A Perilipin Gene from Clonostachys rosea f. Catenulata HL-1-1 Is Related to Sclerotial Parasitism
Abstract
:1. Introduction
2. Results
2.1. Sequence Analysis of ESTs
| Gene | Accession Number | Organism | Blast Homology Search | E-Value |
|---|---|---|---|---|
| Per3 | ABI18161 | Metarhizium anisopliae | Perilipin-like protein | 4e−44 |
| 1-31 | EIW51416 | Trametes versicolor | CHK1 checkpoint-like protein | 4e−16 |
| 2-9 | EEY19892 | Verticillium alfalfae | Endoglucanase | 4e−26 |
| 2-13 | XP_002294430 | Thalassiosira pseudonana | rRNA intron-encoded homing endonuclease | 9e−23 |
| 2-25 | EPE03816 | Ophiostoma piceae | Sodium phosphate | 1e−34 |
| 2-26 | XP_001891758 | Brugia malayi | Transcription factor | 2e−23 |
| 2-27 | CCF47281 | Colletotrichum higginsianum | Shwachman-Bodian-Diamond syndrome protein | 2e−35 |
| 2-29 | NP_690845 | Saccharomyces cerevisiae | Tar1p | 1e−23 |
| 2-49 | XP_751750 | Aspergillus fumigatus | Fatty acid oxygenase PpoA | 2e−41 |
| 2-84 | XP_002567725 | Penicillium chrysogenum | Pc21g06830 | 1e−36 |
| 2-90 | ELA35160 | Colletotrichum gloeosporioides | Trichothecene c-15 hydroxylase | 7e−39 |
| 3-4 | XP_003844958 | Leptosphaeria maculans | Similar to polyketide synthase | 2e−33 |
| 4-33 | EJP62467 | Beauveria bassiana | 4-hydroxyphenylpyruvate dioxygenase | 5e−56 |
| 4-37 | 1101405A | Saccharomyces cerevisiae | Ubiquitin precursor | 5e−86 |
| 4-47 | EFY90398 | Metarhizium acridum | ADP,ATP carrier protein | 6e−23 |
| 4-49 | EQK98455 | Ophiocordyceps sinensis | Glucose-repressible protein | 6e−26 |
| 5-35 | EGC42647 | Ajellomyces capsulatus | Transcript antisense to ribosomal RNA protein | 5e−17 |
| 5-84 | EON96835 | Togninia minima | Heat shock protein 30 | 1e−21 |
| 6-9 | EJP67708 | Beauveria bassiana | Translationally controlled tumor protein-like variant I | 3e−38 |
| 6-35 | EHK46444 | Trichoderma atroviride | Plasma membrane ATPase | 1e−75 |
| 7-13 | EFQ32165 | Colletotrichum graminicola | Cytochrome b561 | 2e−14 |
| 7-32 | EGV64838 | Candida tenuis | Ubiquitin | 2e−139 |
| 7-52 | EGS20521 | Chaetomium thermophilum | Zinc finger domain-containing protein | 2e−5 |
| 7-69 | EKG14599 | Macrophomina phaseolina | MFS transporter | 1e−39 |
| 7-73 | EQB58524 | Colletotrichum gloeosporioides | Aldehyde dehydrogenase | 2e−24 |
| 7-77 | EKD20522 | Marssonina brunnea f. sp. multigermtubi | Metallopeptidase family M24 | 5e−9 |
| 7-82 | EFQ34155 | Glomerella graminicola | Archaeal flagellin N-terminal-like domain-containing protein | 2e−5 |
| 7-85 | ABY21303 | Mytilus trossulus | Cyp-like protein | 2e−13 |
| 8-7 | EGX92208 | Cordyceps militaris | ATP synthase subunit E | 2e−15 |
| 8-16 | EJU02320 | Dacryopinax sp. | Plant senescence-associated protein | 2e−63 |
| 8-20 | EGR52174 | Trichoderma reesei | Glycoside hydrolase family 16 | 2e−26 |
| 8-21 | CCT68298 | Fusarium fujikuroi | Neutral amino acid permease | 1e−5 |
| 8-22 | EFY90747 | Metarhizium acridum | NADH:ubiquinone oxidoreductase 18.4 kD subunit | 8e−36 |
| 8-24 | EGS18562 | Chaetomium thermophilum | Chitin synthase-like protein | 1e−8 |
| 8-25 | BAA10929 | Nicotiana tabacum | Cytochrome P450 like TBP | 9e−21 |
| 8-48 | EFY88095 | Metarhizium acridum | Peptidoglycan binding domain containing protein | 6e−27 |
| 8-70 | XP_003655325 | Thielavia terrestris | Histone H4-like protein | 3e−54 |
| 8-76 | EGY21577 | Verticillium dahliae | Prohibitin-2 | 2e−138 |
| 8-89 | XP_002384050 | Aspergillus flavus | NADH-cytochrome B5 reductase | 3e−60 |
| 9-25 | EKD19306 | Marssonina brunnea f. sp. multigermtubi | 60S ribosomal protein L11 | 3e−9 |
| 9-58 | WP_005077491 | Mycobacterium abscessus | Dienelactone hydrolase | 5e−39 |
| 9-78 | XP_002474926 | Postia placenta | Chloroperoxidase-like protein | 3e−5 |
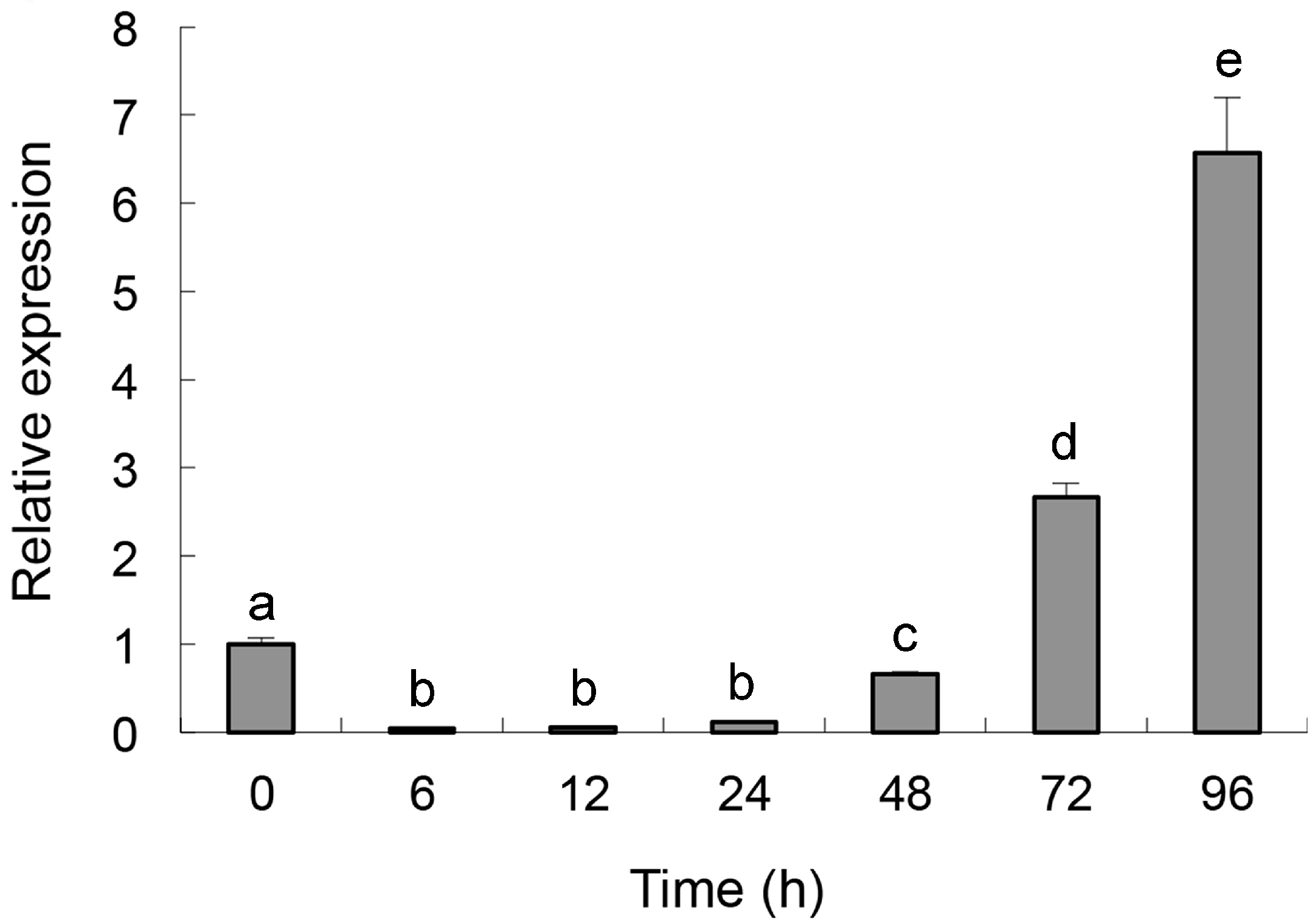
2.2. Quantitative Determination of Per3 Expression
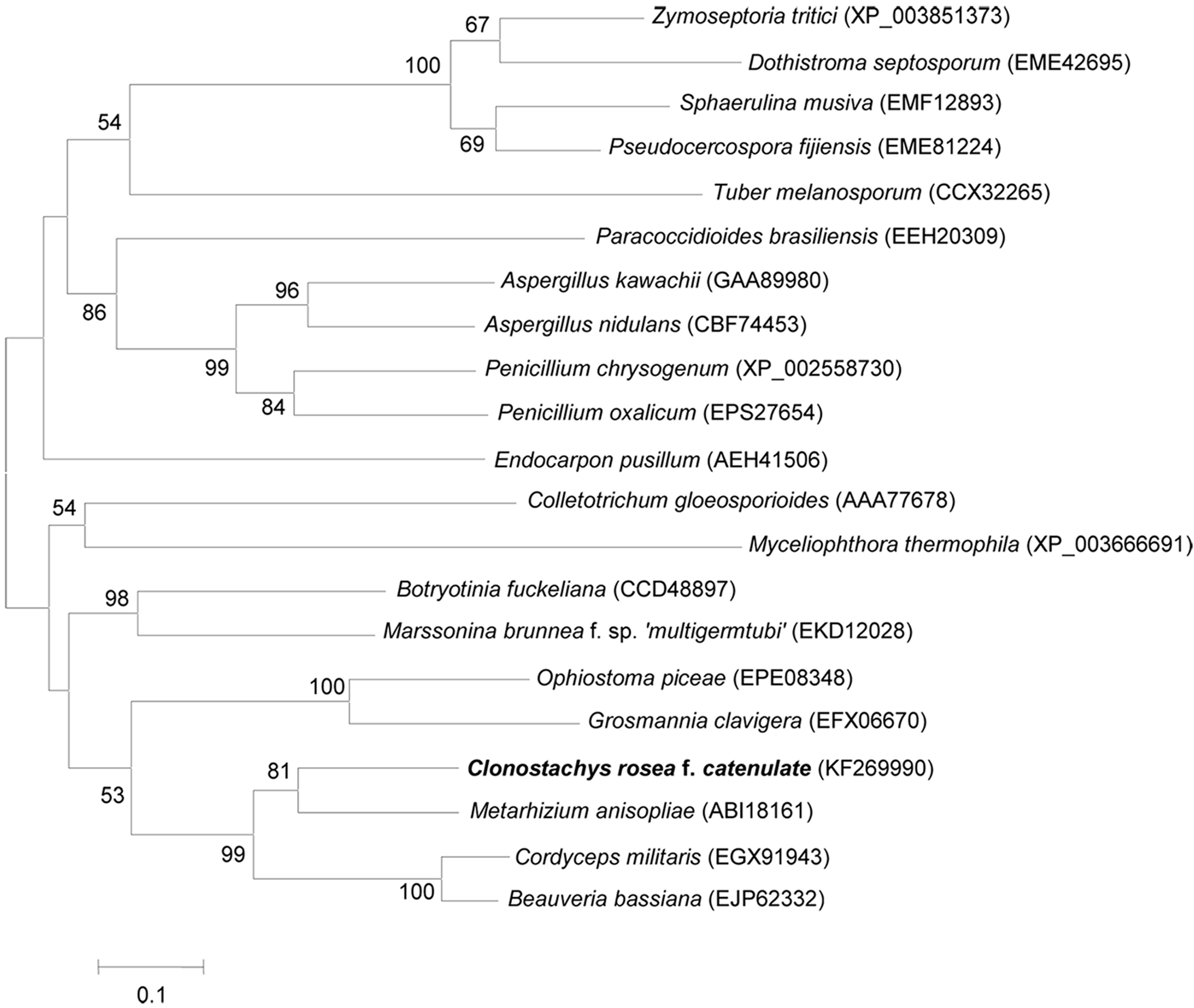
2.3. Cloning and Characterization of Per3
2.4. Overexpression of Per3 in C. rosea f. catenulate HL-1-1
| Strain | Parasitic Rate (%) | |||
|---|---|---|---|---|
| 9 h | 12 h | 15 h | 18 h | |
| HL-1-1 | 3.3 ± 0.0 a | 36.7 ± 0.4 a | 86.7 ± 0.8 a | 100.0 ± 0.0 a |
| Pert 1-2 | 30.0 ± 0.4 b | 96.7 ± 0.7 b | 100.0 ± 0.0 b | 100.0 ± 0.0 a |
| Pert 2-1 | 26.7 ± 0.2 c | 100.0 ± 0.0 c | 100.0 ± 0.0 b | 100.0 ± 0.0 a |

3. Discussion
4. Experimental Section
4.1. Strains
4.2. Preparation of Sclerotia Powder Medium
4.3. DNA Sequencing and Analyzing
4.4. RNA Extraction and cDNA Synthesis
4.5. Real-Time PCR
| Primers | Sequence (5'-3') | Purpose |
|---|---|---|
| SPF | CGTTGTCAAGAAGCCTACCG | Real-time PCR |
| SPR | GAGGCCCTTCTGCTCAATCT | |
| β-tubulin F | CATCTTCAGACCGGTCAGTG | Real-time PCR |
| β-tubulin R | AAGTAGACGTTCATGCGCTC | |
| 3'OPF | AGATTGAGCAGAAGGGCCTC | 3'RACE |
| 3'OPR | TCAACCAGTAAGGCGAGAAT | |
| 5'OPF | AGTGAGGAAGCTGCTAATCC | 5'RACE |
| 5'OPR | ATCTTCTTGATCTCGCTCGA | |
| Per3 F | GAAACTTCTCTTTCGTCTCTATCGA | Amplification of complete DNA and full-length cDNA |
| Per3 R | CTTGCAAGCACAGAAAGAAAATCAA | |
| qdzF | GAATTCCCTTGTATCTCTA | qdz amplification |
| qdzR | AAGAGAAAAGAAAAGAGCA | |
| zzzF | CCGACCGGGGATCCACTTA | zzz amplification |
| zzzR | GGAGTGGGCGCTTACACAG |
4.6. Cloning of Full-Length cDNA of the Per3 Gene
4.7. Bioinformatic Analysis of the Full-Length cDNA of Per3
4.8. Plasmid Construction and Transformation
4.9. Growth and Sporulation of the Transformants
4.10. Mycoparasitism of the Transformants on Sclerotia of S. sclerotiorum
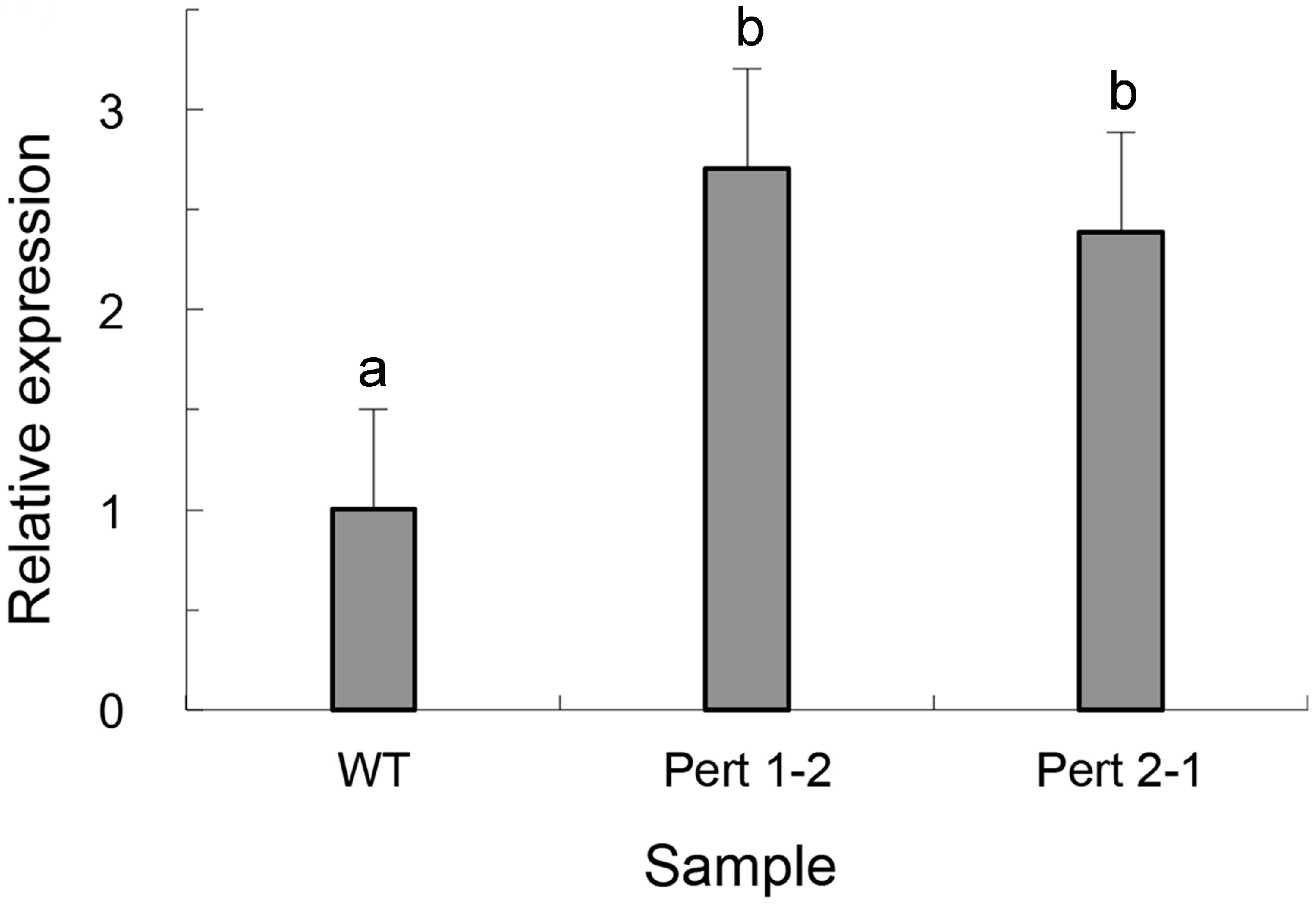
4.11. Transcription Level of Per3 in the Transformants
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McQuilken, M.P.; Gemmell, J.; Lahdenpera, M.L. Gliocladium catenulatum as a potential biological control agent of damping-off in bedding plants. J. Phytopathol. 2001, 149, 171–178. [Google Scholar] [CrossRef]
- Huang, H.C. Gliocladium catenulatum-hyper-parasite of Sclerotinia sclerotiorum and Fusarium species. Can. J. Bot. 1978, 56, 2243–2246. [Google Scholar] [CrossRef]
- Ma, G.Z.; Wu, X.R.; Yang, W.L.; Lu, G.Z. Inhibition of zymotic liquid from different isolates of Gliocladium spp. to three pathogenic fungi. J. Huazhong Agric. Univ. 2004, 23, 96–99. [Google Scholar]
- Chatterton, S.; Punja, Z.K. Chitinase and β-1,3-glucanase enzyme production by the mycoparasite Clonostachys rosea f. catenulata against fungal plant pathogens. Can. J. Microbiol. 2009, 55, 356–367. [Google Scholar]
- Chatterton, S.; Jayaraman, J.; Punja, Z.K. Colonization of cucumber plants by the biocontrol fungus Clonostachys rosea f. catenulate. Biol. Control. 2008, 46, 267–278. [Google Scholar] [CrossRef]
- Chatterton, S.; Punja, Z.K. Interactions between Clonostachys rosea f. catenulate, Fusarium oxysporum and cucumber roots leading to biological control of fusarium root and stem rot. Recent Dev. Manag. Plant Dis. 2009, 1, 93–106. [Google Scholar]
- Rahman, M.; Punja, Z.K. Biological control of damping-off on American ginseing (Panax quinquefolius) by Clonostachys rosea f. catenulate (=Gliocladium catenulatum). Can. J. Plant Pathol. 2007, 29, 203–207. [Google Scholar] [CrossRef]
- Chatterton, S.; Punja, Z.K. Factors influencing colonization of cucumber roots by Clonostachys rosea f. catenulate, a biological disease control agent. Biocontrol. Sci. Technol. 2010, 20, 37–55. [Google Scholar] [CrossRef]
- Steindorff, A.S.; Ramada, M.H.; Coelho, A.S.; Miller, R.N.; Pappas, G.J., Jr.; Ulhoa, C.J.; Noronha, E.F. Identification of mycoparasitism-related genes against the phytopathogens Sclerotinia sclerotiorum through transcriptome and expression profile analysis in Trichoderma harzianum. BMC Genomics 2014, 15, 204. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.S.; St Leger, R.J. A scorpion neurotoxin increases the potency of a fungal insecticide. Nat. Biotechnol. 2007, 25, 1455–1456. [Google Scholar] [CrossRef] [PubMed]
- Pava-Ripoll, M.; Posada, F.J.; Momen, B.; Wang, C.S.; St Leger, R. Increased pathogenicity against coffee berry borer, Hypothenemus hampei (Coleoptera: Curculionidae) by Metarhizium anisopliae expressing the scorpion toxin (AaIT) gene. J. Invertebr. Pathol. 2008, 99, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Cardoza, R.E.; Malmierca, M.G.; Gutiérrez, S. Overexpression of erg1 gene in Trichoderma harzianum CECT 2413: Effect on the induction of tomato defence-related genes. J. Appl. Microbiol. 2014, 117, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Kosawang, C.; Karlsson, M.; Velez, H.; Rasmussen, P.H.; Collinge, D.B.; Jensen, B.; Jensen, D.F. Zearalenone detoxification by zearalenone hydrolase is important for the antagonistic ability of Clonostachys rosea against mycotoxigenic Fusarium graminearum. Fungal Biol. 2014, 118, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Dubey, M.K.; Jensen, D.F.; Karlsson, M. An ATP-binding cassette pleiotropic drug transporter protein is required for xenobiotic tolerance and antagonism in the fungal biocontrol agent Clonostachys rosea. Mol. Plant Microbe Interact. 2014, 27, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.G.; Tu, H.H.; Liu, X.Y.; Tao, N.; Zhang, K.Q. PacC in the nematophagous fungus Clonostachys rosea controls virulence to nematodes. Environ. Microbiol. 2010, 12, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, A.S.; Egan, J.J.; Wek, S.A.; Garty, N.B.; Blanchette-Mackie, E.J.; Londos, C. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J. Biol. Chem. 1991, 266, 11341–11346. [Google Scholar] [PubMed]
- Bickel, P.E.; Tansey, J.T.; Welte, M.A. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochem. Biophys. Acta 2009, 1791, 419–440. [Google Scholar] [PubMed]
- Marcinkiewicz, A.; Gauthier, D.; Garcia, A.; Brasaemle, D.L. The phosphorylation of serine 492 of perilipin a directs lipid droplet fragmentation and dispersion. J. Biol. Chem. 2006, 281, 11901–11909. [Google Scholar] [CrossRef] [PubMed]
- Tansey, J.T.; Sztalryd, C.; Gruia-Gray, J.; Roush, D.L.; Zee, J.V.; Gavrilova, O.; Reitman, M.L.; Deng, C.X.; Li, C.; Kimmel, A.R.; et al. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc. Natl. Acad. Sci. USA 2001, 98, 6494–6499. [Google Scholar]
- Teixeira, L.; Rabouilleb, C.; Rørtha, P.; Ephrussi, A.; Vanzo, N.F. Drosophila perilipin/ADRP homologue Lsd2 regulates lipid metabolism. Mech. Dev. 2003, 120, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.S.; St Leger, R.J. The Metarhizium anisopliae perilipin homolog MPL1 regulates lipid metabolism, appressorial turgor pressure, and virulence. J. Biol. Chem. 2007, 282, 21110–21115. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.Z.; Gao, H.L.; Zhang, Y.H.; Li, S.D.; Xie, B.Y.; Wu, S.J. Purification and characterization of chitinase from Gliocladium catenulatum strain HL-1-1. Afr. J. Microbiol. Res. 2012, 6, 4377–4383. [Google Scholar]
- Gao, H.L.; Li, S.D.; Guo, R.J.; Zhang, Y.H. Screening and analysis of differentially expressed genes in Gliocladium catenulatum parasitizing on sclerotia of Sclerotinia sclerotiorum. Chin. J. Biol. Control. 2009, 25, 48–54. [Google Scholar]
- Giczey, G.; Kerényi, Z.; Fülöp, L.; Hornok, L. Expression of cmg1, an exo-β-1,3-glucanase gene from Coniothyrium minitans, increases during sclerotial parasitism. Appl. Environ. Microbiol. 2001, 67, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Djonovic, S.; Pozo, M.J.; Kenerley, C.M. Tvbgn3, a β-1,6-glucanase from the biocontrol fungus Trichoderma virens, is involved in mycoparasitism and control of Pythium ultimum. Appl. Environ. Microbiol. 2006, 72, 7661–7670. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, A.; Bai, G.; Guo, P.; Xiao, K.; Guenzi, A.C.; Ayoubi, P. Fusarium graminearum-induced changes in gene expression between Fusarium head blight-resistant and susceptible wheat cultivars. Funct. Integr. Genomics 2007, 7, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.; Elfstrand, M.; Stenlid, J.; Olson, A. A fungal cytochrome P450 is expressed during the interaction between the fungal pathogen Heterobasidion annosum sensu lato and conifer trees. DNA Seq. 2008, 19, 115–120. [Google Scholar] [PubMed]
- Carpenter, M.A.; Stewart, A.; Ridgway, H.J. Identification of novel Trichoderma hamatum genes expressed during mycoparasitism using subtractive hybridization. FEMS Microbiol. Lett. 2005, 251, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Morissette, D.C.; Dauch, A.; Beech, R.; Masson, L.; Brousseau, R.; Jabaji-Hare, S. Isolation of mycoparasitic-related transcripts by SSH during interaction of the mycoparasite Stachybotrys elegans with its host Rhizoctonia solani. Curr. Genet. 2008, 53, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Atanasova, L.; Le Crom, S.; Gruber, S.; Coulpier, F.; Seidl-Seiboth, V.; Kubicek, C.P.; Druzhinina, I.S. Comparative transcriptomics reveals different strategies of Trichoderma mycoparasitism. BMC Genomics 2013, 14, 121. [Google Scholar] [CrossRef]
- Dugan, F.M.; Lupien, S.L.; Hernandez-Bello, M.; Peever, T.L.; Chen, W. Fungi resident in chickpea debris and their suppression of growth and reproduction of Didymella rabiei under laboratory conditions. J. Phytopathol. 2005, 153, 431–439. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species-opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Faber, B.C.; Cleutjens, K.B.; Niessen, R.L.; Aarts, P.L.; Boon, W.; Greenberg, A.S.; Kitslaar, P.J.; Tordoir, J.H.; Daemen, M.J. Identification of genes potentially involved in rupture of human atherosclerotic plaques. Circ. Res. 2001, 89, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Arimura, N.; Horiba, T.; Imagawa, M.; Shimizu, M.; Sato, R. The peroxisome proliferator-activated receptor gamma regulates expression of the perilipin gene in adipocytes. J. Biol. Chem. 2004, 279, 10070–10076. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.H.; Yamaguchi, T.; Hirose, F.; Osumi, T. Perilipin, a critical regulator of fat storage and breakdown, is a target gene of estrogen receptor-related receptor α. Biochem. Biophys. Res. Commun. 2008, 368, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Welte, M.A.; Cermelli, S.; Griner, J.; Viera, A.; Guo, Y.; Kim, D.H.; Gindhart, J.G.; Gross, S.P. Regulation of lipid-droplet transport by the perilipin homolog LSD2. Curr. Biol. 2005, 15, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, M.H.; Li, S.D.; Luo, M. Cloning and functional analysis of endoglucanase gene from Gliocladium catenulatum HL-1-1. Chin. J. Biol. Control. 2013, 29, 74–82. [Google Scholar]
- Ewing, B.; Green, P. Base-calling of automated sequencer traces using Phred. II. error probabilities. Genome Res. 1998, 8, 186–194. [Google Scholar]
- Mamarabadi, M.; Jensen, B.; Jensen, D.F.; Lubeck, M. Real-time RT-PCR expression analysis of chitinase and endoglucanase genes in the three-way interaction between the biocontrol strain Clonostachys rosea IK726, Botrytis cinerea and strawberry. FEMS Microbiol. Lett. 2008, 285, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆Ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Bjellqvist, B.; Basse, B.; Olsen, E.; Celis, J.E. Reference points for comparisons of two-dimensional maps of proteins from different human cell types defined in a pH scale where isoelectric points correlate with polypeptide compositions. Electrophoresis 1994, 15, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein identification and analysis tools on the ExPASy Server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Petersen, T.N.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar]
- Hofmann, K.; Stoffel, W. TMbase-A database of membrane spanning proteins segments. Biol. Chem. Hoppe Seyler 1993, 374, 166. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Punt, P.J.; Oliver, R.P.; Dingemanse, M.A.; Pouwels, P.H.; van den Hondel, C.A.M.J.J. Transformation of Aspergillus based on the hygromycin B resistance marker from Escherichia coli. Gene 1987, 56, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.Q.; Jiang, J.; Sun, M.H.; Xie, X.M.; Li, S.D. Construction of Clonostachys rosea 67-1 genetic transformation system by restriction enzyme-mediated integration (REMI). Chin. J. Biol. Control. 2013, 29, 263–269. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.-B.; Li, S.-D.; Zhong, Z.-M.; Sun, M.-H. A Perilipin Gene from Clonostachys rosea f. Catenulata HL-1-1 Is Related to Sclerotial Parasitism. Int. J. Mol. Sci. 2015, 16, 5347-5362. https://doi.org/10.3390/ijms16035347
Sun Z-B, Li S-D, Zhong Z-M, Sun M-H. A Perilipin Gene from Clonostachys rosea f. Catenulata HL-1-1 Is Related to Sclerotial Parasitism. International Journal of Molecular Sciences. 2015; 16(3):5347-5362. https://doi.org/10.3390/ijms16035347
Chicago/Turabian StyleSun, Zhan-Bin, Shi-Dong Li, Zeng-Ming Zhong, and Man-Hong Sun. 2015. "A Perilipin Gene from Clonostachys rosea f. Catenulata HL-1-1 Is Related to Sclerotial Parasitism" International Journal of Molecular Sciences 16, no. 3: 5347-5362. https://doi.org/10.3390/ijms16035347






