The Possible Physical Barrier and Coastal Dispersal Strategy for Japanese Grenadier Anchovy, Coilia nasus in the East China Sea and Yellow Sea: Evidence from AFLP Markers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
| Populations | Number of Individuals | Date of Collection | Number of Loci | Number of Polymorphic Loci | Proportion of Polymorphic Loci | Nei’s Genetic Diversity |
|---|---|---|---|---|---|---|
| Coilia mystus | 12 | May 2005 | 286 | 193 | 67.48% | 0.1435 |
| Zhenjiang (ZJ) | 20 | July 2004 | 282 | 143 | 50.71% | 0.1016 |
| Nantong (NT) | 17 | October 2004 | 279 | 125 | 44.80% | 0.0808 |
| Dongying (DY) | 20 | May 2004 | 272 | 116 | 42.65% | 0.0750 |
| Kashima (KA) | 12 | March 2003 | 230 | 69 | 30.00% | 0.0554 |
| Yanagawa (YA) | 9 | March 2003 | 231 | 73 | 31.60% | 0.0584 |
| Total | 93 | – | 371 | 310 | 85.36% | – |
| Primer Combinations | E-AGA/M-CAG | E-AGA/M-CTA | E-AGG/M-CAC | E-ACC/M-CAG | E-AGT/M-CTT | Total |
|---|---|---|---|---|---|---|
| Number of loci | 105 | 87 | 72 | 42 | 65 | 371 |
| Number of polymorphic loci | 87 | 75 | 59 | 37 | 52 | 310 |
| Proportion of polymorphic loci | 85.71% | 86.20% | 81.94% | 88.10% | 80.00% | 83.56% |
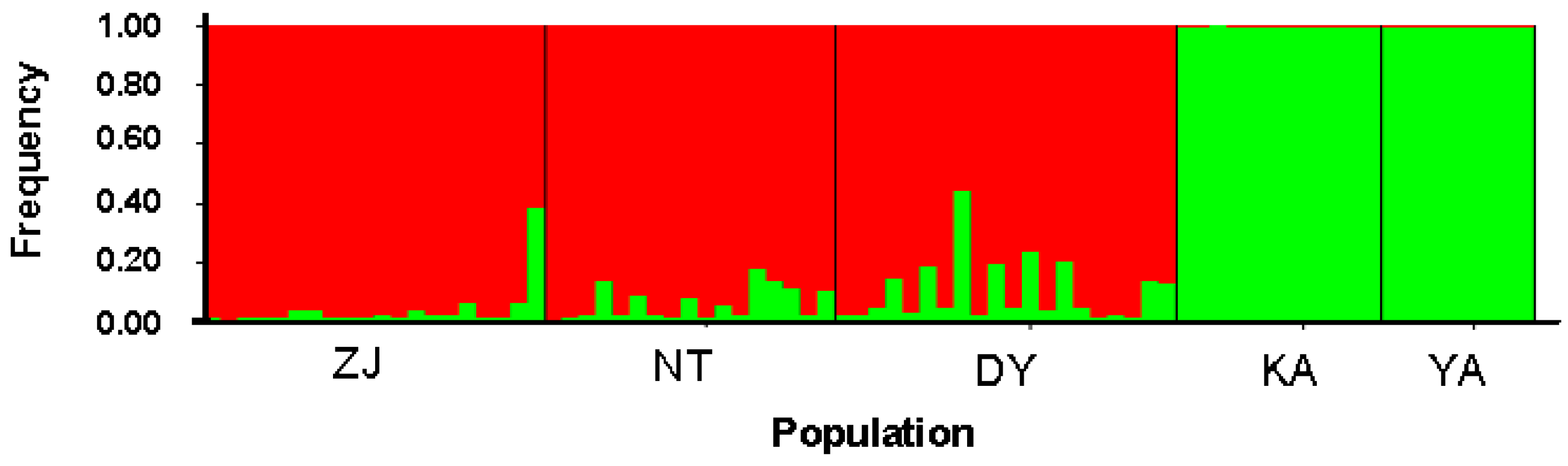
| Source of Variation | Variance Components | Percentage of Variance | F/φ-Statistics | p |
|---|---|---|---|---|
| One gene pool | ||||
| Among populations | 3.6003 | 17.77 | 0.1777 | 0.00 |
| Within populations | 16.6565 | 82.23 | ||
| Two gene pools (DY, NT, ZJ) (Ka, Ya) | ||||
| Between groups | 3.2468 | 14.84 | 0.1484 | 0.00 |
| Among populations within groups | 1.9707 | 9.01 | 0.1058 | 0.00 |
| Within populations | 16.6565 | 76.15 | 0.2385 | 0.00 |
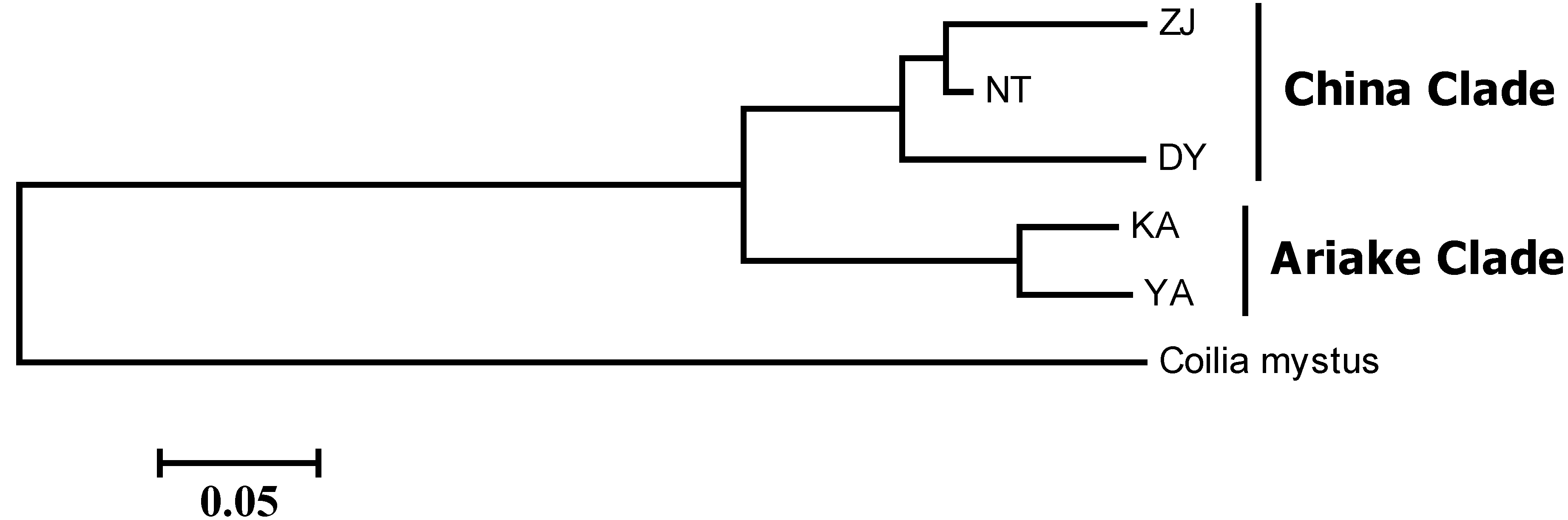
| Populations | Coilia mystus | Zhenjiang | Nantong | Dongying | Kashima | Yanagawa |
|---|---|---|---|---|---|---|
| Coilia mystus | – | 0.6406 | 0.6360 | 0.6404 | 0.6616 | 0.6550 |
| Zhenjiang | 0.6715 | – | 0.1102 | 0.1148 | 0.1337 | 0.1246 |
| Nantong | 0.6880 | 0.0746 | – | 0.0920 | 0.1141 | 0.1002 |
| Dongying | 0.6946 | 0.1660 | 0.0789 | – | 0.0962 | 0.0841 |
| Kashima | 0.7136 | 0.2580 | 0.2203 | 0.2333 | – | 0.0551 |
| Yanagawa | 0.7093 | 0.2749 | 0.2302 | 0.2717 | 0.0732 * | – |
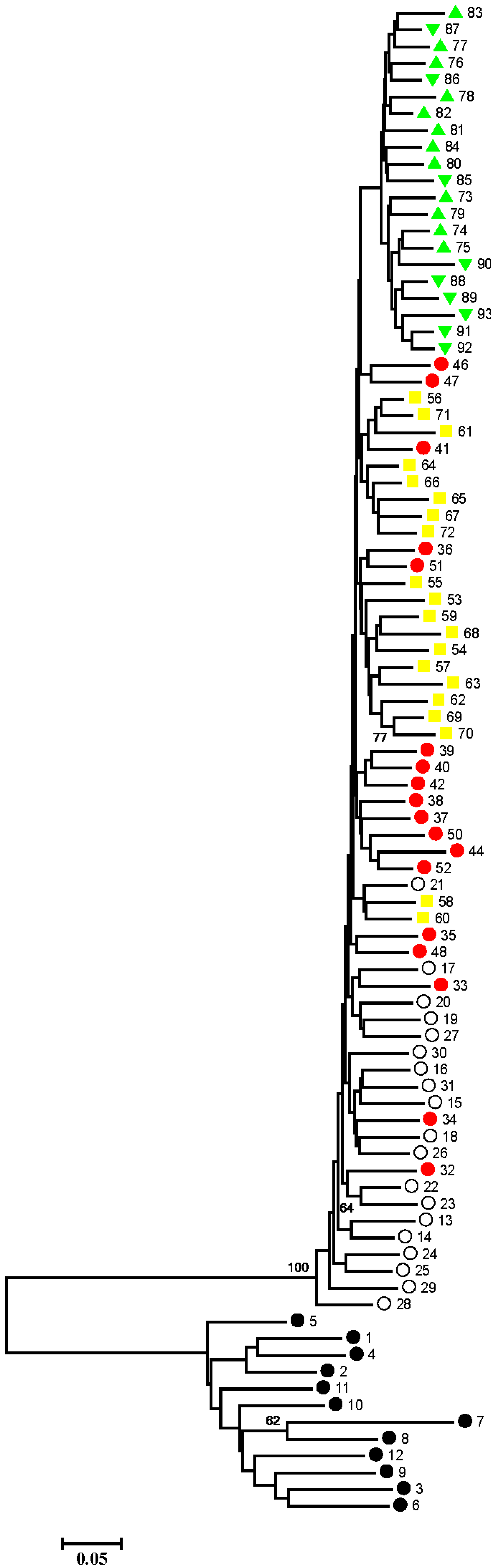

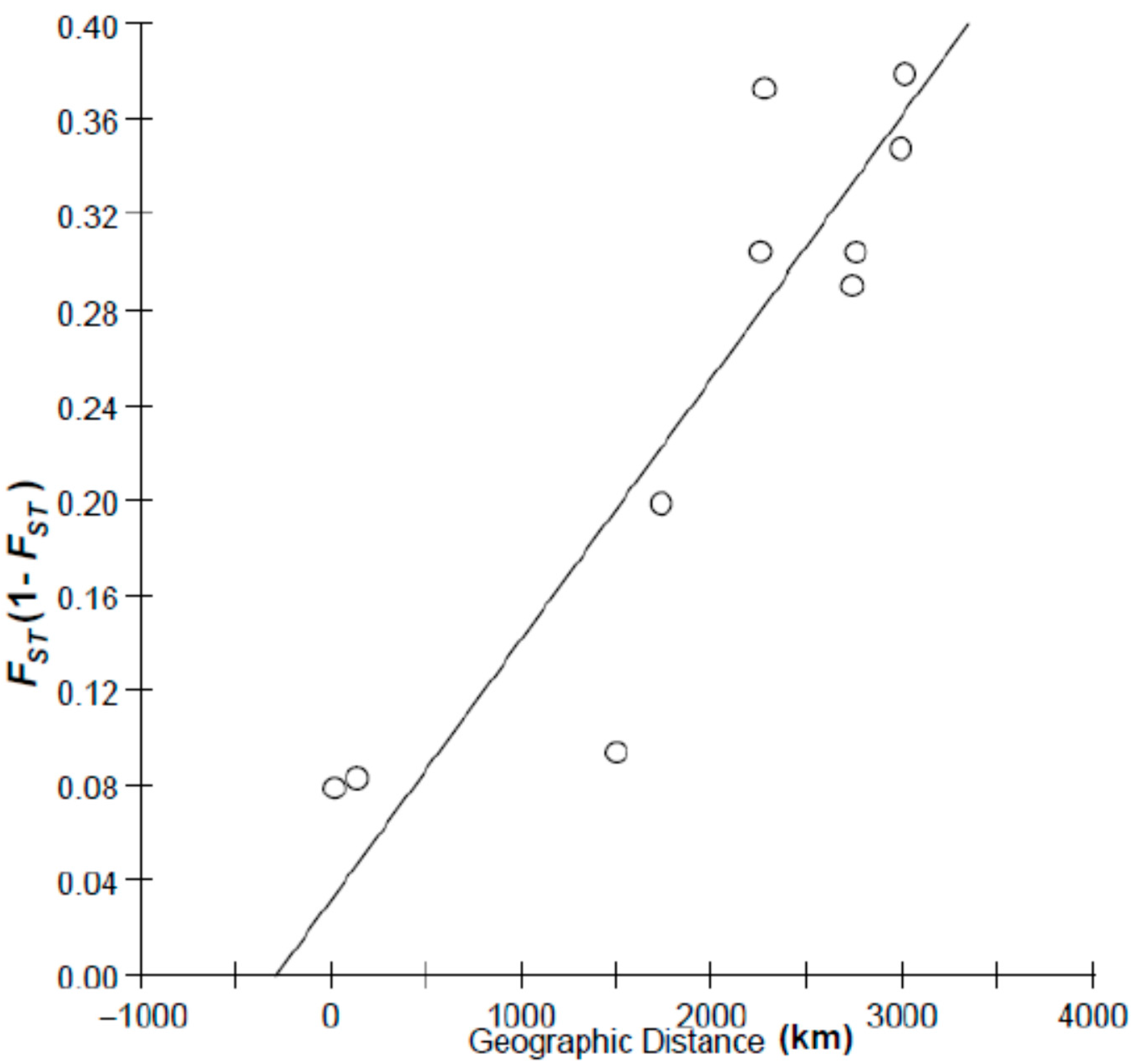
2.2. Discussion
3. Materials & Methods
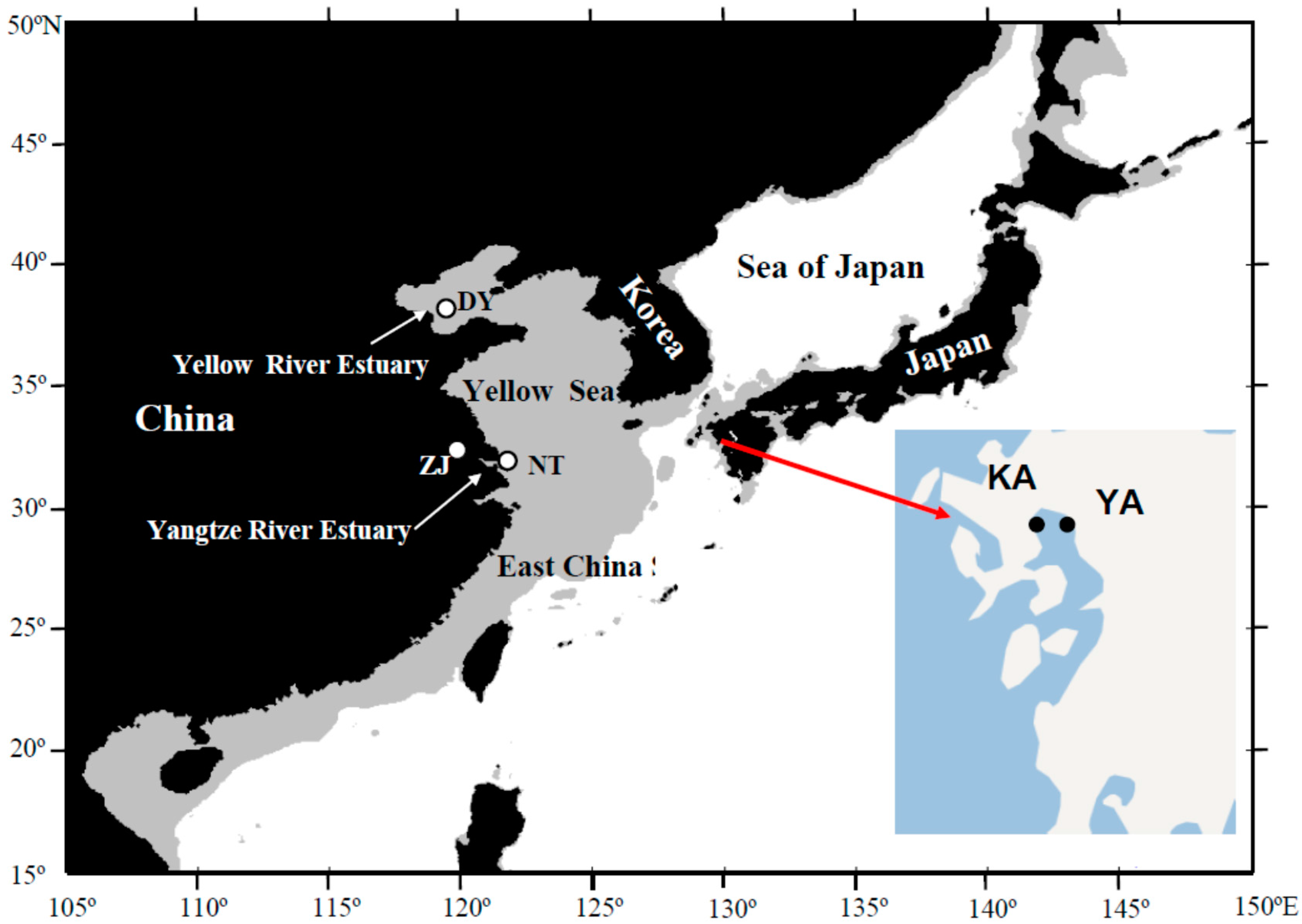
3.1. Sample Collection
3.2. AFLP Analysis
| Primer | Sequence |
|---|---|
| Adapters | |
| EcoRI-adapter | 5'-CTCGTAGACTGCGTACC-3' |
| 5'-AATTGGTACGCAGTCTAC-3' | |
| MseI-adapter | 5'-GACGTGAGTCCTGAG-3' |
| 5'-TACTCAGGACTCAT-3' | |
| Pre-Amplification Primer | |
| EcoRI | 5'-GACTGCGTACCAATTC-3' |
| MseI | 5'-GATGAGTCCTGAGTAA-3' |
| Selective Amplification Primer | |
| E-AGA/M-CAG | 5'-GACTGCGTACCAATTCAGA-3' |
| 5'-GATGAGTCCTGAGTAACAG-3' | |
| E-AGA/M-CTA | 5'-GACTGCGTACCAATTCAGA-3' |
| 5'-GATGAGTCCTGAGTAACTA-3' | |
| E-AGG/M-CAC | 5'-GACTGCGTACCAATTCAGG-3' |
| 5'-GATGAGTCCTGAGTAACAC-3' | |
| E-ACC/M-CAG | 5'-GACTGCGTACCAATTCACC-3' |
| 5'-GATGAGTCCTGAGTAACAG-3' | |
| E-AGT/M-CTT | 5'-GACTGCGTACCAATTCAGT-3' |
| 5'-GATGAGTCCTGAGTAACTT-3' | |
3.3. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Palumbi, S.R. Genetic divergence, reproductive isolation, and marine speciation. Annu. Rev. Ecol. Syst. 1994, 25, 547–572. [Google Scholar] [CrossRef]
- Hansen, J.A.K.O.B.; Nielsen, E.E.; GrØnkjer, P.; Loeschcke, V. Evolutionary mechanisms shaping the genetic population structure of marine fishes lessons from the European flounder (Platichthys flesus L.). Mol. Ecol. 2007, 16, 3104–3118. [Google Scholar] [CrossRef] [PubMed]
- Riginos, C.; Victor, B.C. Larval spatial distributions and other early life-history characteristics predict genetic differentiation in eastern Pacific blennioid fishes. Proc. R. Soc. Lond. B 2001, 268, 1931–1936. [Google Scholar] [CrossRef]
- Pelc, R.A.; Warner, R.R.; Gaines, S.D. Geographical patterns of genetic structure in marine species with contrasting life histories. J. Biogeogr. 2009, 36, 1881–1890. [Google Scholar] [CrossRef]
- Dawson, M.N. Incipient speciation of Catostylus mosaicus (Scyphozoa, Rhizostomeae, Catostylidae), comparative phylogeography and biogeography in southeast Australia. J. Biogeogr. 2005, 32, 515–533. [Google Scholar] [CrossRef]
- Bilton, D.T.; Paula, J.; Bishop, J.D.D. Dispersal, genetic differentiation and speciation in estuarine organisms. Estuar. Coast. Shelf Sci. 2002, 55, 937–952. [Google Scholar] [CrossRef]
- Watts, R.J.; Johnson, M.S. Estuaries, lagoons and enclosed embayments: Habitats that enhance population subdivision of inshore fishes. Mar. Freshw. Res. 2004, 55, 641–651. [Google Scholar] [CrossRef]
- Ni, G.; Li, Q.I.; Kong, L.; Yu, H. Comparative phylogeography in marginal seas of the Northwestern Pacific. Mol. Ecol. 2004, 23, 534–548. [Google Scholar] [CrossRef]
- Avise, J.C.; Arnold, J.; Ball, R.M.; Bermingham, E.; Lamb, T.; Neigel, J.E.; Reeb, C.A.; Saunders, N.C. Intraspecific phylogeography: The mitochondrial DNA bridge between population genetics and systematics. Ann. Rev. Ecol. Syst. 1987, 18, 489–522. [Google Scholar]
- Dong, Y.W.; Wang, H.S.; Han, G.D.; Ke, C.H.; Zhan, X.; Nakano, T.; Williams, G.A. The impact of Yangtze River discharge, ocean currents and historical events on the biogeographic pattern of Cellana toreuma along the China coast. PLoS One 2012, 7, e36178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marko, P.B. What’s larvae got to do with it? Disparate patterns of post-glacial population structure in two benthic marine gastropods with identical dispersal potential. Mol. Ecol. 2004, 13, 597–611. [Google Scholar]
- Whitehead, P.J.P.; Nelson, G.J.; Wongratana, T. FAO Species Catalogue, vol. 7: Clupeoid Fishes of the World (Suborder Clupeoidei). An Annotated and Illustrated Catalogue of the Herrings, Sardines, Pilchards, Sprats, Shads, Anchovies and Wolf-herrings. Part 2: Engraulidae; FAO: Rome, Italy, 1988. [Google Scholar]
- Zhang, S.Y. Fauna Sinica Osteichthyes: Acipenseriformes, Elopiformes, Clupeiformes, Gonorhynchiformes; Science Press: Beijing, China, 2001. [Google Scholar]
- Liu, D.; Guo, H.Y.; Tang, W.Q.; Yang, J.Q. Comparative evolution of S7 intron 1 and ribosomal internal transcribed spacer in Coilia nasus (Clupeiformes: Engraulidae). Int. J. Mol. Sci. 2002, 13, 3085–3100. [Google Scholar] [CrossRef]
- Yuan, C.M. Observations on the breeding migration of Coilia ectenes. Bull. Biol. 1987, 12, 1–3. [Google Scholar]
- Takita, T. The spawning and the early life history of the Engraulid fish Coilia. sp. distributed in Ariake Sound. Bull. Fac. Fish. Nagasaki Univ. 1967, 23, 107–122. [Google Scholar]
- Li, S.F.; Cheng, J.H.; Yan, L.P. Spatial structures of fish communities on the continental shelf of the East China Sea. Acta Ecol. Sin. 2007, 27, 4377–4386. [Google Scholar]
- Ma, C.Y.; Cheng, Q.Q.; Zhang, Q.Y.; Zhao, Y.L. Genetic variation of Coilia ectenes (Clupeiformes: Engraulidae) revealed by the complete cytochrome b sequences of mitochondrial DNA. J. Exp. Mar. Biol. Ecol. 2009, 385, 14–19. [Google Scholar] [CrossRef]
- Yang, Q.L.; Gao, T.X.; Miao, Z.Q. Differentiation between populations of Japanese grenadier anchovy (Coilia nasus) in Northwestern Pacific based on ISSR markers: Implications for biogeography. Biochem. Syst. Ecol. 2011, 39, 286–296. [Google Scholar] [CrossRef]
- Gao, T.X.; Wan, Z.Z.; Song, N.; Zhang, X.M.; Han, Z.Q. Evolutionary mechanisms shaping the genetic population structure of coastal fish: Insight from populations of Coilia nasus in Northwestern Pacific. Mitochondrial DNA 2014, 25, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.M.; Qin, A.L. On the classification of Coilia. from Japan. J. Nanjing Univ. 1985, 21, 318–326. [Google Scholar]
- Vos, P.; Hogers, R.; Bleeker, M.; Reijans, M.; van de Lee, T.; Hornes, M.; Frijters, A.; Pot, J.; Peleman, J.; Kuiper, M.; et al. AFLP: A new technique for DNA fingerprinting. Nucleic Acids. Res. 1995, 23, 4407–4414. [Google Scholar] [CrossRef]
- Liu, J.Z.; Cordes, J.F. DNA marker technologies and their applications in aquaculture genetics. Aquaculture 2004, 238, 1–37. [Google Scholar] [CrossRef]
- Palumbi, S.R. Marine reserves and ocean neighborhoods: The spatial scale of marine populations and their management. Annu. Rev. Envion. Resour. 2004, 29, 31–68. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Lambeck, K.; de Deckker, P.; Johnston, P.; Fifield, L. Timing of the last glacial maximum from observed sea-level minima. Nature 2000, 406, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Gao, T.X.; Wu, S.F.; Zhang, Y.P. Pleistocene isolation in the Northwestern Pacific marginal seas and limited dispersal in a marine fish, Chelon haematocheilus (Temminck & Schlegel, 1845). Mol. Ecol. 2007, 16, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.J. Development of Microsatellite DNA Markers and Population Genetics for Sand Lance (Ammodytes personatus) and Roughskin Sculpin (Trachidermus fasciatus). Ph.D. Thesis, Ocean University of China, Qingdao, China, June 2012. [Google Scholar]
- Liu, J.X.; Gao, T.X.; Yokogawa, K.; Zhang, Y.P. Differential population structuring and demographic history of two closely related fish species, Japanese sea bass (Lateolabrax japonicus) and spotted sea bass (Lateolabrax maculatus) in Northwestern Pacific. Mol. Phylogenet. Evol. 2006, 39, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.Q.; Gao, T.X.; Yanagimoto, T.; Sakurai, Y. Deep phylogeographic break among white croaker Pennahia argentata (Sciaenidae, Perciformes) populations in Northwestern Pacific. Fish. Sci. 2008, 74, 770–780. [Google Scholar] [CrossRef]
- Han, Z.Q.; Shui, B.N.; Wang, Z.Y.; Miao, Z.Q.; Gao, T.X. Analysis of genetic structure of white croaker using amplified fragment length polymorphism (AFLP) markers. Afr. J. Biotechnol. 2009, 8, 4308–4315. [Google Scholar]
- Shaw, P.W.; Arkhipkin, A.I.; Al-Khairulla, H. Genetic structuring of Patagonian toothfish populations in the Southwest Atlantic Ocean: The effect of the Antarctic Polar Front and deep-water troughs as barriers to genetic exchange. Mol. Ecol. 2004, 13, 3293–3303. [Google Scholar] [CrossRef] [PubMed]
- Shui, B.N.; Han, Z.Q.; Gao, T.X.; Miao, Z.Q.; Yanagimoto, T. Mitochondrial DNA variation in the East China Sea and Yellow Sea populations of Japanese Spanish mackerel Scomberomorus niphonius. Fish. Sci. 2009, 75, 593–600. [Google Scholar] [CrossRef]
- Liu, J.X.; Gao, T.X.; Zhuang, Z.M.; Jin, X.S.; Yokogawa, K.; Zhang, Y.P. Late Pleistocene divergence and subsequent population expansion of two closely related fish species, Japanese anchovy (Engraulis japonicus) and Australian anchovy (Engraulis australis). Mol. Phylogenet. Evol. 2006, 40, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jayasankar, P.; Khoo, S.K. AFLP fingerprinting reveals genetic variability in common carp stocks from Indonesia. Asian Fish. Sci. 2000, 13, 139–147. [Google Scholar]
- Merril, C.R.; Switzer, R.C.; van Keuren, M.L. Trace polypeptides in cellular extracts and human body fluid detected by two-dimensional electrophoresis and a highly sensitive silver stain. Proc. Natl. Acad. Sci. USA 1979, 76, 4335–4339. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Li, W.H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc. Natl. Acad. Sci. USA 1979, 76, 5269–5273. [Google Scholar] [CrossRef] [PubMed]
- Vekemans, X. AFLP-SURV version 1.0. Distributed by the Author. Laboratoire de Génétique et Ecologie Végétale, Université Libre de Bruxelles: Bruxelles, Belgium, 2002. [Google Scholar]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [PubMed]
- Falush, D.; Stephens, M.; Pritchard, J.K. Inference of population structure using multilocus genotype data: Dominant markers and null alleles. Mol. Ecol. Notes 2007, 7, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Slatkin, M. Isolation by distance in equilibrium and non-equilibrium populations. Evolution 1993, 47, 264–279. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Z.-Q.; Han, G.; Wang, Z.-Y.; Gao, T.-X. The Possible Physical Barrier and Coastal Dispersal Strategy for Japanese Grenadier Anchovy, Coilia nasus in the East China Sea and Yellow Sea: Evidence from AFLP Markers. Int. J. Mol. Sci. 2015, 16, 3283-3297. https://doi.org/10.3390/ijms16023283
Han Z-Q, Han G, Wang Z-Y, Gao T-X. The Possible Physical Barrier and Coastal Dispersal Strategy for Japanese Grenadier Anchovy, Coilia nasus in the East China Sea and Yellow Sea: Evidence from AFLP Markers. International Journal of Molecular Sciences. 2015; 16(2):3283-3297. https://doi.org/10.3390/ijms16023283
Chicago/Turabian StyleHan, Zhi-Qiang, Gang Han, Zhi-Yong Wang, and Tian-Xiang Gao. 2015. "The Possible Physical Barrier and Coastal Dispersal Strategy for Japanese Grenadier Anchovy, Coilia nasus in the East China Sea and Yellow Sea: Evidence from AFLP Markers" International Journal of Molecular Sciences 16, no. 2: 3283-3297. https://doi.org/10.3390/ijms16023283





