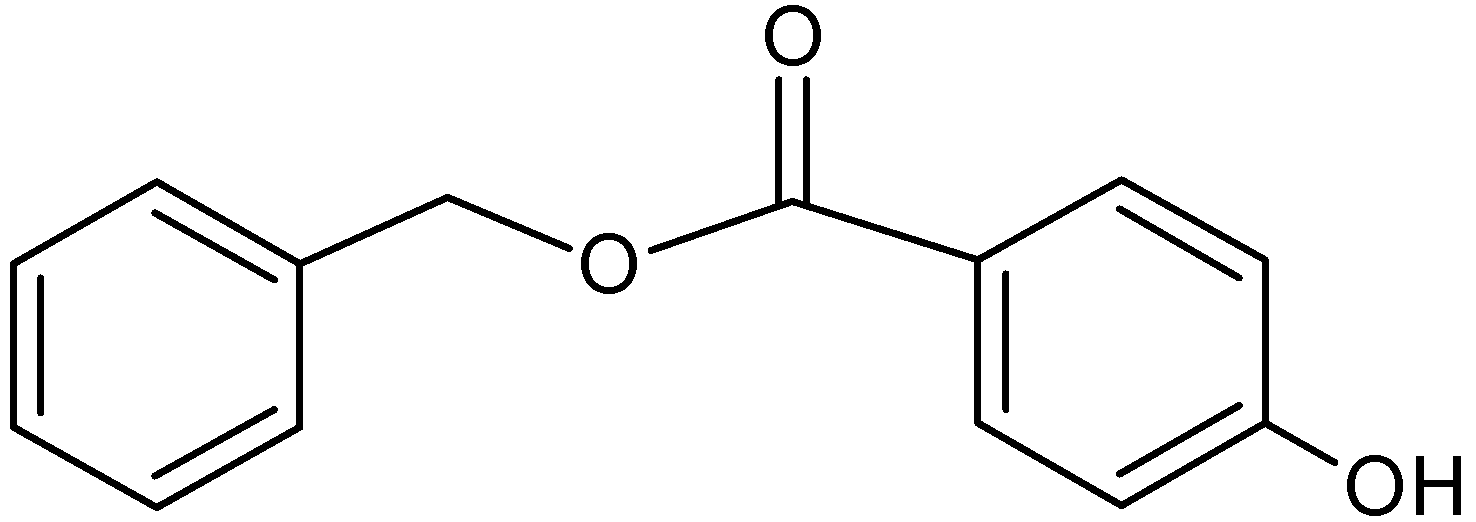
Exploiting β-Cyclodextrin in Molecular Imprinting for Achieving Recognition of Benzylparaben in Aqueous Media
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of MAA-β-CD Monomer
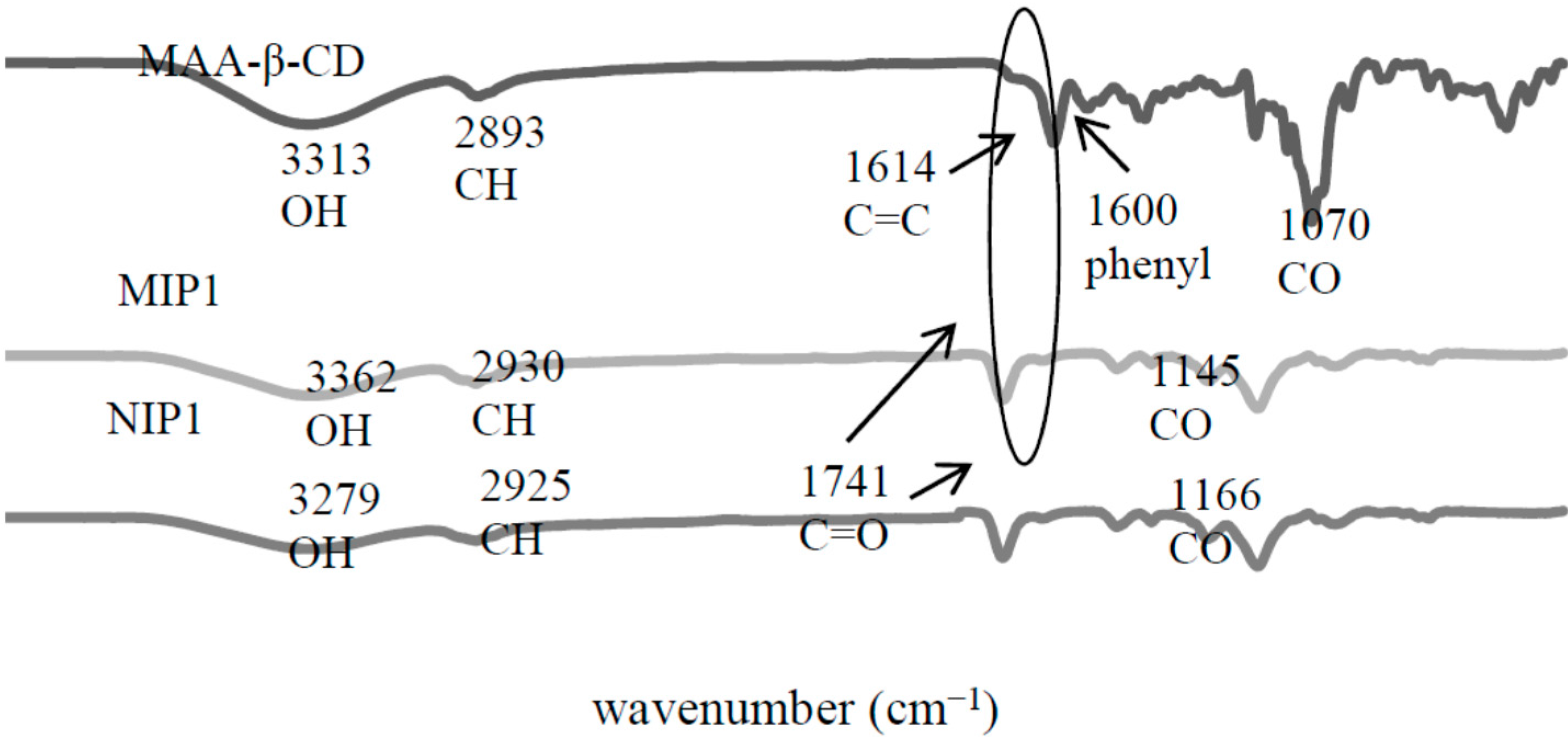
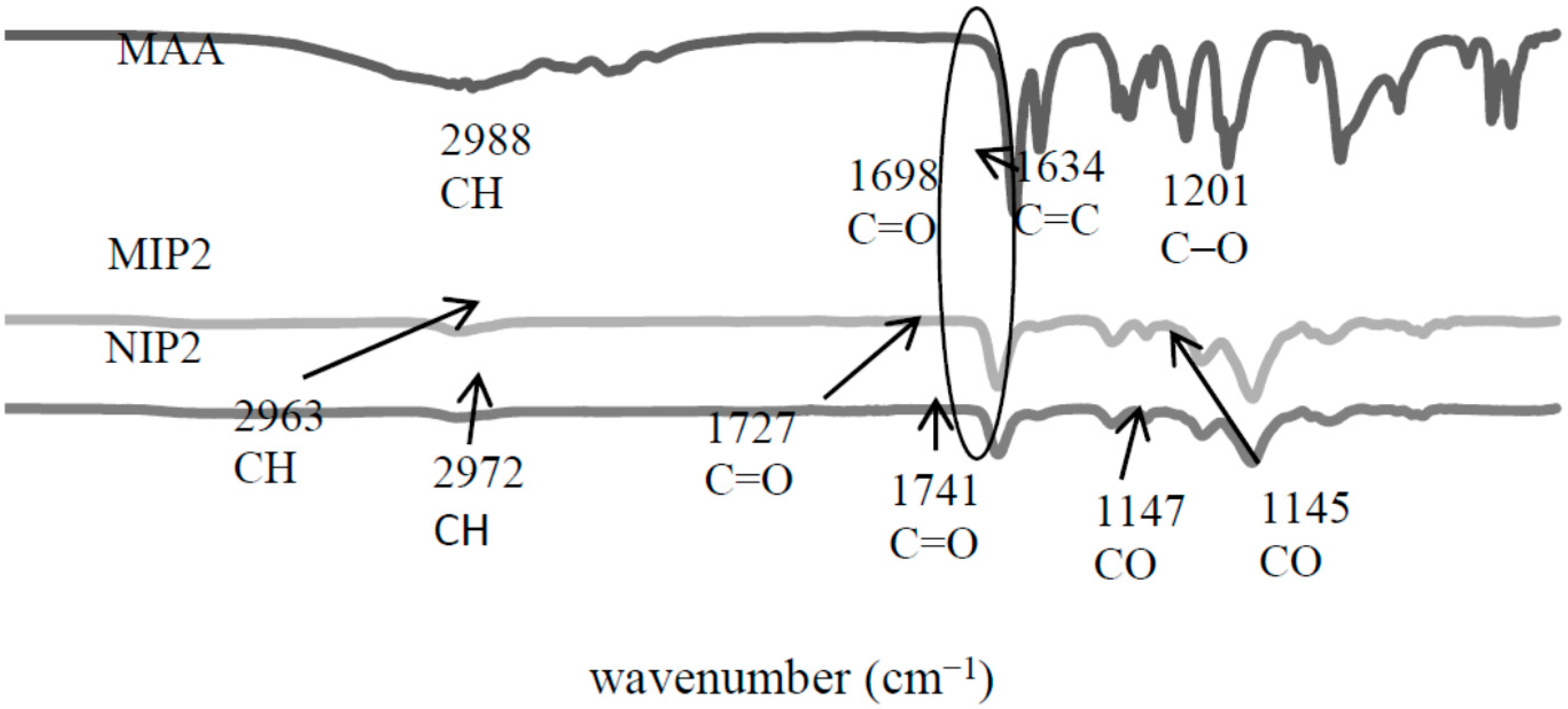
2.2. Characterization of MIPs/NIPs
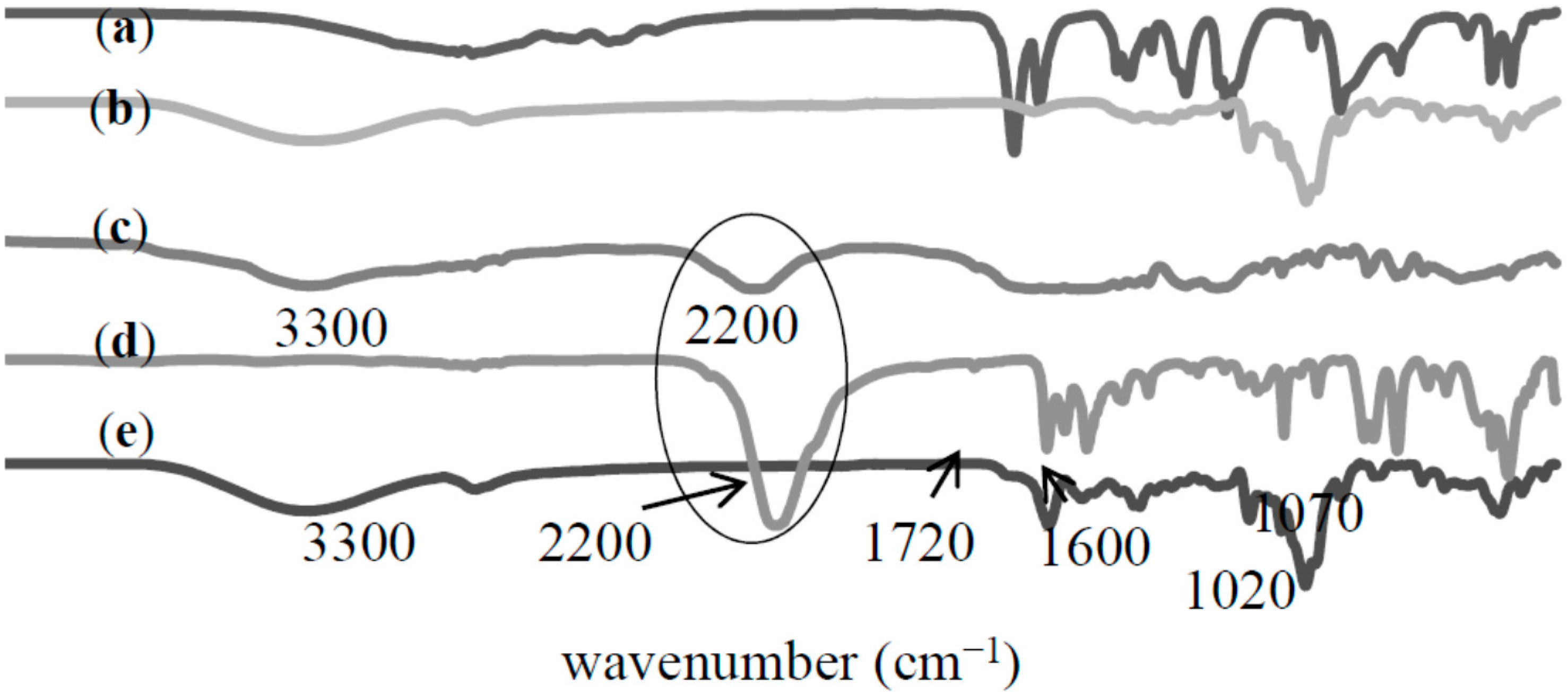
2.2.1. Fourier Transform Infrared (FTIR) Analysis
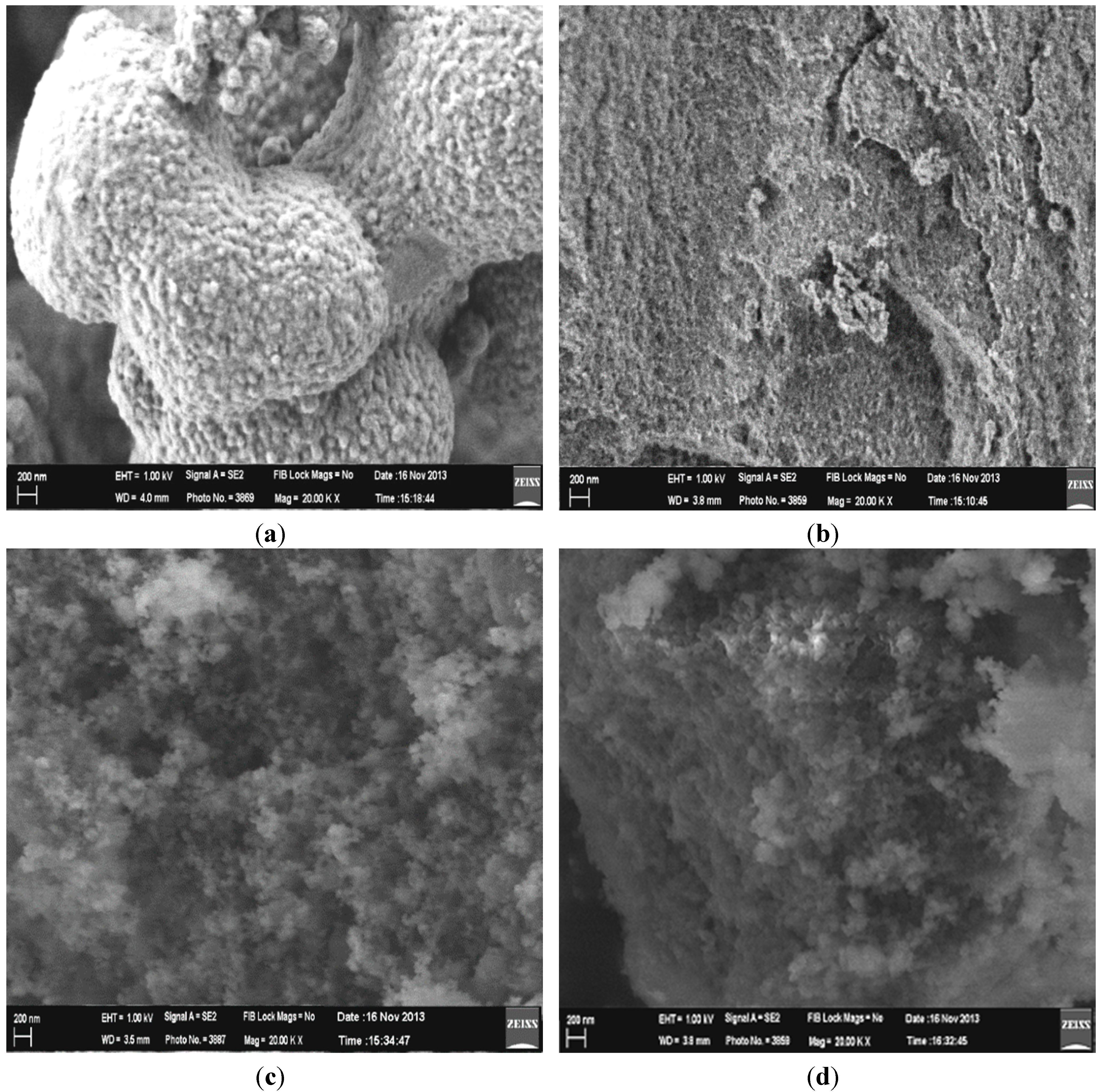
2.2.2. Field Emission Scanning Electron Microscopy (FESEM) Analysis
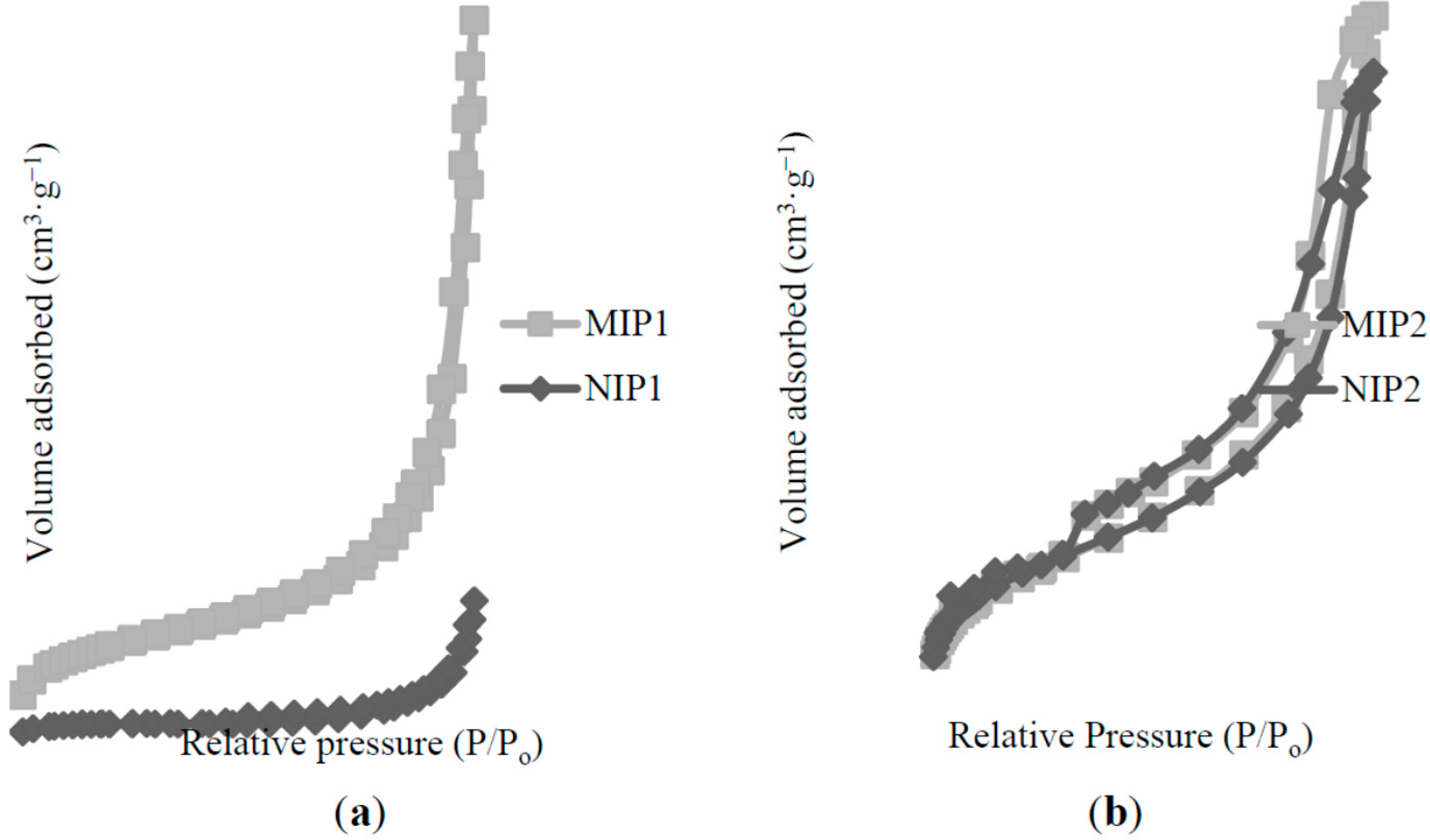
2.2.3. Particle Size and Brunauer-Emmett-Teller (BET) Analysis
| Samples | Surface Area, S (m2/g) | Pore Diameter, dp (nm) | Pore Volume, Vp (cm3/g) |
|---|---|---|---|
| MIP1 | 11.31 | 8.67 | 4.20 × 10−4 |
| NIP1 | 2.22 | 8.21 | 5.75 × 10−4 |
| MIP2 | 426.9 | 7.14 | 0.7 |
| NIP2 | 415.0 | 6.01 | 0.6 |
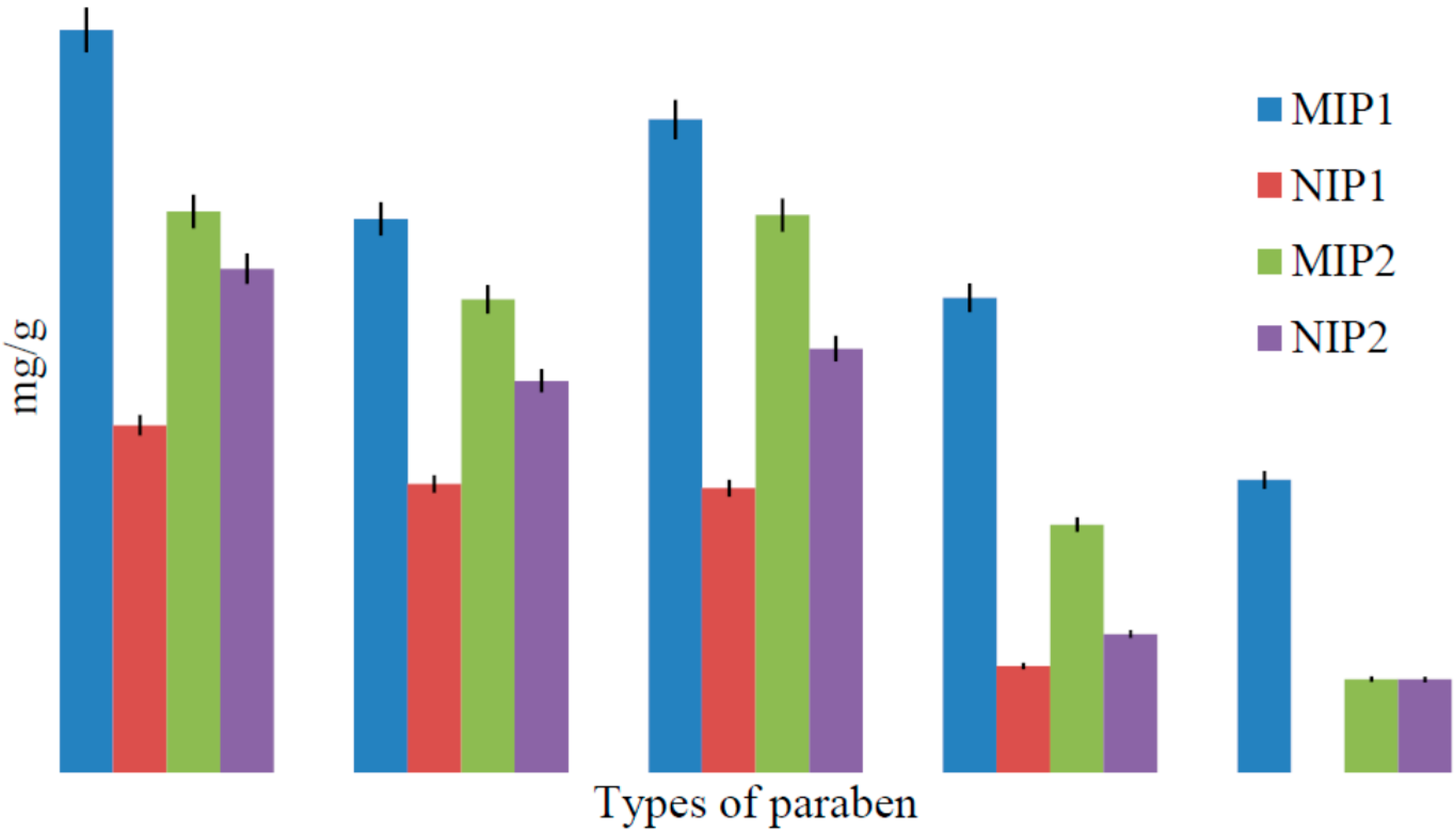
2.3. Binding Characteristics of the Polymers for BzP
| Polymers | Kd (L/g) | IF |
|---|---|---|
| MIP1 | 1.901 ± 0.025 | 7.979 |
| NIP1 | 0.238 ± 0.044 | - |
| MIP2 | 1.518 ± 0.071 | 1.733 |
| NIP2 | 0.876 ± 0.054 | - |
| Parabens | MIP1 | NIP1 | IF | MIP2 | NIP2 | IF |
|---|---|---|---|---|---|---|
| Kd (L/g) | Kd (L/g) | Kd (L/g) | Kd (L/g) | |||
| Benzylparaben (BzP) | 1.901 ± 0.025 | 0.238 ± 0.044 | 7.979 | 1.518 ± 0.071 | 0.876 ± 0.054 | 1.733 |
| Butylparaben (BuP) | 0.675 ± 0.031 | 0.113 ± 0.018 | 5.991 | 0.787 ± 0.097 | 0.477 ± 0.002 | 1.649 |
| Propylparaben (PrP) | 1.048 ± 0.063 | 0.189 ± 0.014 | 5.538 | 0.419 ± 0.035 | 0.239 ± 0.012 | 1.751 |
| Ethylparaben (EtP) | 0.389 ± 0.002 | 0.049 ± 0.004 | 7.832 | 0.210 ± 0.040 | 0.201 ± 0.049 | 1.044 |
| Methylparaben (MeP) | 0.171 ± 0.007 | - | - | 0.059 ± 0.003 | - | - |
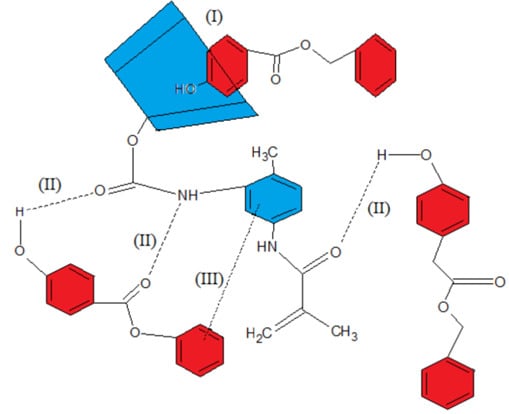
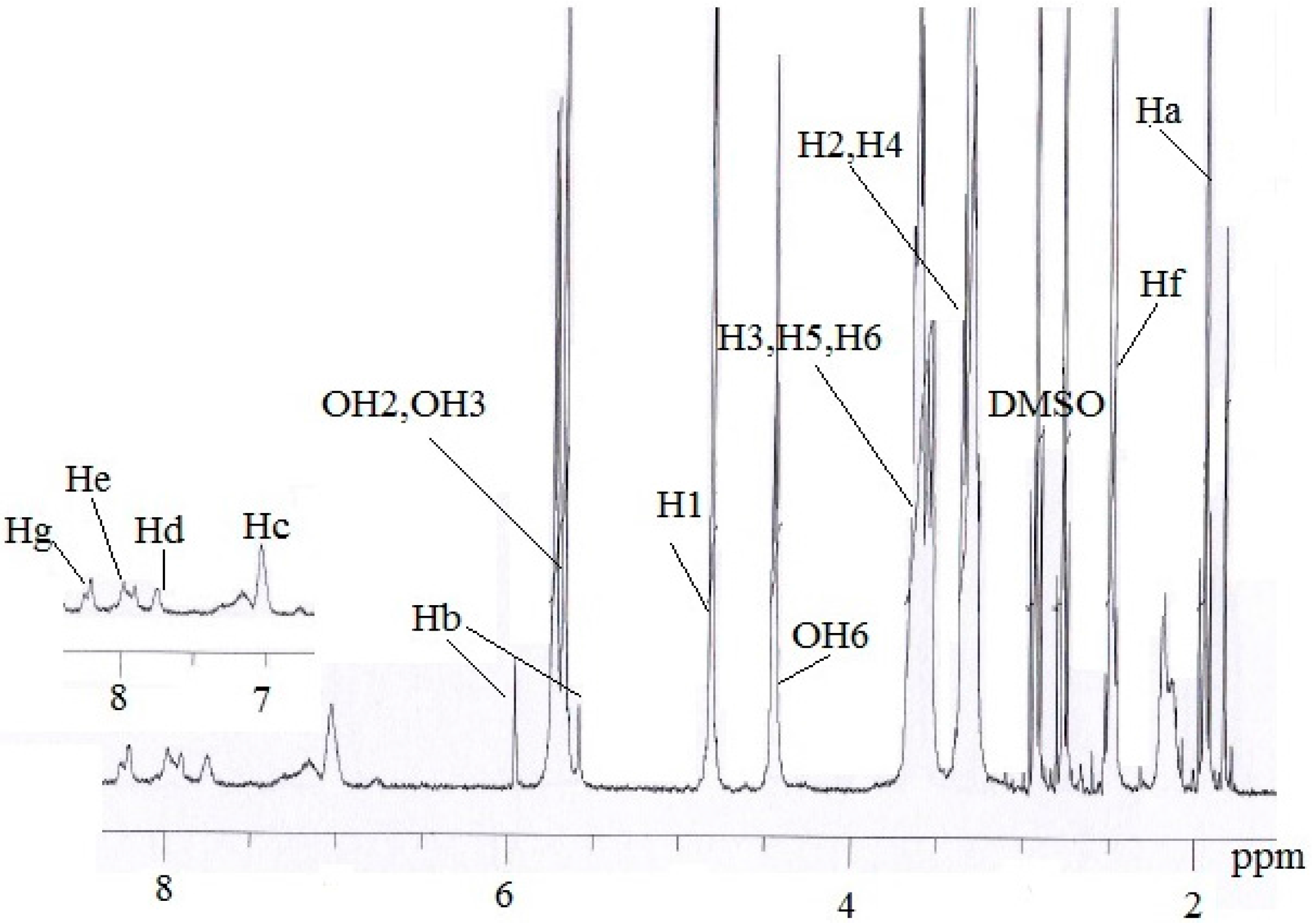
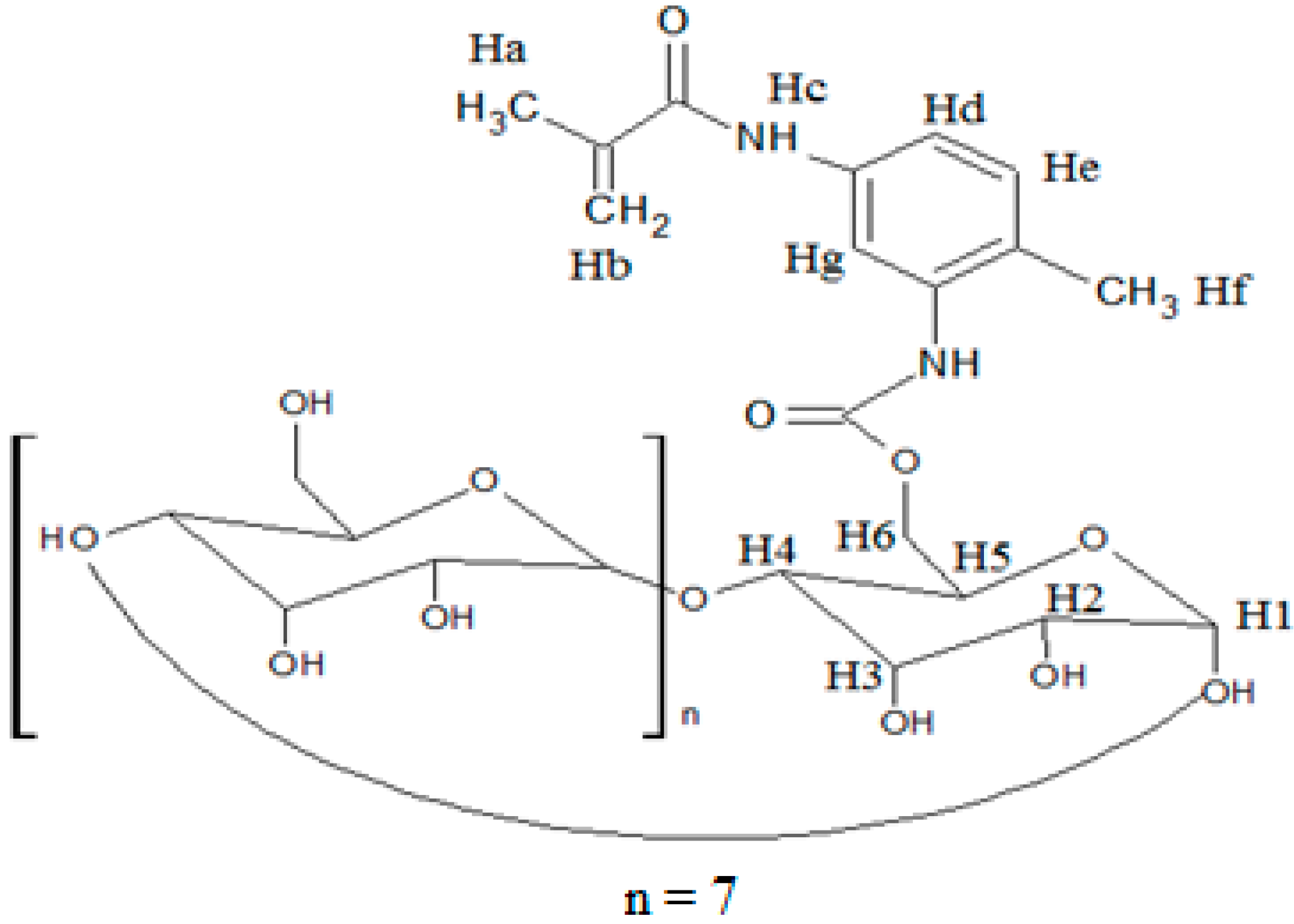
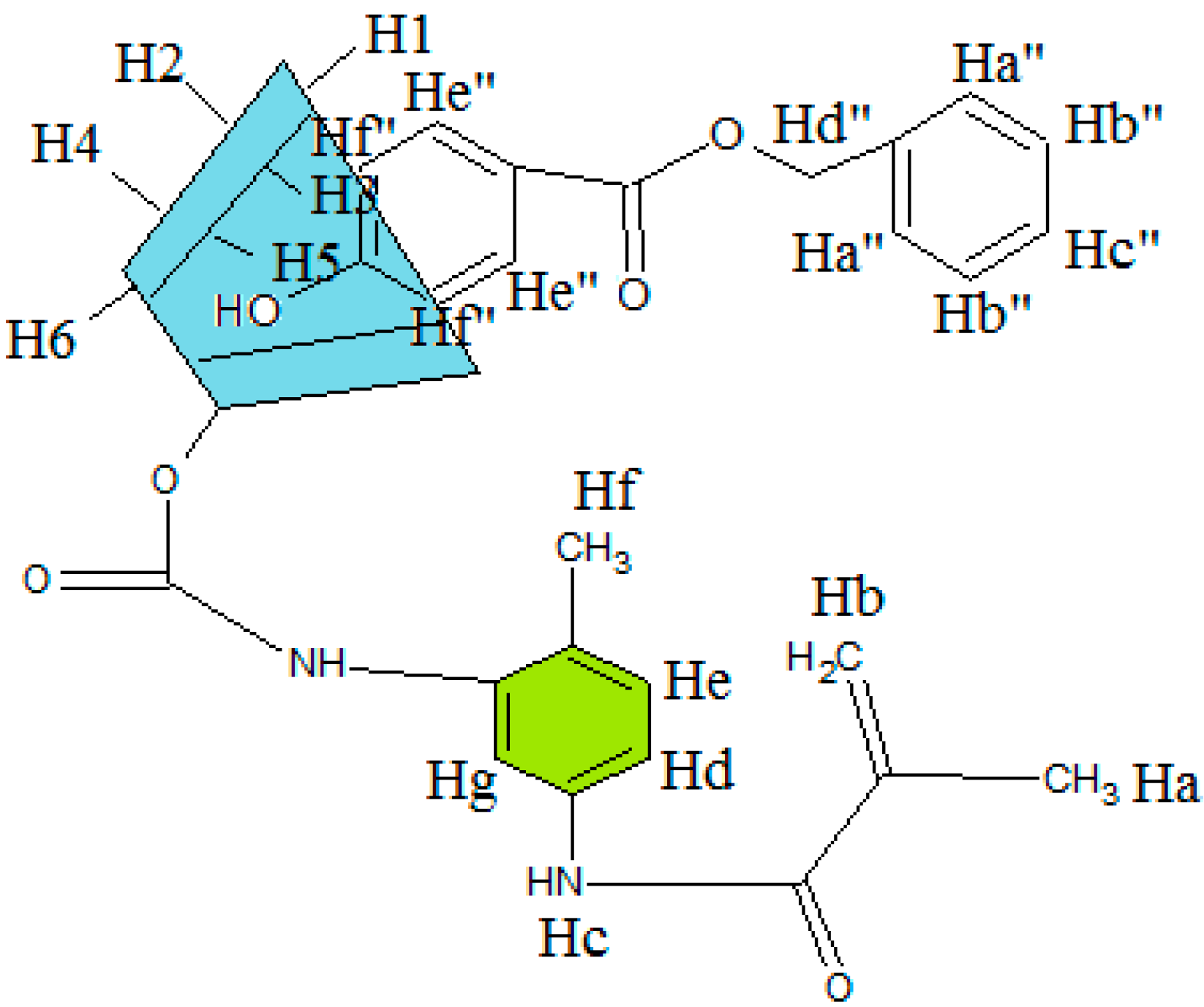
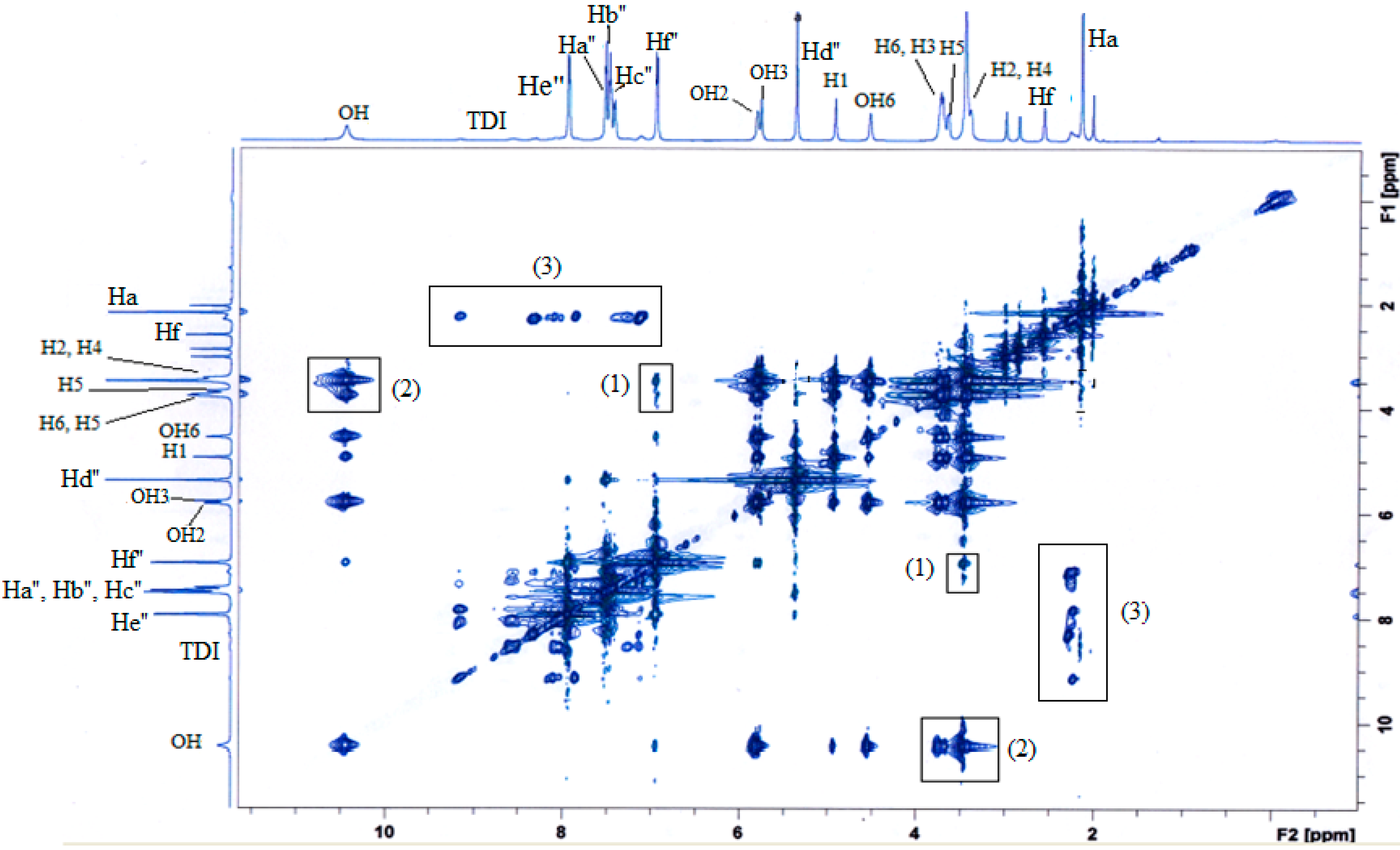
2.4. Inclusion Complex of MAA-β-CD-BzP
| H Proton | δ (MAA-β-CD-BzP) | δ (MAA-β-CD) | δ (BzP) | Δδ |
|---|---|---|---|---|
| Ha | 1.9750 | 1.9750 | - | 0.0000 |
| Hb | 5.6673 | 5.7356 | - | −0.0683 |
| Hc | 7.7481 | 7.7248 | - | 0.0233 |
| Hd | 7.9079 | 7.9205 | - | −0.0126 |
| He | 8.2088 | 8.2204 | - | −0.0116 |
| Hf | 2.5205 | 2.5205 | - | 0.0000 |
| Hg | 8.4547 | 8.4229 | - | 0.0318 |
| H1 | 4.8124 | 4.8118 | - | 0.0006 |
| H2 | 3.2900 | 3.3272 | - | −0.0372 |
| H3 | 3.5365 | 3.6213 | - | −0.0848 |
| H4 | 3.3205 | 3.3620 | - | −0.0415 |
| H5 | 3.3510 | 3.6012 | - | −0.2502 |
| H6 | 3.6213 | 3.6482 | - | −0.0269 |
| OH2 | 5.7332 | 5.7356 | - | −0.0024 |
| OH3 | 5.7155 | 5.7179 | - | −0.0024 |
| OH6 | 4.4469 | 4.4487 | - | −0.0018 |
| Hc'' | 7.3856 | - | 7.3844 | 0.0012 |
| Hd'' | 5.2762 | - | 5.2786 | −0.0024 |
| He'' | 7.8463 | - | 7.8493 | −0.0030 |
| Hf'' | 6.8517 | - | 6.8559 | −0.0042 |
| OH | 10.3426 | - | 10.3481 | −0.0055 |
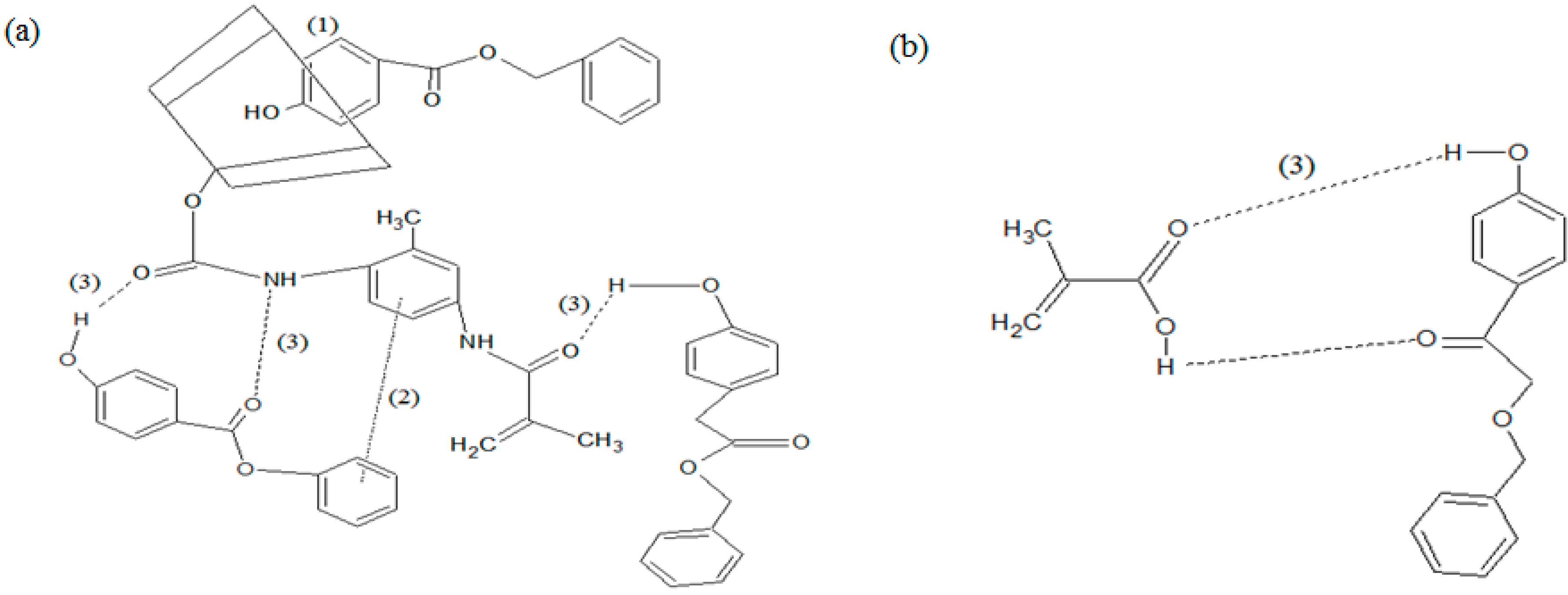
3. Experimental Section
3.1. Materials and Methods
3.2. Instruments
3.3. Synthesis of MAA-β-CD Monomer

3.4. Synthesis of MAA-β-CD-BzP Complex

3.5. Synthesis of MIPs/NIPs
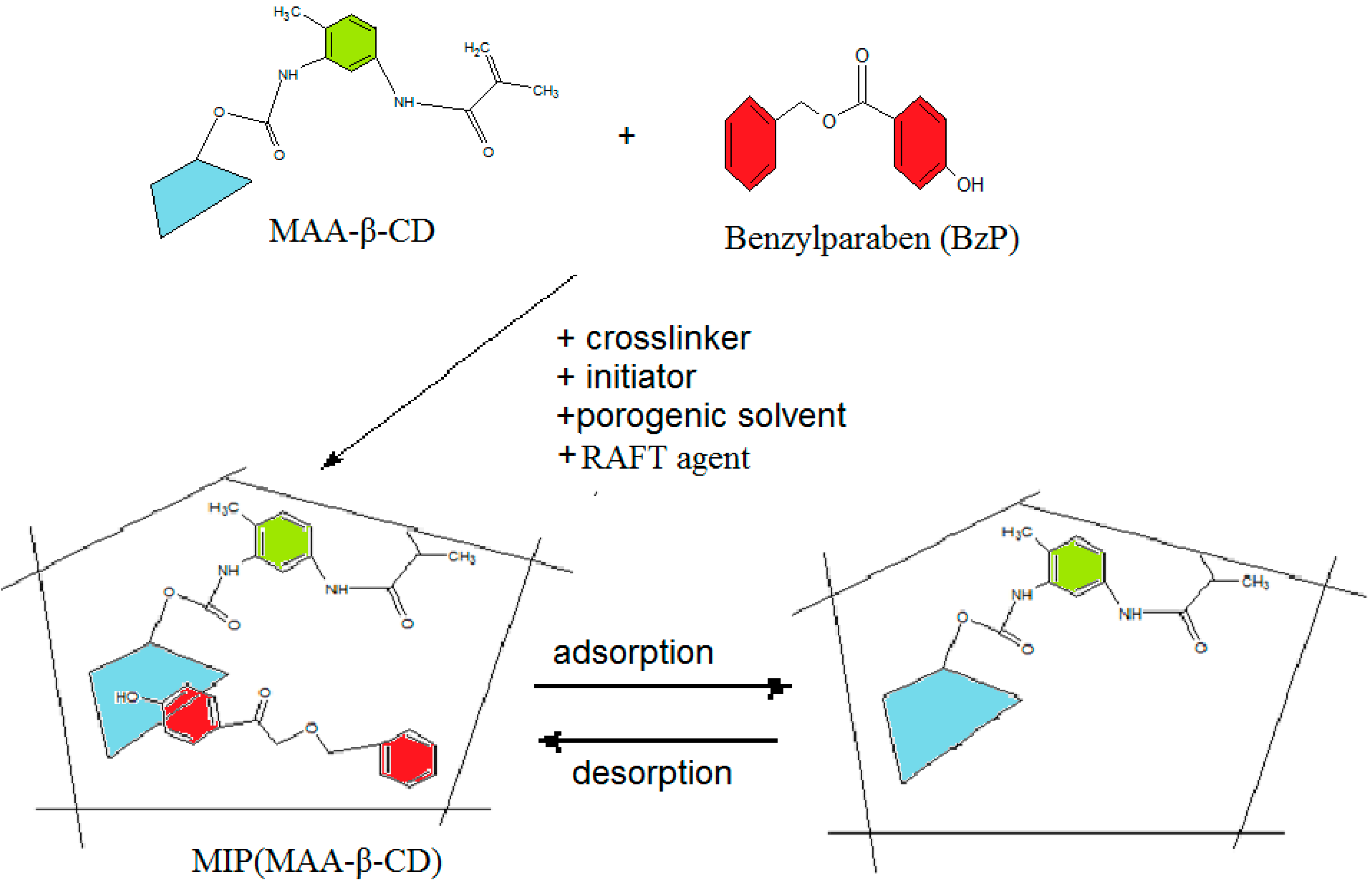
3.6. Binding Experiments
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alexander, C.; Andersson, H.S.; Andersson, L.I.; Ansell, R.J.; Kirsch, N.; Nicholls, I.A.; O’Mahony, J.; Whitcombe, M.J. Molecular imprinting science and technology: A survey of the literature for the years up to and including 2003. J. Mol. Recognit. 2006, 19, 106–180. [Google Scholar] [CrossRef] [PubMed]
- Puoci, F.; Cirillo, G.; Curcio, M.; Iemma, F.; Spizzirri, U.G.; Picci, N. Molecularly imprinted solid phase extraction for the selective HPLC determination of α-tocopherol in bay leaves. Anal. Chim. Acta 2007, 593, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zou, H.; Chen, X.; Luo, Q.; Kong, L. Molecularly imprinted monolithic stationary phases for liquid chromatographic separation of enantiomers and diastereomers. J. Chromatogr. A 2003, 984, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, D.; Bossi, A.; Whitcombe, M.J.; Chianella, I.; Fowler, S.A.; Subrahmanyam, S.; Piletska, E.V.; Piletsky, S.A. Electrochemical sensor for catechol and dopamine based on a catalytic molecularly imprinted polymer-conducting polymer hybrid recognition element. Anal. Chem. 2009, 81, 3576–3584. [Google Scholar] [CrossRef] [PubMed]
- Hillberg, A.L.; Brain, K.R.; Allender, C.J. Design and evaluation of thin and flexible theophylline imprinted polymer membrane materials. J. Mol. Recognit. 2009, 22, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Antwi-Boampong, S.; Mani, K.S.; Carlan, J.; BelBruno, J.J. A selective molecularly imprinted polymer-carbon nanotube sensor for cotinine sensing. J. Mol. Recognit. 2014, 27, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, K. Grafting of β-cyclodextrin-modified 2-hydroxyethyl methacrylate onto polyurethane. J. Appl. Polym. Sci. 1996, 60, 2245–2249. [Google Scholar] [CrossRef]
- Tang, S.; Kong, L.; Ou, J.; Liu, Y.; Li, X.; Zou, H. Application of cross-linked β-cyclodextrin polymer for adsorption of aromatic amino acids. J. Mol. Recognit. 2006, 19, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Kuang, D.; Liu, L.; Deng, Q. Selective adsorption of norfloxacin in aqueous media by an imprinted polymer based on hydrophobic and electrostatic interactions. J. Pharm. Biomed. Anal. 2007, 45, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Hishiya, T.; Asanuma, H.; Komiyama, M. Molecular imprinting of cyclodextrin on silica-gel support for the stationary phase of high-performance-liquid-chromatography. J. Incl. Phenom. 2001, 41, 149–153. [Google Scholar] [CrossRef]
- Asanuma, H.; Akiyama, T.; Kajiya, K.; Hishiya, T.; Komiyama, M. Molecular imprinting of cyclodextrin in water for the recognition of nanometer-scaled guests. Anal. Chim. Acta 2001, 435, 25–33. [Google Scholar] [CrossRef]
- Hishiya, T.; Akiyama, T.; Asanuma, H.; Komiyama, M. Molecular imprinting of cyclodextrins leading to synthetic antibodies. J. Incl. Phenom. 2002, 44, 365–367. [Google Scholar] [CrossRef]
- Ma, X.; Liu, J.; Zhang, Z.; Wang, L.; Chen, Z.; Xiang, S. The cooperative utilization of imprinting, electro-spinning and a pore-forming agent to synthesise β-cyclodextrin polymers with enhanced recognition of naringin. RSC Adv. 2013, 3, 25396–25402. [Google Scholar] [CrossRef]
- Xu, Z.F.; Wen, G.; Kuang, D.Z.; Zhang, F.X.; Tang, S.P. Selective separation of deltamethrin by molecularly imprinted polymers using a β-cyclodextrin derivative as the functional monomer. J. Environ. Sci. Health B 2013, 48, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ferronato, C.; Deng, N.; Chovelon, J.-M. Study of benzylparaben photocatalytic degradation by TiO2. Appl. Catal. B Environ. 2011, 104, 353–360. [Google Scholar] [CrossRef]
- Tay, K.S.; Rahman, N.A.; Abas, M.R.B. Ozonation of parabens in aqueous solution: Kinetics and mechanism of degradation. Chemosphere 2010, 81, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Darbre, P.; Aljarrah, A.; Miller, W.; Coldham, N.; Sauer, M.; Pope, G. Concentrations of parabens in human breast tumours. J. Appl. Toxicol. 2004, 24, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Tavares, R.S.; Martins, F.C.; Oliveira, P.J.; Ramalho-Santos, J.; Peixoto, F.P. Parabens in male infertility—Is there a mitochondrial connection? Reprod. Toxicol. 2009, 27, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Beltran, A.; Marcé, R.M.; Cormack, P.A.G.; Borrull, F. Synthetic approaches to parabens molecularly imprinted polymers and their applications to the solid-phase extraction of river water samples. Anal. Chim. Acta 2010, 677, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Wang, Z.; Ma, L.; Zhang, M.; Wang, J.; Dai, X.; Yan, Y. Selective adsorption of methylparaben by submicrosized molecularly imprinted polymer: Batch and dynamic flow mode studies. Ind. Eng. Chem. Res. 2012, 51, 14915–14924. [Google Scholar] [CrossRef]
- Benijts, T.; Lambert, W.; de Leenheer, A. Analysis of multiple endocrine disruptors in environmental waters via wide-spectrum solid-phase extraction and dual-polarity ionization LC-ion trap-MS/MS. Anal. Chem. 2003, 76, 704–711. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Radical addition—Fragmentation chemistry in polymer synthesis. Polymer 2008, 49, 1079–1131. [Google Scholar] [CrossRef]
- Xu, S.; Li, J.; Chen, L. Molecularly imprinted polymers by reversible addition—Fragmentation chain transfer precipitation polymerization for preconcentration of atrazine in food matrices. Talanta 2011, 85, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.P.; Mohamad, S.; Abas, M.R.B. Removal of parabens from aqueous solution using β-cyclodextrin cross-linked polymer. Int. J. Mol. Sci. 2010, 11, 3459–3471. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-F.; Chen, C.-F.; Yen, S.-H. Synthesis and characterization of novel sulfobetaines derived from 2,4-tolylene diisocyanate. J. Appl. Polym. Sci. 2001, 82, 3447–3459. [Google Scholar] [CrossRef]
- Chiang, W.Y.; Chan, S.C. Preparation and properties of photocurable unsaturated oligoester acrylourethanes. J. Appl. Polym. Sci. 1987, 34, 127–141. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Lazaridis, N.K.; Bikiaris, D.N. Optimization of chitosan and β-cyclodextrin molecularly imprinted polymer synthesis for dye adsorption. Carbohydr. Polym. 2013, 91, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Hu, W.; Pan, J.; Zhou, H.; Guan, W.; Wang, X.; Dai, J.; Xu, L. Selective adsorption and separation of BPA from aqueous solution using novel molecularly imprinted polymers based on kaolinite/Fe3O4 composites. Chem. Eng. J. 2011, 171, 603–611. [Google Scholar] [CrossRef]
- González, G.P.; Hernando, P.F.; Alegria, J. A morphological study of molecularly imprinted polymers using the scanning electron microscope. Anal. Chim. Acta 2006, 557, 179–183. [Google Scholar] [CrossRef]
- Spivak, D.A. Optimization, evaluation, and characterization of molecularly imprinted polymers. Adv. Drug Deliv. Rev. 2005, 57, 1779–1794. [Google Scholar] [CrossRef] [PubMed]
- Asouhidou, D.D.; Triantafyllidis, K.S.; Lazaridis, N.K.; Matis, K.A. Adsorption of remazol red 3BS from aqueous solutions using APTES-and cyclodextrin-modified HMS-type mesoporous silicas. Colloids Surf. A Physicochem. Eng. Asp. 2009, 346, 83–90. [Google Scholar] [CrossRef]
- Yang, M.; Gu, W.; Sun, L.; Zhang, F.; Ling, Y.; Chu, X.; Wang, D. Study on the molecularly imprinted polymers with methyl-testosterone as the template. Talanta 2010, 81, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Kayaci, F.; Aytac, Z.; Uyar, T. Surface modification of electrospun polyester nanofibers with cyclodextrin polymer for the removal of phenanthrene from aqueous solution. J. Hazard. Mater. 2013, 261, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Mei, Z.; Wei, X. Study on sorption of chlorophenols from aqueous solutions by an insoluble copolymer containing β-cyclodextrin and polyamidoamine units. Chem. Eng. J. 2012, 192, 138–145. [Google Scholar] [CrossRef]
- Alahmadi, S.M.; Mohamad, S.; Maah, M.J. Synthesis and characterization of mesoporous silica functionalized with calix[4]arene derivatives. Int. J. Mol. Sci. 2012, 13, 13726–13736. [Google Scholar] [CrossRef] [PubMed]
- Sing, K.S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Wilson, L.D.; Mohamed, M.H.; Headley, J.V. Surface area and pore structure properties of urethane-based copolymers containing β-cyclodextrin. J. Colloid Interface Sci. 2011, 357, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, Q.; Liu, T.; Sun, J.; Jiao, Y.; Xia, Y.; Xia, L.; Wang, Z.; Zhang, W.; Wang, K. Equilibrium, kinetic and thermodynamic studies on the adsorption of phenol onto graphene. Mater. Res. Bull. 2012, 47, 1898–1904. [Google Scholar] [CrossRef]
- Ma, X.; Li, L.; Yang, L.; Su, C.; Wang, K.; Yuan, S.; Zhou, J. Adsorption of heavy metal ions using hierarchical CaCO3-maltose meso/macroporous hybrid materials: Adsorption isotherms and kinetic studies. J. Hazard. Mater. 2012, 209, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Toth, B.; Laszlo, K.; Horvai, G. Chromatographic behavior of silica—Polymer composite molecularly imprinted materials. J. Chromatogr. A 2005, 1100, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M. Physical adsorption characterization of nanoporous materials. Chem. Ing. Tech. 2010, 82, 1059–1073. [Google Scholar] [CrossRef]
- Hu, T.-P.; Zhang, Y.-M.; Zheng, L.-H.; Fan, G.-Z. Molecular recognition and adsorption performance of benzothiophene imprinted polymer on silica gel surface. J. Fuel Chem. Technol. 2010, 38, 722–729. [Google Scholar] [CrossRef]
- Pan, G.; Zu, B.; Guo, X.; Zhang, Y.; Li, C.; Zhang, H. Preparation of molecularly imprinted polymer microspheres via reversible addition—Fragmentation chain transfer precipitation polymerization. Polymer 2009, 50, 2819–2825. [Google Scholar] [CrossRef]
- Schneider, H.-J.; Hacket, F.; Rüdiger, V.; Ikeda, H. NMR studies of cyclodextrins and cyclodextrin complexes. Chem. Rev. 1998, 98, 1755–1786. [Google Scholar] [CrossRef] [PubMed]
- Cwiertnia, B.; Hladon, T.; Stobiecki, M. Stability of diclofenac sodium in the inclusion complex with β-cyclodextrin in the solid state. J. Pharm. Pharmacol. 1999, 51, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, S.; Surikumaran, H.; Raoov, M.; Marimuthu, T.; Chandrasekaram, K.; Subramaniam, P. Conventional study on novel dicationic ionic liquid inclusion with β-cyclodextrin. Int. J. Mol. Sci. 2011, 12, 6329–6345. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Guan, Y.; Xiao, H. Preparation and characterization of inclusion complexes of a cationic β-cyclodextrin polymer with butylparaben or triclosan. Int. J. Pharm. 2008, 357, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Nabuurs, T.; Pears, D.; Overbeek, A. Defect free coatings from two-pack isocyanate curable acrylic dispersions. Prog. Org. Coat. 1999, 35, 129–140. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asman, S.; Mohamad, S.; Sarih, N.M. Exploiting β-Cyclodextrin in Molecular Imprinting for Achieving Recognition of Benzylparaben in Aqueous Media. Int. J. Mol. Sci. 2015, 16, 3656-3676. https://doi.org/10.3390/ijms16023656
Asman S, Mohamad S, Sarih NM. Exploiting β-Cyclodextrin in Molecular Imprinting for Achieving Recognition of Benzylparaben in Aqueous Media. International Journal of Molecular Sciences. 2015; 16(2):3656-3676. https://doi.org/10.3390/ijms16023656
Chicago/Turabian StyleAsman, Saliza, Sharifah Mohamad, and Norazilawati Muhamad Sarih. 2015. "Exploiting β-Cyclodextrin in Molecular Imprinting for Achieving Recognition of Benzylparaben in Aqueous Media" International Journal of Molecular Sciences 16, no. 2: 3656-3676. https://doi.org/10.3390/ijms16023656