The Anti-TNF-α Antibody Infliximab Inhibits the Expression of Fat-Transporter-Protein FAT/CD36 in a Selective Hepatic-Radiation Mouse Model
Abstract
:1. Introduction
2. Results
2.1. Real Time PCR Analysis of Tumor Necrosis Factor-α (TNF-α) in Irradiated Mice Liver

2.2. Changes in Triglycerides (TG) Level in Mice Livers and Serum after Irradiation

2.3. Early Accumulation of Fat in Irradiated Mice Liver Tissue
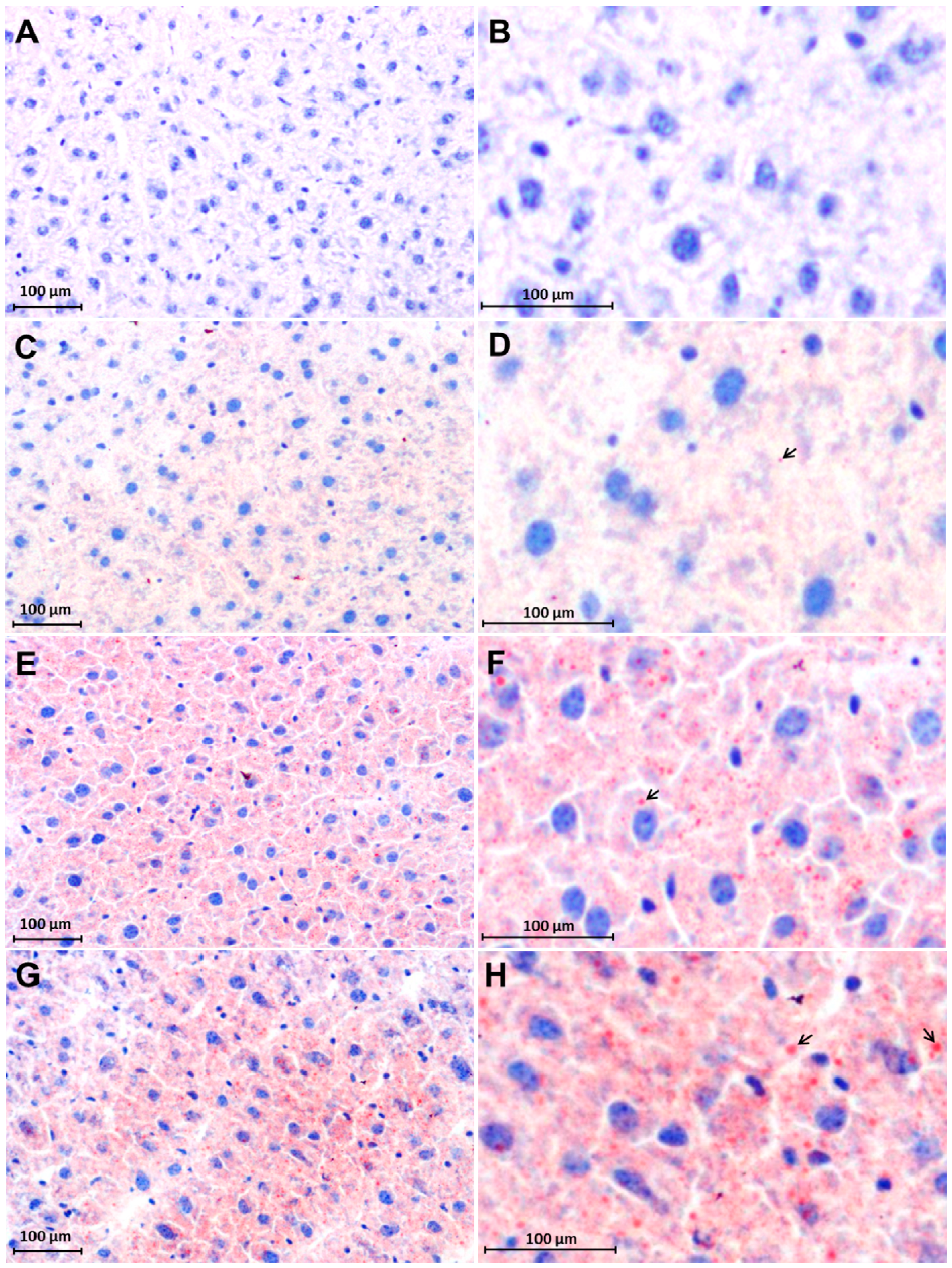
2.4. Changes in FAT/CD36 Protein Level in Mice Livers by Western Blot Analysis

2.5. Localization of FAT/CD36 in the Liver Using Immunofluorescent Staining
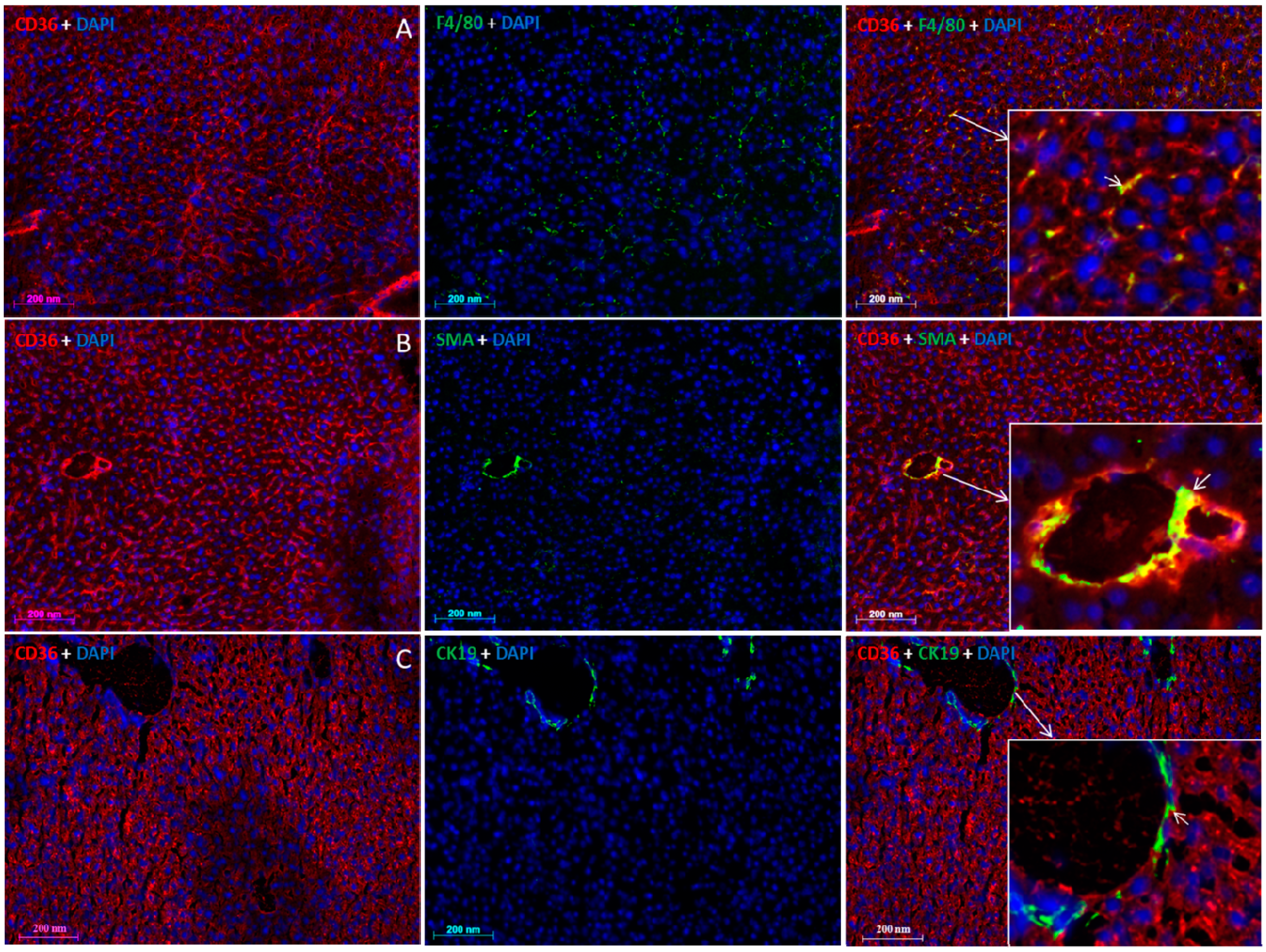

3. Discussion
4. Material and Methods
4.1. Materials
4.2. Animal Model
4.3. Animal Groups of Different Treatments
| Group 1 (G1) | Group 2 (G2) | Group 3 (G3) | Group 4 (G4) | Group 5 (G5) | Group 6 (G6) |
|---|---|---|---|---|---|
| Sham-irradiated | 25-Gy | 25-Gy + TNF-α | 25-Gy + IFX | TNF-α | IFX |
4.4. Staining of Triglycerides in Irradiated Mice Liver Tissue
4.5. Immunofluorescent Double-Staining
4.6. Triglyceride Profile in Serum and Liver Tissue of Mice after Irradiation
4.7. RNA Isolation and Real-Time PCR Analysis
4.8. Protein Extraction from Liver Tissue
4.9. Western Blot Analysis
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Reddy, J.K.; Rao, M.S. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G852–G858. [Google Scholar] [CrossRef] [PubMed]
- Tamura, S.; Shimomura, I. Contribution of adipose tissue and de novo lipogenesis to nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Hayhurst, G.P.; Lee, Y.H.; Lambert, G.; Ward, J.M.; Gonzalez, F.J. Hepatocyte nuclear factor 4α (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol. Cell. Biol. 2001, 21, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Hubscher, S.G. Histological assessment of non-alcoholic fatty liver disease. Histopathology 2006, 49, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Berk, P.D. Regulatable fatty acid transport mechanisms are central to the pathophysiology of obesity, fatty liver, and metabolic syndrome. Hepatology 2008, 48, 1362–1376. [Google Scholar] [CrossRef] [PubMed]
- Koonen, D.P.; Jacobs, R.L.; Febbraio, M.; Young, M.E.; Soltys, C.L.; Ong, H.; Vance, D.E.; Dyck, J.R. Increased hepatic CD36 expression contributes to dyslipidemia associated with diet-induced obesity. Diabetes 2007, 56, 2863–2871. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.L.; Febbraio, M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci. Signal. 2009, 2, re3. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008, 4, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Storch, J.; Thumser, A.E. The fatty acid transport function of fatty acid-binding proteins. Biochim. Biophys. Acta 2000, 1486, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Bonen, A.; Han, X.X.; Tandon, N.N.; Glatz, J.F.; Lally, J.; Snook, L.A.; Luiken, J.J. FAT/CD36 expression is not ablated in spontaneously hypertensive rats. J. Lipid Res. 2009, 50, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Luiken, J.J.; Koonen, D.P.; Willems, J.; Zorzano, A.; Becker, C.; Fischer, Y.; Tandon, N.N.; van der Vusse, G.J.; Bonen, A.; Glatz, J.F. Insulin stimulates long-chain fatty acid utilization by rat cardiac myocytes through cellular redistribution of FAT/CD36. Diabetes 2002, 51, 3113–3119. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Nakata, T.; Oka, T.; Ogawa, T.; Okamoto, F.; Kusaka, Y.; Sohmiya, K.; Shimamoto, K.; Itakura, K. Defect in human myocardial long-chain fatty acid uptake is caused by FAT/CD36 mutations. J. Lipid Res. 2001, 42, 751–759. [Google Scholar] [PubMed]
- Su, X.; Abumrad, N.A. Cellular fatty acid uptake: A pathway under construction. Trends Endocrinol. Metab. 2009, 20, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Glatz, J.F.; Luiken, J.J.; Bonen, A. Membrane fatty acid transporters as regulators of lipid metabolism: Implications for metabolic disease. Physiol. Rev. 2010, 90, 367–417. [Google Scholar] [CrossRef] [PubMed]
- Baillie, A.G.; Coburn, C.T.; Abumrad, N.A. Reversible binding of long-chain fatty acids to purified FAT, the adipose CD36 homolog. J. Membr. Biol. 1996, 153, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Coburn, C.T.; Knapp, F.F., Jr.; Febbraio, M.; Beets, A.L.; Silverstein, R.L.; Abumrad, N.A. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J. Biol. Chem. 2000, 275, 32523–32529. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T.S.; Robertson, J.M.; Anscher, M.S.; Jirtle, R.L.; Ensminger, W.D.; Fajardo, L.F. Hepatic toxicity resulting from cancer treatment. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.K.; Seol, M.A.; Park, H.R.; Jung, U.; Roh, C. Ionising radiation triggers fat accumulation in white adipose tissue. Int. J. Radiat. Biol. 2011, 87, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Rodningen, O.K.; Borresen-Dale, A.L.; Alsner, J.; Hastie, T.; Overgaard, J. Radiation-induced gene expression in human subcutaneous fibroblasts is predictive of radiation-induced fibrosis. Radiother. Oncol. 2008, 86, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H.; Smith, C.W.; Clemens, M.G.; Ganey, P.E.; Roth, R.A. Mechanisms of inflammatory liver injury: Adhesion molecules and cytotoxicity of neutrophils. Toxicol. Appl. Pharmacol. 1996, 139, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Ramadori, G.; Armbrust, T. Cytokines in the liver. Eur. J. Gastroenterol. Hepatol. 2001, 13, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, H.; Sheikh, N.; Saile, B.; Reuter, F.; Rave-Frank, M.; Hermann, R.M.; Dudas, J.; Hille, A.; Hess, C.F.; Ramadori, G. x-Irradiation in rat liver: Consequent upregulation of hepcidin and downregulation of hemojuvelin and ferroportin-1 gene expression. Radiology 2007, 242, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Malik, I.A.; Moriconi, F.; Sheikh, N.; Naz, N.; Khan, S.; Dudas, J.; Mansuroglu, T.; Hess, C.F.; Rave-Frank, M.; Christiansen, H.; et al. Single-dose γ-irradiation induces up-regulation of chemokine gene expression and recruitment of granulocytes into the portal area but not into other regions of rat hepatic tissue. Am. J. Pathol. 2010, 176, 1801–1815. [Google Scholar] [CrossRef] [PubMed]
- Naz, N.; Ahmad, S.; Cameron, S.; Moriconi, F.; Rave-Frank, M.; Christiansen, H.; Hess, C.F.; Ramadori, G.; Malik, I.A. Differential regulation of ferritin subunits and iron transport proteins: An effect of targeted hepatic X-irradiation. Biomed. Res. Int. 2013, 2013, 353106. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, H.; Batusic, D.; Saile, B.; Hermann, R.M.; Dudas, J.; Rave-Frank, M.; Hess, C.F.; Schmidberger, H.; Ramadori, G. Identification of genes responsive to γ radiation in rat hepatocytes and rat liver by cDNA array gene expression analysis. Radiat. Res. 2006, 165, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Lauzier, B.; Merlen, C.; Vaillant, F.; McDuff, J.; Bouchard, B.; Beguin, P.C.; Dolinsky, V.W.; Foisy, S.; Villeneuve, L.R.; Labarthe, F.; et al. Post-translational modifications, a key process in CD36 function: Lessons from the spontaneously hypertensive rat heart. J. Mol. Cell Cardiol. 2011, 51, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Martius, G.; Alwahsh, S.M.; Rave-Frank, M.; Hess, C.F.; Christiansen, H.; Ramadori, G.; Malik, I.A. Hepatic fat accumulation and regulation of FAT/CD36: An effect of hepatic irradiation. Int. J. Clin. Exp. Pathol. 2014, 7, 5379–5392. [Google Scholar] [PubMed]
- Lavoie, J.M.; Gauthier, M.S. Regulation of fat metabolism in the liver: Link to non-alcoholic hepatic steatosis and impact of physical exercise. Cell. Mol. Life Sci. 2006, 63, 1393–1409. [Google Scholar] [CrossRef]
- Li, Z.; Yang, S.; Lin, H.; Huang, J.; Watkins, P.A.; Moser, A.B.; de Simone, C.; Song, X.Y.; Diehl, A.M. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology 2003, 37, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Manco, M.; Marcellini, M.; Giannone, G.; Nobili, V. Correlation of serum TNF-α levels and histologic liver injury scores in pediatric nonalcoholic fatty liver disease. Am. J. Clin. Pathol. 2007, 127, 954–960. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Lee, J.H.; Febbraio, M.; Xie, W. The emerging roles of fatty acid translocase/CD36 and the aryl hydrocarbon receptor in fatty liver disease. Exp. Biol. Med. (Maywood) 2011, 236, 1116–1121. [Google Scholar] [CrossRef]
- Zhou, J.; Febbraio, M.; Wada, T.; Zhai, Y.; Kuruba, R.; He, J.; Lee, J.H.; Khadem, S.; Ren, S.; Li, S.; et al. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARγ in promoting steatosis. Gastroenterology 2008, 134, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Ge, F.; Zhou, S.; Hu, C.; Lobdell, H.; Berk, P.D. Insulin- and leptin-regulated fatty acid uptake plays a key causal role in hepatic steatosis in mice with intact leptin signaling but not in ob/ob or db/db mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G855–G866. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sambandam, N.; Han, X.; Gross, R.W.; Courtois, M.; Kovacs, A.; Febbraio, M.; Finck, B.N.; Kelly, D.P. CD36 deficiency rescues lipotoxic cardiomyopathy. Circ. Res. 2007, 100, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.J.; Seong, J.; Lee, I.J.; Han, K.H.; Chon, C.Y.; Ahn, S.H. Radiation-induced hepatic toxicity after radiotherapy combined with chemotherapy for hepatocellular carcinoma. Hepatol. Res. 2007, 37, 906–913. [Google Scholar] [CrossRef]
- Campbell, S.E.; Tandon, N.N.; Woldegiorgis, G.; Luiken, J.J.; Glatz, J.F.; Bonen, A. A novel function for fatty acid translocase (FAT)/CD36: Involvement in long chain fatty acid transfer into the mitochondria. J. Biol. Chem. 2004, 279, 36235–36241. [Google Scholar] [CrossRef] [PubMed]
- Rosca, M.G.; Vazquez, E.J.; Chen, Q.; Kerner, J.; Kern, T.S.; Hoppel, C.L. Oxidation of fatty acids is the source of increased mitochondrial reactive oxygen species production in kidney cortical tubules in early diabetes. Diabetes 2012, 61, 2074–2083. [Google Scholar] [CrossRef] [PubMed]
- Yamamori, T.; Yasui, H.; Yamazumi, M.; Wada, Y.; Nakamura, Y.; Nakamura, H.; Inanami, O. Ionizing radiation induces mitochondrial reactive oxygen species production accompanied by upregulation of mitochondrial electron transport chain function and mitochondrial content under control of the cell cycle checkpoint. Free Radic. Biol. Med. 2012, 53, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martius, G.; Cameron, S.; Rave-Fränk, M.; Hess, C.F.; Wolff, H.A.; Malik, I.A. The Anti-TNF-α Antibody Infliximab Inhibits the Expression of Fat-Transporter-Protein FAT/CD36 in a Selective Hepatic-Radiation Mouse Model. Int. J. Mol. Sci. 2015, 16, 4682-4697. https://doi.org/10.3390/ijms16034682
Martius G, Cameron S, Rave-Fränk M, Hess CF, Wolff HA, Malik IA. The Anti-TNF-α Antibody Infliximab Inhibits the Expression of Fat-Transporter-Protein FAT/CD36 in a Selective Hepatic-Radiation Mouse Model. International Journal of Molecular Sciences. 2015; 16(3):4682-4697. https://doi.org/10.3390/ijms16034682
Chicago/Turabian StyleMartius, Gesa, Silke Cameron, Margret Rave-Fränk, Clemens F. Hess, Hendrik A. Wolff, and Ihtzaz A. Malik. 2015. "The Anti-TNF-α Antibody Infliximab Inhibits the Expression of Fat-Transporter-Protein FAT/CD36 in a Selective Hepatic-Radiation Mouse Model" International Journal of Molecular Sciences 16, no. 3: 4682-4697. https://doi.org/10.3390/ijms16034682






