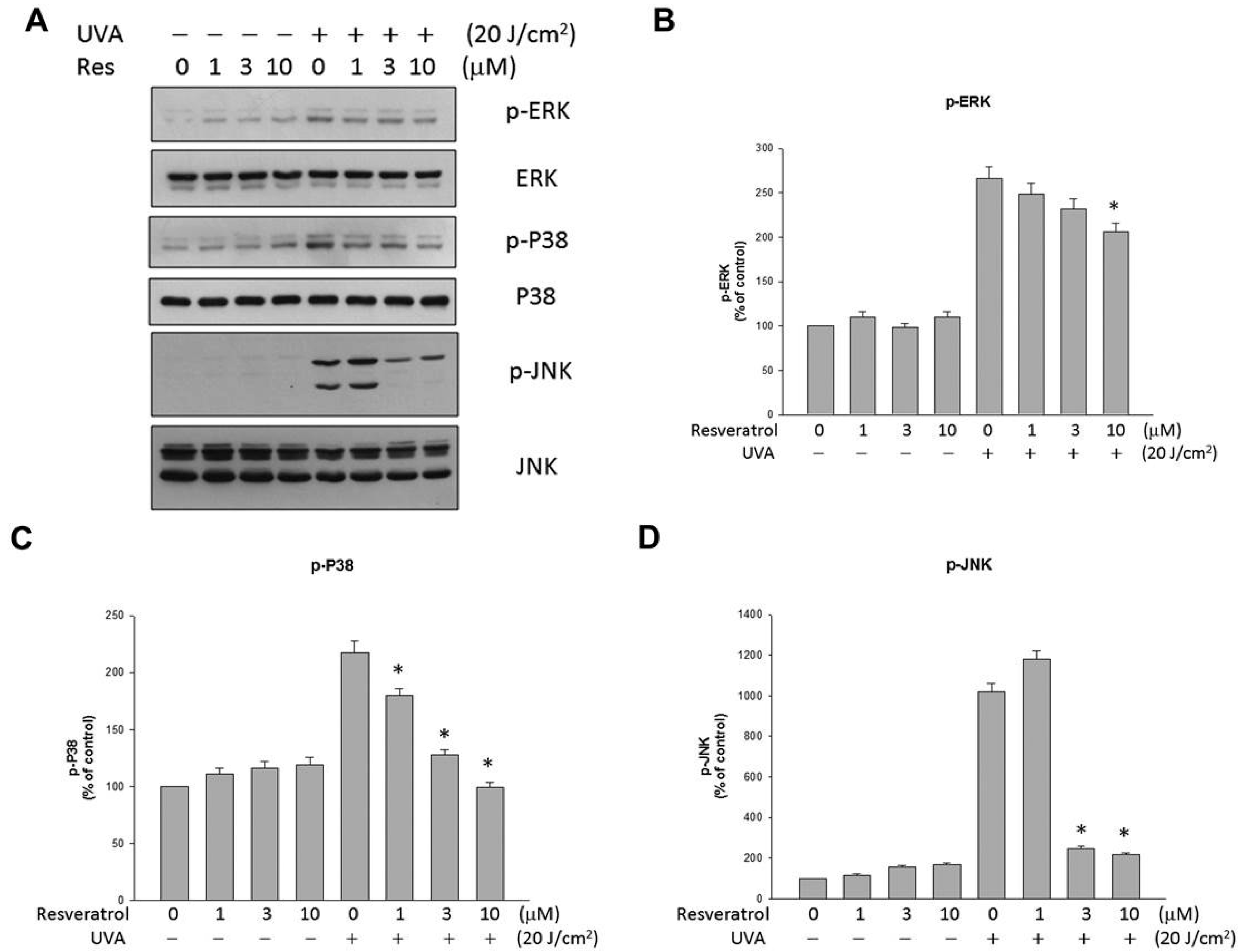
2.4. Resveratrol Suppressed UVA-Induced MAPK Activation
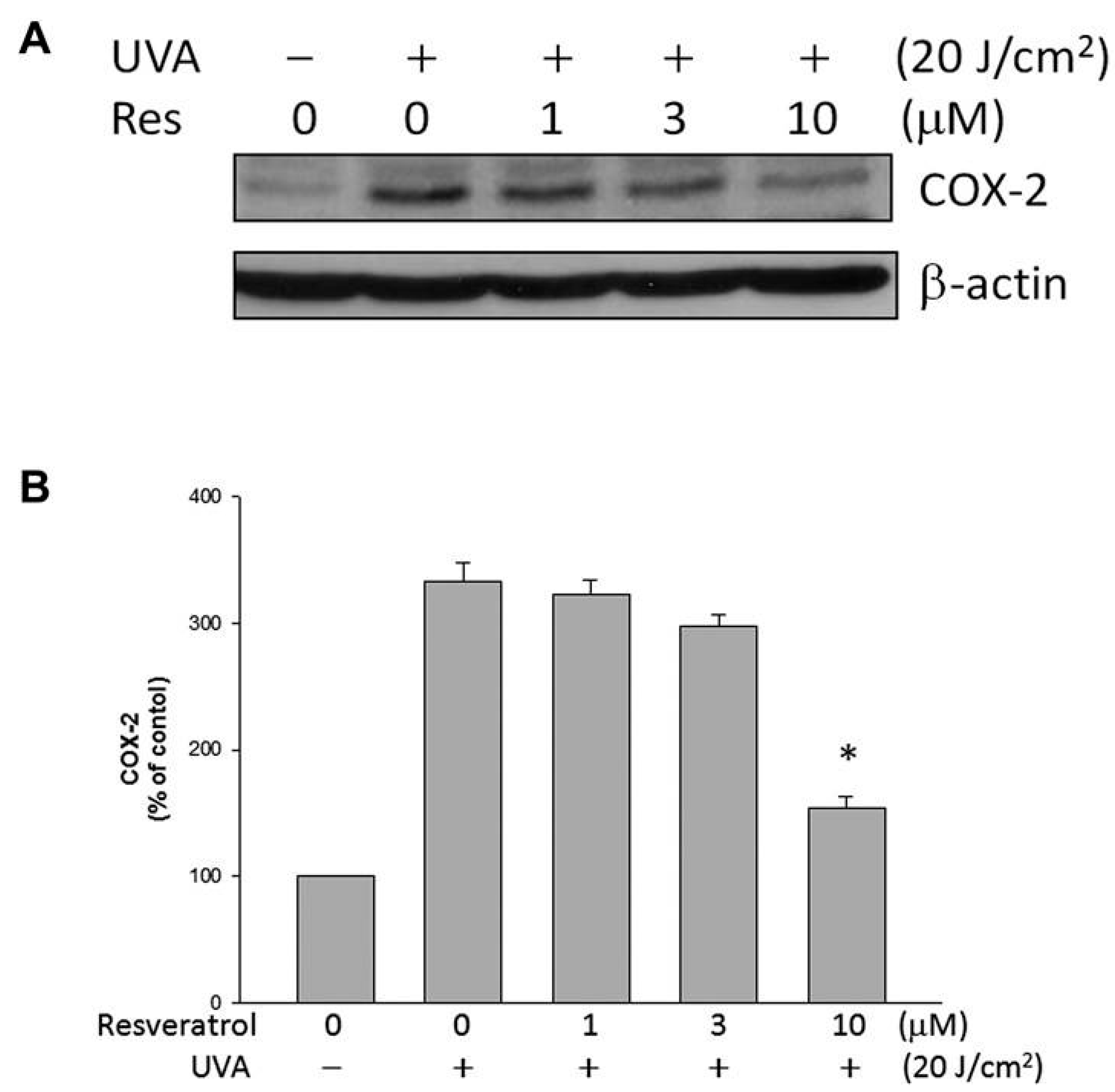
2.5. Resveratrol Lowered UVA-Induced COX-2 Expression
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Cell Preparation and UV Radiation
4.3. Cell Viability Assays
4.4. Flow Cytometric Analysis
4.5. Western Blot Analysis
4.6. Data Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Murthy, R.K.; Ravi, K.; Balaiya, S.; Brar, V.S.; Chalam, K.V. Lutein protects retinal pigment epithelium from cytotoxic oxidative stress. Cutan. Ocul. Toxicol. 2014, 33, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Glickman, R.D. Ultraviolet phototoxicity to the retina. Eye Contact Lens 2011, 37, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Roduit, R.; Schorderet, D.F. MAP kinase pathways in UV-induced apoptosis of retinal pigment epithelium ARPE19 cells. Apoptosis 2008, 13, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.E. Ultraviolet radiation as a risk factor for cataract and macular degeneration. Eye Contact Lens 2011, 37, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef] [PubMed]
- Bressler, N.M. Age-related macular degeneration is the leading cause of blindness. JAMA 2004, 291, 1900–1901. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.M.; Huang, J.H.; Lin, H.H.; Chiang, H.S.; Chen, B.H.; Hong, J.Y.; Hung, C.F. Protective effects of (−)-epigallocatechin gallate on UVA-induced damage in ARPE19 cells. Mol. Vis. 2008, 14, 2528–2534. [Google Scholar] [PubMed]
- Yao, J.; Bi, H.E.; Sheng, Y.; Cheng, L.B.; Wendu, R.L.; Wang, C.H.; Cao, G.F.; Jiang, Q. Ultraviolet (UV) and hydrogen peroxide activate ceramide-ER stress-AMPK signaling axis to promote retinal pigment epithelium (RPE) cell apoptosis. Int. J. Mol. Sci. 2013, 14, 10355–10368. [Google Scholar] [CrossRef] [PubMed]
- Artigas, J.M.; Felipe, A.; Navea, A.; Artigas, C.; Garcia-Domene, M.C. Spectral transmittance of intraocular lenses under natural and artificial illumination: criteria analysis for choosing a suitable filter. Ophthalmology 2011, 118, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Brockmann, C.; Schulz, M.; Laube, T. Transmittance characteristics of ultraviolet and blue-light-filtering intraocular lenses. J. Cataract Refract. Surg. 2008, 34, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Yan, X. From resveratrol to its derivatives: New sources of natural antioxidant. Curr. Med. Chem. 2013, 20, 1005–1017. [Google Scholar] [PubMed]
- Suzuki, Y.; Ito, S.; Sasaki, R.; Asahi, M.; Ishida, Y. Resveratrol suppresses cell proliferation via inhibition of STAT3 phosphorylation and Mcl-1 and cIAP-2 expression in HTLV-1-infected T cells. Leuk. Res. 2013, 37, 1674–1679. [Google Scholar] [CrossRef] [PubMed]
- Svajger, U.; Jeras, M. Anti-inflammatory effects of resveratrol and its potential use in therapy of immune-mediated diseases. Int. Rev. Immunol. 2012, 31, 202–222. [Google Scholar] [CrossRef] [PubMed]
- Ogas, T.; Kondratyuk, T.P.; Pezzuto, J.M. Resveratrol analogs: Promising chemopreventive agents. Ann. N. Y. Acad. Sci. 2013, 1290, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Raederstorff, D.; Kunz, I.; Schwager, J. Resveratrol, from experimental data to nutritional evidence: The emergence of a new food ingredient. Ann. N. Y. Acad. Sci. 2013, 1290, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; George, J.; Ahmad, N. Resveratrol-based combinatorial strategies for cancer management. Ann. N. Y. Acad. Sci. 2013, 1290, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Pintea, A.; Rugina, D.; Pop, R.; Bunea, A.; Socaciu, C.; Diehl, H.A. Antioxidant effect of trans-resveratrol in cultured human retinal pigment epithelial cells. J. Ocul. Pharmacol. Ther. 2011, 27, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Sheu, S.J.; Liu, N.C.; Ou, C.C.; Bee, Y.S.; Chen, S.C.; Lin, H.C.; Chan, J.Y. Resveratrol stimulates mitochondrial bioenergetics to protect retinal pigment epithelial cells from oxidative damage. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6426–6438. [Google Scholar] [CrossRef]
- Chao, S.C.; Hu, D.N.; Yang, P.Y.; Lin, C.Y.; Nien, C.W.; Yang, S.F.; Roberts, J.E. Ultraviolet-A irradiation upregulated urokinase-type plasminogen activator in pterygium fibroblasts through ERK and JNK pathways. Investig. Ophthalmol. Vis. Sci. 2013, 54, 999–1007. [Google Scholar] [CrossRef]
- Mikulski, D.; Gorniak, R.; Molski, M. A theoretical study of the structure-radical scavenging activity of trans-resveratrol analogues and cis-resveratrol in gas phase and water environment. Eur. J. Med. Chem. 2010, 45, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- King, R.E.; Kent, K.D.; Bomser, J.A. Resveratrol reduces oxidation and proliferation of human retinal pigment epithelial cells via extracellular signal-regulated kinase inhibition. Chem. Biol. Interact. 2005, 151, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Sheu, S.J.; Bee, Y.S.; Chen, C.H. Resveratrol and large-conductance calcium-activated potassium channels in the protection of human retinal pigment epithelial cells. J. Ocul. Pharmacol. Ther. 2008, 24, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.M.; Chang, H.H.; Wang, V.C.; Huang, C.L.; Hung, C.F. Inhibitory effects of resveratrol on PDGF-BB-induced retinal pigment epithelial cell migration via PDGFRβ, PI3K/Akt and MAPK pathways. PLoS One 2013, 8, e56819. [Google Scholar] [CrossRef] [PubMed]
- Sheu, S.J.; Wu, T.T. Resveratrol protects against ultraviolet A-mediated inhibition of the phagocytic function of human retinal pigment epithelial cells via large-conductance calcium-activated potassium channels. Kaohsiung J. Med. Sci. 2009, 25, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, Y.; Miyanishi, A.; Mizuno, H.; Kato, S.; Aoshima, H.; Kokubo, K.; Miwa, N. Super-highly hydroxylated fullerene derivative protects human keratinocytes from UV-induced cell injuries together with the decreases in intracellular ROS generation and DNA damages. J. Photochem. Photobiol. B. 2011, 102, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Baumler, W.; Regensburger, J.; Knak, A.; Felgentrager, A.; Maisch, T. UVA and endogenous photosensitizers—The detection of singlet oxygen by its luminescence. Photochem. Photobiol. Sci. 2012, 11, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; Yang, Y.C.; Cheng, H.C.; Wu, A.C.; Chen, S.L.; Chen, H.K.; Tsao, Y.P. Activation of mitogen-activated protein kinases is essential for hydrogen peroxide -induced apoptosis in retinal pigment epithelial cells. Apoptosis 2006, 11, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.L.; Yang, L.; Lu, F.; Xiao, H.; Xu, R.; Wang, L.; Zhu, F.; Zhang, Y. UVA, UVB and UVC induce differential response signaling pathways converged on the eIF2α phosphorylation. Photochem. Photobiol. 2011, 87, 1092–1104. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.L.; Fang, J.Y.; Chen, M.; Wu, C.J.; Huang, C.C.; Hung, C.F. Chrysin protects epidermal keratinocytes from UVA- and UVB-induced damage. J. Agric. Food Chem. 2011, 59, 8391–8400. [Google Scholar] [CrossRef] [PubMed]
- Tsoyi, K.; Park, H.B.; Kim, Y.M.; Chung, J.I.; Shin, S.C.; Lee, W.S.; Seo, H.G.; Lee, J.H.; Chang, K.C.; Kim, H.J. Anthocyanins from black soybean seed coats inhibit UVB-induced inflammatory cylooxygenase-2 gene expression and PGE2 production through regulation of the nuclear factor-κB and phosphatidylinositol 3-kinase/Akt pathway. J. Agric. Food Chem. 2008, 56, 8969–8974. [Google Scholar] [CrossRef] [PubMed]
- Lim do, Y.; Lee, M.H.; Shin, S.H.; Chen, H.; Ryu, J.; Shan, L.; Li, H.; Bode, A.M.; Zhang, W.D.; Dong, Z. (+)-2-(1-Hydroxyl-4-oxocyclohexyl) ethyl caffeate suppresses solar UV-induced skin carcinogenesis by targeting PI3K, ERK1/2, and p38. Cancer Prev. Res. (Phila.) 2014, 7, 856–865. [Google Scholar] [CrossRef]
- Klettner, A. Oxidative stress induced cellular signaling in RPE cells. Front. Biosci. (Sch. Ed.) 2012, 4, 392–411. [Google Scholar] [CrossRef]
- Quyen, B.T.; Choi, H.K.; Kang, K.W. Pin1 is required for ultraviolet A-stimulated cyclooxygenase-2 induction in mouse epidermal cells. Cancer Lett. 2013, 335, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, M.; Nishiyama, C.; Takagi, A.; Nakano, N.; Hara, M.; Ikeda, S.; Okumura, K.; Ogawa, H. Cyclooxygenase-2 inhibition restores ultraviolet B-induced downregulation of ATP2A2/SERCA2 in keratinocytes: Possible therapeutic approach of cyclooxygenase-2 inhibition for treatment of Darier disease. Br. J. Dermatol. 2012, 166, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mansmann, U.R. Modeling of non-steroidal anti-inflammatory drug effect within signaling pathways and miRNA-regulation pathways. PLoS One 2013, 8, e72477. [Google Scholar] [CrossRef] [PubMed]
- Salvado, M.D.; Alfranca, A.; Haeggstrom, J.Z.; Redondo, J.M. Prostanoids in tumor angiogenesis: Therapeutic intervention beyond COX-2. Trends Mol. Med. 2012, 18, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Yanni, S.E.; Barnett, J.M.; Clark, M.L.; Penn, J.S. The role of PGE2 receptor EP4 in pathologic ocular angiogenesis. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5479–5486. [Google Scholar] [CrossRef]
- Hu, W.; Criswell, M.H.; Ottlecz, A.; Cornell, T.L.; Danis, R.P.; Lambrou, G.N.; Ciulla, T.A. Oral administration of lumiracoxib reduces choroidal neovascular membrane development in the rat laser-trauma model. Retina 2005, 25, 1054–64. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Yanagi, Y.; Tamaki, Y.; Uchida, S.; Muranaka, K. COX-2-selective inhibitor, etodolac, suppresses choroidal neovascularization in a mice model. Biochem. Biophys. Res. Commun. 2004, 325, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, K.A.; Toma, H.S.; Cai, J.; Penn, J.S.; Sternberg, P.; Kim, S.J. Reduced choroidal neovascular membrane formation in cyclooxygenase-2 null mice. Investig. Ophthalmol. Vis. Sci. 2011, 52, 701–707. [Google Scholar] [CrossRef]
- Maloney, S.C.; Fernandes, B.F.; Castiglione, E.; Antecka, E.; Martins, C.; Marshall, J.C.; di Cesare, S.; Logan, P.; Burnier, M.N., Jr. Expression of cyclooxygenase-2 in choroidal neovascular membranes from age-related macular degeneration patients. Retina 2009, 29, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.D.; Di Battista, J.A. The cardinal role of the phospholipase A2/cyclooxygenase-2/prostaglandin E synthase/prostaglandin E2 (PCPP) axis in inflammostasis. Inflamm. Res. 2011, 60, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, C.-M.; Huang, C.-H.; Li, H.-J.; Hsiao, C.-Y.; Su, C.-C.; Lee, P.-L.; Hung, C.-F. Protective Effects of Resveratrol against UVA-Induced Damage in ARPE19 Cells. Int. J. Mol. Sci. 2015, 16, 5789-5802. https://doi.org/10.3390/ijms16035789
Chan C-M, Huang C-H, Li H-J, Hsiao C-Y, Su C-C, Lee P-L, Hung C-F. Protective Effects of Resveratrol against UVA-Induced Damage in ARPE19 Cells. International Journal of Molecular Sciences. 2015; 16(3):5789-5802. https://doi.org/10.3390/ijms16035789
Chicago/Turabian StyleChan, Chi-Ming, Cheng-Hua Huang, Hsin-Ju Li, Chien-Yu Hsiao, Ching-Chieh Su, Pei-Lan Lee, and Chi-Feng Hung. 2015. "Protective Effects of Resveratrol against UVA-Induced Damage in ARPE19 Cells" International Journal of Molecular Sciences 16, no. 3: 5789-5802. https://doi.org/10.3390/ijms16035789