Systems Pharmacology Dissection of the Anti-Inflammatory Mechanism for the Medicinal Herb Folium Eriobotryae
Abstract
:1. Introduction
2. Results and Discussion
2.1. Active Compounds Identification
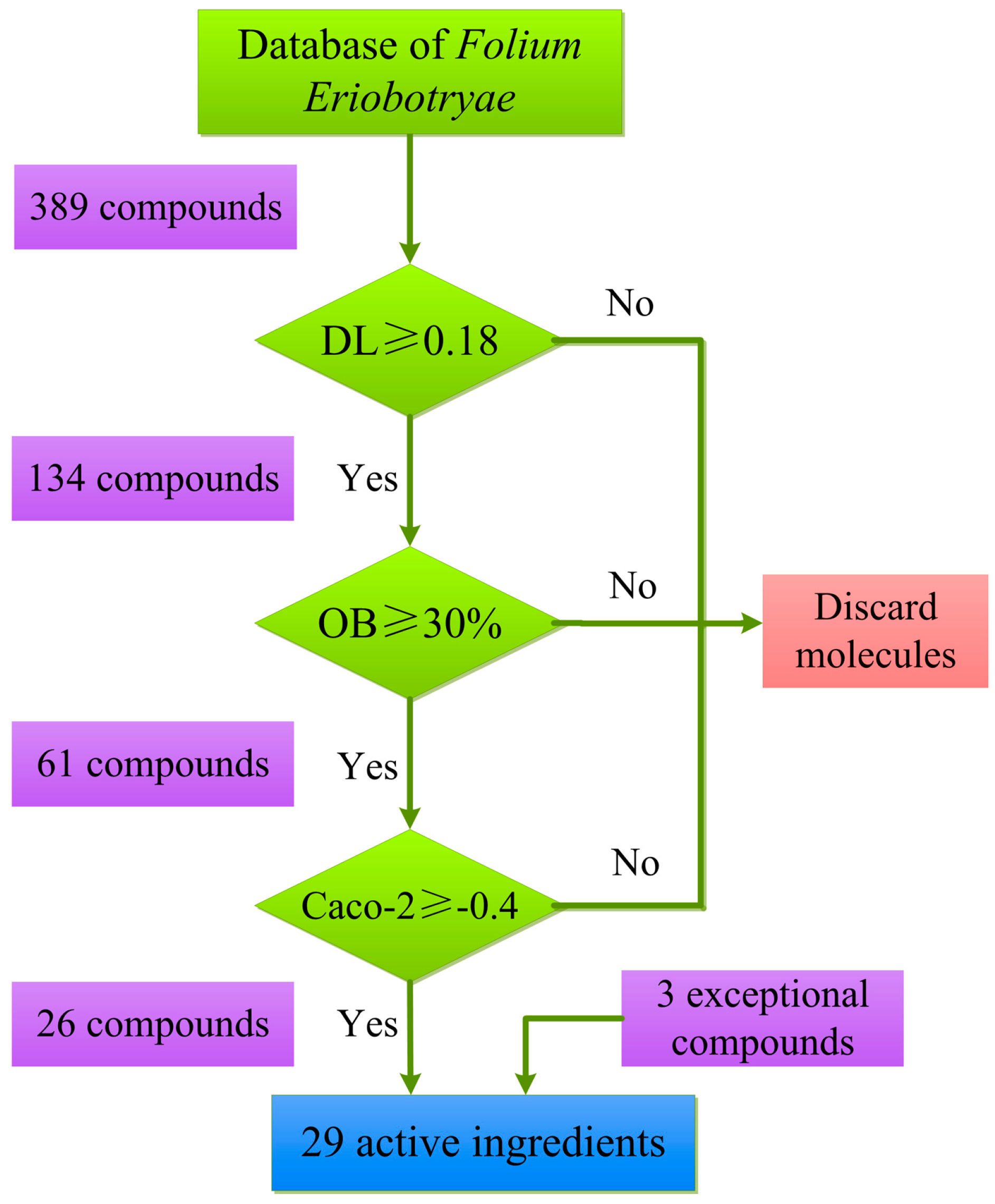
2.1.1. Drug-likeness Evaluation
2.1.2. Oral Bioavailability Prediction
2.1.3. Caco-2 Permeability Screening
2.1.4. Exceptional Molecules
| No. | Ingredient | Structure | OB (%) | Caco-2 | DL | Bioactivity (PMID) |
|---|---|---|---|---|---|---|
| M_01 | β-sitosterol | 
| 45.04 | 1.33 | 0.75 | anti-inflammation (6967611); antipyretic (6967611); anthelminthic & anti-mutagenic activities (12203259); anti-oxidative (16508147) |
| M_02 | tormentic acid | 
| 33.73 | −0.20 | 0.71 | anti-inflammation (12007601); anti-HIV (12907261); antidiabetic (11830140); antitumor-promoting (14745168) |
| M_03 | sanguisorba,officinalis-7 | 
| 35.32 | −0.25 | 0.71 | |
| M_04 | rutin | 
| 35.45 | −1.91 | 0.28 | anti-inflammation (11680812); antioxidant (10904145) |
| M_05 | quercetin | 
| 31.36 | 0.02 | 0.28 | antioxidant (9414116); anti-inflammation (18549926); pro-apoptotic (14688022) |
| M_06 | pomolic acid | 
| 75.45 | 0.26 | 0.73 | anti-inflammation (18260049); anticancer (22223345); anti-apoptotic (15694664); anti-HIV (9748372) |
| M_07 | oleanolic acid | 
| 66.91 | 0.61 | 0.76 | anti-inflammation (1359067); antioxidant (22891614); anti-HIV (9748372); antidiabetic (18066109); hepatoprotection (18066109); antibacterial (18069238) |
| M_08 | methyl ursolate | 
| 47.63 | 0.92 | 0.74 | anti-inflammation (16204964) |
| M_09 | methyl maslinate | 
| 42.79 | 0.32 | 0.72 | |
| M_10 | methyl betulinate | 
| 43.41 | 0.85 | 0.76 | |
| M_11 | methyl arjunolate | 
| 56.25 | −0.05 | 0.70 | anti-inflammation (16204964) |
| M_12 | maslinic acid | 
| 55.84 | 0.04 | 0.74 | anti-inflammation (3769076); anti-HIV (8759159); antioxidant (12802735); anti-tumor (20509140); antiangiogenic (21175131) |
| M_13 | linguersinol | 
| 48.60 | −0.17 | 0.54 | |
| M_14 | kaempferol | 
| 65.70 | 0.27 | 0.24 | anti-inflammation (17666881); analgesic (17666881); antioxidant (17551714); anti-bacteria (15234754) |
| M_15 | delta 7-stigmastenol | 
| 40.40 | 1.30 | 0.75 | |
| M_16 | 2α-hydroxy ursolic acid | 
| 39.30 | 0.03 | 0.74 | anti-cancer (15922841) |
| M_17 | arjunolic acid | 
| 43.27 | −0.27 | 0.72 | antioxidant (18273903) |
| M_18 | 3-O-trans-p-coumaroyltormentic acid | 
| 46.94 | −0.17 | 0.34 | anti-inflammation (16204964) |
| M_19 | 3-O-trans-caffeoyltormentic acid | 
| 36.33 | −0.07 | 0.32 | |
| M_20 | 3-O-cis-p-coumaroyltormentic acid | 
| 33.35 | −0.19 | 0.34 | anti-inflammation (16204964) |
| M_21 | 3-epiursolic acid | 
| 39.92 | 0.51 | 0.75 | |
| M_22 | 2α,3α-dihydroxyursolic acid | 
| 37.73 | 0.25 | 0.74 | |
| M_23 | 2α,3α-dihydroxyurs-12-en-28-oic acid | 
| 45.16 | 0.21 | 0.74 | |
| M_24 | 2α,3α,23-trihydroxyolean-12-en-28-oic acid | 
| 41.34 | −0.27 | 0.72 | anti-inflammation (16204964) |
| M_25 | 2α,3α,19α-trihydroxy-12-oleanen-28-oic acid | 
| 30.03 | −0.18 | 0.72 | |
| M_26 | 2α,19α-dihydroxy-3-oxo-urs-12-en-28-oic acid | 
| 46.95 | −0.30 | 0.71 | anti-HIV (12907261) |
| M_27 | ursolic acid | 
| 9.95 | 0.79 | 0.66 | anti-inflammation (19051345); anti-HIV (8759159); hepatoprotection (8847885); antiangiogenic (21175131); anti-cancer (15922841); antibacterial (18069238) |
| M_28 | chlorogenic acid | 
| 63.12 | −1.07 | 0.33 | antioxidant (12771329); anticarcinogenic (12771329); anti-inflammation (17077520); analgesic (17077520) |
| M_29 | caffeic acid | 
| 51.08 | 0.24 | 0.05 | antioxidant (16243424); anticoagulant (19678956); anti-inflammation (19678956) |
2.2. Target Identification
| No. | Protein Name | Gene Name | UniProt ID |
|---|---|---|---|
| P_01 | 3-hydroxy-3-methylglutaryl-Coenzyme A reductase | HMGCR | P04035 |
| P_02 | Aldose reductase | AKR1B1 | P15121 |
| P_03 | Arachidonate 15-lipoxygenase | ALOX15 | P16050 |
| P_04 | Arachidonate 5-lipoxygenase | ALOX5 | P09917 |
| P_05 | Baculoviral IAP repeat-containing protein 5 | BIRC5 | O15392 |
| P_06 | C-C motif chemokine 2 | CCL2 | P13500 |
| P_07 | CD40 ligand | CD40LG | P29965 |
| P_08 | Cell division protein kinase 2 | CDK2 | P24941 |
| P_09 | Cholinesterase | BCHE | P06276 |
| P_10 | C-X-C motif chemokine 10 | CXCL10 | P02778 |
| P_11 | C-X-C motif chemokine 2 | CXCL2 | P19875 |
| P_12 | Cytochrome P450 1A2 | CYP1A2 | P05177 |
| P_13 | Epidermal growth factor receptor | EGFR | P00533 |
| P_14 | E-selectin | SELE | P16581 |
| P_15 | Glycogen synthase kinase-3 beta | GSK3B | P49841 |
| P_16 | Heme oxygenase 1 | HMOX1 | P09601 |
| P_17 | Inhibitor of nuclear factor kappa-B kinase subunit alpha | CHUK | O15111 |
| P_18 | Inhibitor of nuclear factor kappa-B kinase subunit beta | IKBKB | O14920 |
| P_19 | Intercellular adhesion molecule 1 | ICAM1 | P05362 |
| P_20 | Interleukin-1 alpha | IL1A | P01583 |
| P_21 | Interleukin-1 beta | IL1B | P01584 |
| P_22 | Interleukin-10 | IL10 | P22301 |
| P_23 | Interleukin-2 | IL2 | P60568 |
| P_24 | Interleukin-6 | IL6 | P05231 |
| P_25 | Interleukin-8 | IL8 | P10145 |
| P_26 | Mitogen-activated protein kinase 14 | MAPK14 | Q16539 |
| P_27 | Mitogen-activated protein kinase 8 | MAPK8 | P45983 |
| P_28 | Muscarinic acetylcholine receptor M1 | CHRM1 | P11229 |
| P_29 | NF-kappa-B inhibitor alpha | NFKBIA | P25963 |
| P_30 | Nitric-oxide synthase, endothelial | NOS3 | P29474 |
| P_31 | Ornithine decarboxylase | ODC1 | P11926 |
| P_32 | Osteopontin | SPP1 | P10451 |
| P_33 | Peroxisome proliferator-activated receptor alpha | PPARA | Q07869 |
| P_34 | Peroxisome proliferator-activated receptor delta | PPARD | Q03181 |
| P_35 | Peroxisome proliferator-activated receptor gamma | PPARG | P37231 |
| P_36 | Phospholipase A2 | PLA2G1B | Q9BS22 |
| P_37 | Poly [ADP-ribose] polymerase 1 | PARP1 | P09874 |
| P_38 | Prostaglandin G/H synthase 1 | COX1 | P23219 |
| P_39 | Prostaglandin G/H synthase 2 | COX2 | P35354 |
| P_40 | Signal transducer and activator of transcription 1-alpha/beta | STAT1 | P42224 |
| P_41 | Transcription factor AP-1 | JUN | P05412 |
| P_42 | Transcription factor p65 | RELA | Q96F54 |
| P_43 | Tumor necrosis factor | TNF | P01375 |
2.3. Network Construction and Analysis
2.3.1. Anti-Inflammatory D-T Network for Western Medicines
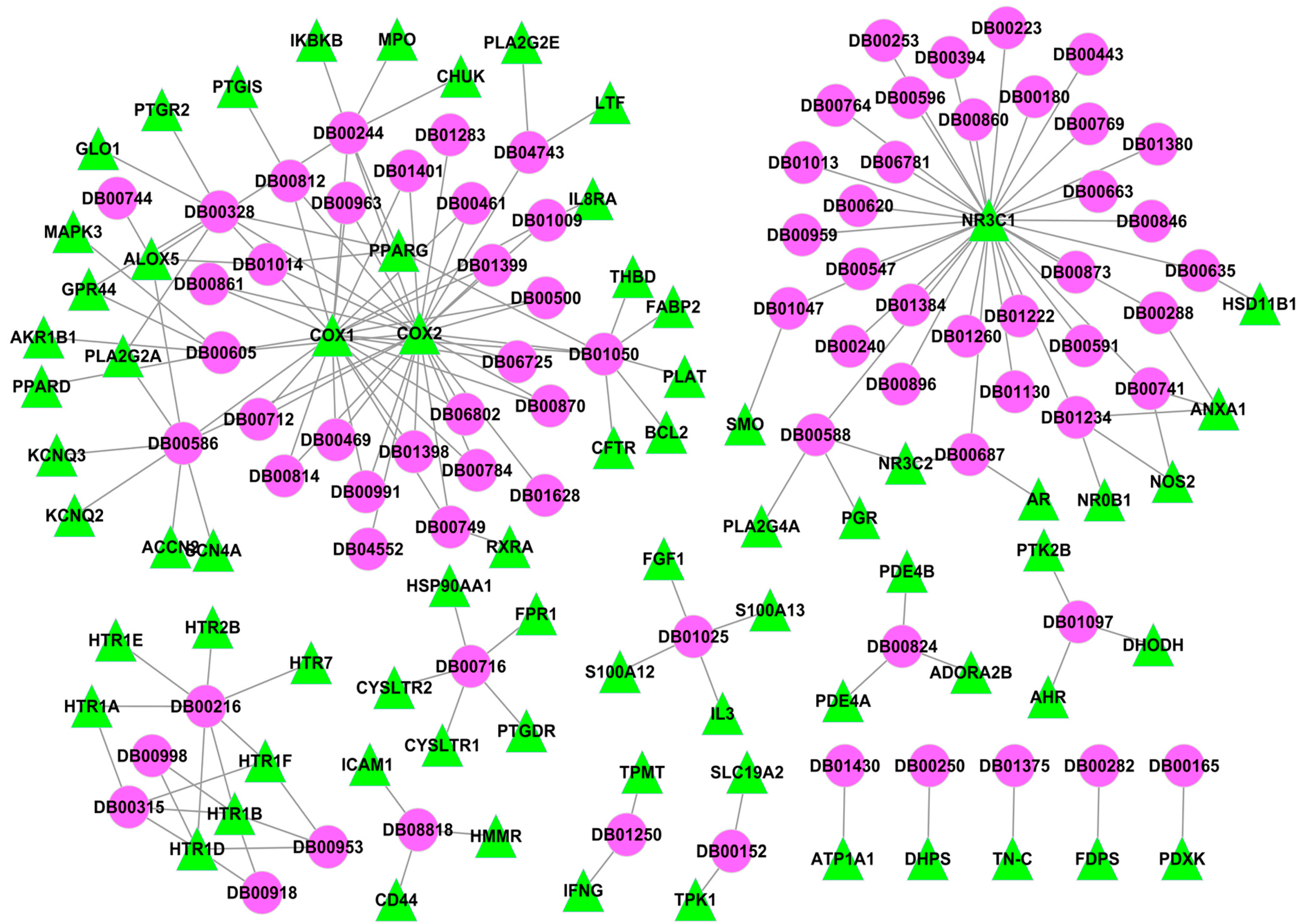
2.3.2. Anti-Inflammatory I-T Network for Folium Eriobotryae

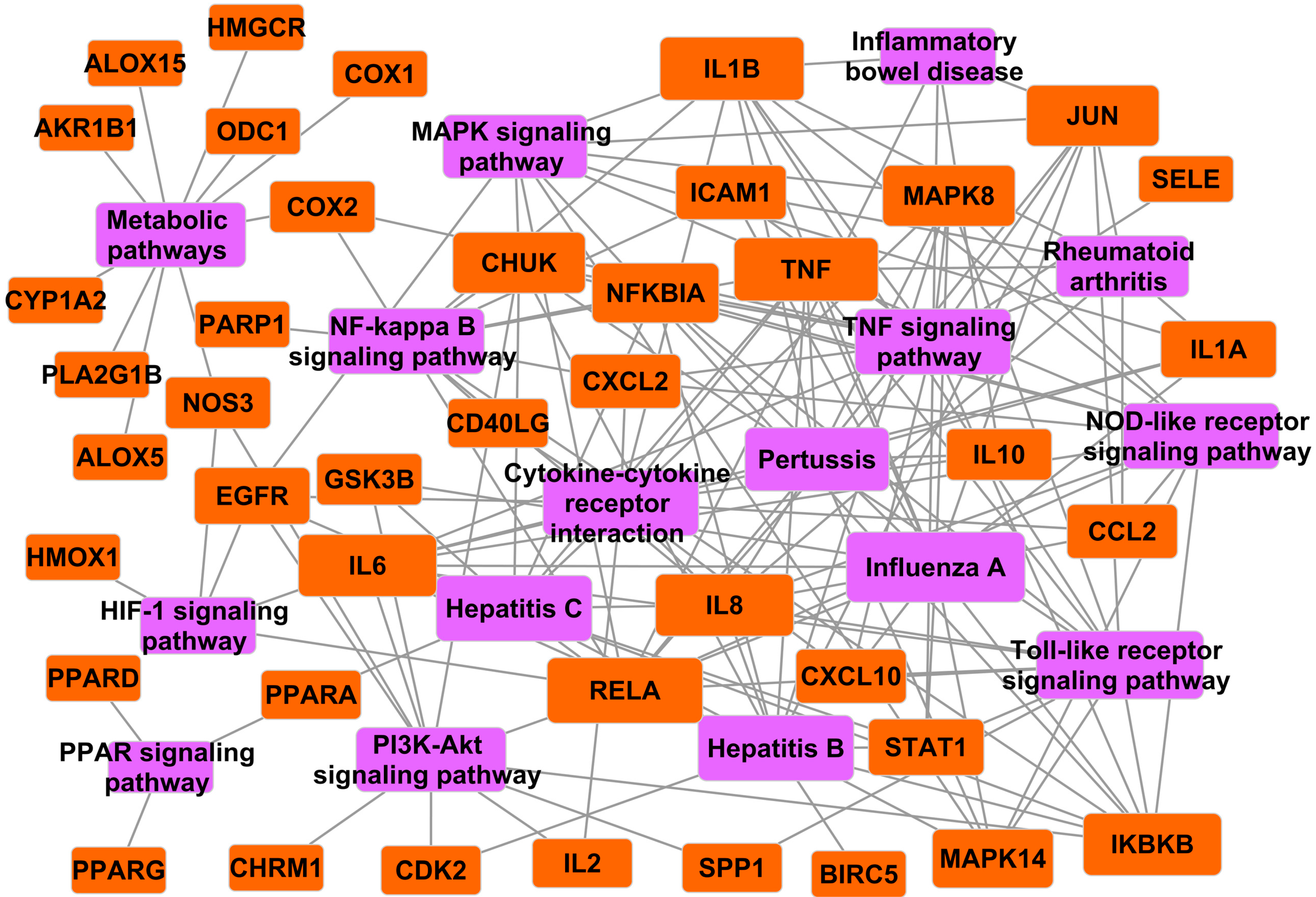
2.3.3. Target-Pathway Network of Folium Eriobotryae
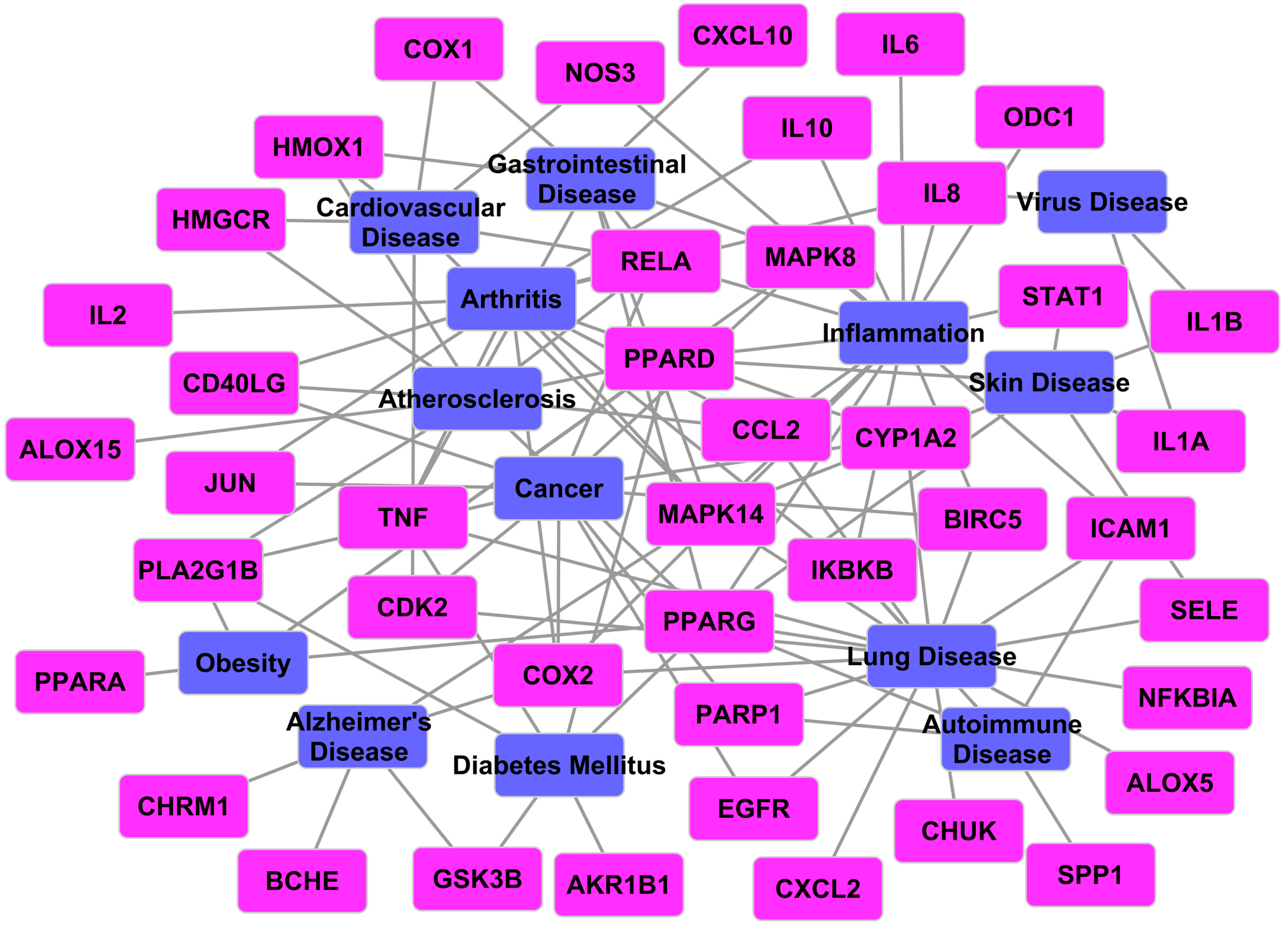
2.3.4. Target-Disease Network
| Actions | References (PMID) |
|---|---|
| Anti-inflammation | Banno N, et al. [38]; Huang Y, et al. [74]; Uto T, et al. [75]; Ge JF, et al. [73]; Kim SH, et al. [76]; Shimizu M, et al. [31]; Choi YG, et al. [77]; Cha DS, et al. [78] |
| Anti-cancer | Banno N, et al. [38]; Ito H, et al. [61]; Ito H, et al. [79]; Kim SH, et al. [80] |
| Treatment of cardiovascular diseases | Not proved |
| Treating lung diseases | Ge JF, et al. [73]; Lee CH, et al. [18] |
| Anti-diabetes | Li WL, et al. [71]; Lü H, et al. [70]; Chen J, et al. [81]; Qa'dan F, et al. [82]; De Tommasi N, et al. [83]; Chen J, et al. [72]; Tanaka K [84]; Noreen W, et al. [85]; Gumy C, et al. [86]; Kim SH, et al. [80]; Lü H, et al. [87]; Chen J, et al. [72] |
| Treating skin diseases | Shimizu M, et al. [72]; De Tommasi N, et al. [88] |
| Antirheumatic activity | Not proved |
| Neuroprotective effects | Kim MJ, et al. [89] |
| Treatment of gastroenteric disorders | Ito H, et al. [61] |
| Anti-atherosclerosis | Not proved |
| Treatment of autoimmune disease | Not proved |
| Antiviral effect | N De Tommasi, et al. [88] |
| Anti-obesity | Not proved |
3. Materials and Methods
3.1. Database Establishment
3.2. Pharmacokinetic Prediction
3.3. Target Identification
3.4. Network Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tracey, K.J. The inflammatory reflex. Nature 2002, 420, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Crofford, L.J. Use of NSAIDs in treating patients with arthritis. Arthritis Res. Ther. 2013, 15. [Google Scholar] [CrossRef]
- Li, R.W.; Myers, S.P.; Leach, D.N.; Lin, G.D.; Leach, G. A cross-cultural study: Anti-inflammatory activity of Australian and Chinese plants. J. Ethnopharmacol. 2003, 85, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Martinez Gonzalez, J.; Badimon, L. Mechanisms underlying the cardiovascular effects of COX-inhibition: Benefits and risks. Curr. Pharm. Des. 2007, 13, 2215–2227. [Google Scholar]
- Gilroy, D.W.; Lawrence, T.; Perretti, M.; Rossi, A.G. Inflammatory resolution: New opportunities for drug discovery. Nat. Rev. Drug Discov. 2004, 3, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Wang, M.; Cheng, Y.; FitzGerald, G.A. Cardiovascular hazard and non-steroidal anti-inflammatory drugs. Curr. Opin. Pharmacol. 2005, 5, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M. Harmless herbs? A review of the recent literature. Am. J. Med. 1998, 104, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.C.; Chen, C.F.; Chiou, W.F. Andrographolide prevents oxygen radical production by human neutrophils: Possible mechanism(s) involved in its anti-inflammatory effect. Br. J. Pharmacol. 2002, 135, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Sangwan, N.S.; Sangwan, R.S. Andrographis paniculata (Kalmegh): A review. Pharmacog. Rev. 2007, 1, 283–298. [Google Scholar]
- Lee, K.C.; Chang, H.H.; Chung, Y.H.; Lee, T.Y. Andrographolide acts as an anti-inflammatory agent in LPS-stimulated RAW264.7 macrophages by inhibiting STAT3-mediated suppression of the NF-κB pathway. J. Ethnopharmacol. 2011, 135, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Li, H.J.; Chen, J.; Guo, C.W.; Li, P. Rapid and simple method for screening of natural antioxidants from Chinese herb Flos Lonicerae japonicae by DPPH-HPLC-DAD-TOF/MS. J. Sep. Sci. 2008, 31, 3519–3526. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Pan, H.; Li, M.; Miao, X.; Ding, H. Lonicera japonica Thunb.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2011, 138, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Kang, O.H.; Choi, J.G.; Lee, J.H.; Kwon, D.Y. Luteolin isolated from the flowers of Lonicera japonica suppresses inflammatory mediator release by blocking NF-κB and MAPKs activation pathways in HMC-1 cells. Molecules 2010, 15, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Qiusheng, Z.; Xiling, S.; Gang, L.; Meng, S.; Changhai, W. Protective effects of luteolin-7-glucoside against liver injury caused by carbon tetrachloride in rats. Pharmazie 2004, 59, 286–288. [Google Scholar] [PubMed]
- Ito, H.; Kobayashi, E.; Li, S.H.; Hatano, T.; Sugita, D.; Kubo, N.; Shimura, S.; Itoh, Y.; Yoshida, T. Megastigmane glycosides and an acylated triterpenoid from Eriobotrya japonica. J. Nat. Prod. 2001, 64, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Fu, W.; Zheng, Q.S.; Ju, B.; Xu, B. Antioxidative and cardioprotective activities of total flavonoids from Moldavian balm (Dracocephalum moldavica L.). In Proceedings of the 2010 International Conference on Bioinformatics and Biomedical Technology (ICBBT), Chengdu, China, 16–18 April 2010.
- Li, Y.L.; Yang, X.W.; Li, S.M.; Shen, Y.H.; Zeng, H.W.; Liu, X.H.; Tang, J.; Zhang, W.D. Terpenoid constituents of Abies chensiensis with potential anti-inflammatory activity. J. Nat. Prod. 2009, 72, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Wu, S.L.; Chen, J.C.; Li, C.C.; Lo, H.Y.; Cheng, W.Y.; Lin, J.G.; Chang, Y.H.; Hsiang, C.Y.; Ho, T.Y. Eriobotrya japonica leaf and its triterpenes inhibited lipopolysaccharide-induced cytokines and inducible enzyme production via the nuclear factor-kappaB signaling pathway in lung epithelial cells. Am. J. Chin. Med. 2008, 36, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007, 2007, 45673. [Google Scholar] [CrossRef]
- Yang, M.; Cheng, C.; Yang, J.; Guo, D.A. Metabolite profiling and characterization for medicinal herbal remedies. Curr. Drug Metable 2012, 13, 535–557. [Google Scholar] [CrossRef]
- Lu, A.P.; Bian, Z.X.; Chen, K.J. Bridging the traditional Chinese medicine pattern classification and biomedical disease diagnosis with systems biology. Chin. J. Integr. Med. 2012, 18, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.I.; Iyengar, R. Network analyses in systems pharmacology. Bioinformatics 2009, 25, 2466–2472. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Liu, C.; Jiang, J.; Lu, W.; Li, W.; Liu, G.; Zhou, W.; Huang, J.; Tang, Y. Prediction of drug-target interactions and drug repositioning via network-based inference. PLoS Comput. Biol. 2012, 8, e1002503. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, B.; Zhang, N. Network target for screening synergistic drug combinations with application to traditional Chinese medicine. BMC Syst. Biol. 2011, 5. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.; Lowes, S.; Hirst, B.H. The ABCs of drug transport in intestine and liver: Efflux proteins limiting drug absorption and bioavailability. Eur. J. Pharm. Sci. 2004, 21, 25–51. [Google Scholar] [CrossRef] [PubMed]
- Walters, W.P.; Murcko, M.A. Prediction of 'drug-likeness'. Adv. Drug Deliv. Rev. 2002, 54, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Sun, H.; Liu, J.; Cheng, K.; Zhang, P.; Zhang, L.; Hao, J.; Zhang, L.; Ni, P.; Zographos, S.E. Naturally occurring pentacyclic triterpenes as inhibitors of glycogen phosphorylase: Synthesis, structure-activity relationships, and X-ray crystallographic studies. J. Med. Chem. 2008, 51, 3540–3554. [Google Scholar] [CrossRef] [PubMed]
- Price, K.; Johnson, I.; Fenwick, G.; Malinow, M. The chemistry and biological significance of saponins in foods and feedingstuffs. Crit. Rev. Food Sci. Nutr. 1987, 26, 27–135. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.B.; Singh, S.; Bani, S.; Gupta, B.D.; Banerjee, S.K. Anti-inflammatory activity of oleanolic acid in rats and mice. J. Pharm. Pharmacol. 1992, 44, 456–458. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Fukumura, H.; Tsuji, H.; Tanaami, S.; Hayashi, T.; Morita, N. Anti-inflammatory constituents of topically applied crude drugs. I. Constituents and anti-inflammatory effect of Eriobotrya japonica Lindl. Chem. Pharm. Bull. 1986, 34, 2614–2617. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.M.; Cai, S.Q.; Cui, J.R.; Wang, R.Q.; Tu, P.F.; Hattori, M.; Daneshtalab, M. The cytotoxic activity of ursolic acid derivatives. Eur. J. Med. Chem. 2005, 40, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Muegge, I. Selection criteria for drug-like compounds. Med. Res. Rev. 2003, 23, 302–321. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, M.D.; Almeida, M.C.; Lopes, N.P.; de Souza, G.E. Evaluation of the anti-inflammatory, analgesic and antipyretic activities of the natural polyphenol chlorogenic acid. Biol. Pharm. Bull. 2006, 29, 2236–2240. [Google Scholar] [CrossRef] [PubMed]
- Schinella, G.; Aquila, S.; Dade, M.; Giner, R.; Recio Mdel, C.; Spegazzini, E.; de Buschiazzo, P.; Tournier, H.; Rios, J.L. Anti-inflammatory and apoptotic activities of pomolic acid isolated from Cecropia pachystachya. Planta Med. 2008, 74, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; de Las Heras, B.; Villar, A. Anti-inflammatory and immunomodulating properties of a sterol fraction from Sideritis foetens CLEM. Biol. Pharm. Bull. 2001, 24, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.J. Effect of olive oil minor components on oxidative stress and arachidonic acid mobilization and metabolism by macrophages RAW 264.7. Free Radic. Biol. Med. 2003, 35, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Banno, N.; Akihisa, T.; Tokuda, H.; Yasukawa, K.; Taguchi, Y.; Akazawa, H.; Ukiya, M.; Kimura, Y.; Suzuki, T.; Nishino, H. Anti-inflammatory and antitumor-promoting effects of the triterpene acids from the leaves of Eriobotrya japonica. Biol. Pharm. Bull. 2005, 28, 1995–1999. [Google Scholar] [CrossRef] [PubMed]
- Metodiewa, D.; Kochman, A.; Karolczak, S. Evidence for antiradical and antioxidant properties of four biologically active N,N-Diethylaminoethyl ethers of flavaone oximes: A comparison with natural polyphenolic flavonoid rutin action. IUBMB Life 1997, 41, 1067–1075. [Google Scholar] [CrossRef]
- Yang, L.; Sun, Z.; Zu, Y.; Zhao, C.; Sun, X.; Zhang, Z.; Zhang, L. Physicochemical properties and oral bioavailability of ursolic acid nanoparticles using supercritical anti-solvent (SAS) process. Food Chem. 2012, 132, 319–325. [Google Scholar] [CrossRef]
- Ju, J.H.; Zhou, L.; Zheng, R.X.; Wang, C.L.; Yang, J.S. Determination of ursolic acid in Eriobotrya japonica by HPLC analysis. China Pharm. J. 2003, 38, 657–658. [Google Scholar]
- Gonthier, M.P.; Verny, M.A.; Besson, C.; Remesy, C.; Scalbert, A. Chlorogenic acid bioavailability largely depends on its metabolism by the gut microflora in rats. J. Nutr. 2003, 133, 1853–1859. [Google Scholar] [PubMed]
- Shan, J.; Fu, J.; Zhao, Z.; Kong, X.; Huang, H.; Luo, L.; Yin, Z. Chlorogenic acid inhibits lipopolysaccharide-induced cyclooxygenase-2 expression in RAW264. 7 cells through suppressing NF-κB and JNK/AP-1 activation. Int. Immunopharmacol. 2009, 9, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Kobayashi, S. Transepithelial transport of chlorogenic acid, caffeic acid, and their colonic metabolites in intestinal caco-2 cell monolayers. J. Agric. Food Chem. 2004, 52, 2518–2526. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Chao, P.C.; Hsu, C.C.; Yin, M.C. Anti-inflammatory and anti-coagulatory activities of caffeic acid and ellagic acid in cardiac tissue of diabetic mice. Nutr. Metable 2009, 6. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [PubMed]
- Li, P.; Qi, L.W.; Liu, E.H.; Zhou, J.L.; Wen, X.D. Analysis of Chinese herbal medicines with holistic approaches and integrated evaluation models. Trac-Trend Anal. Chem. 2008, 27, 66–77. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Chen, X.; Pan, Y.; Zhang, S.; Wang, Y. Systems pharmacology dissection of multi-scale mechanisms of action for herbal medicines in stroke treatment and prevention. PLoS One 2014, 9, e102506. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zheng, C.; Li, Y.; Wang, Y.; Lu, A.; Yang, L. Systems pharmacology in drug discovery and therapeutic insight for herbal medicines. Brief. Bioinf. 2013, 15, 710–733. [Google Scholar] [CrossRef]
- Vane, J.; Botting, R. New insights into the mode of action of anti-inflammatory drugs. Inflamm. Res. 1995, 44, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gerstenfeld, L.C.; Thiede, M.; Seibert, K.; Mielke, C.; Phippard, D.; Svagr, B.; Cullinane, D.; Einhorn, T.A. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J. Orthop. Res. 2003, 21, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Fonseca, L.M. The benefits of the multi-target approach in drug design and discovery. Bioorg. Med. Chem. 2006, 14, 896–897. [Google Scholar] [CrossRef] [PubMed]
- Albert, R.; Jeong, H.; Barabási, A.L. Error and attack tolerance of complex networks. Nature 2000, 406, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, M.A.; Goh, K.I.; Cusick, M.E.; Barabási, A.L.; Vidal, M. Drug-target network. Nat. Biotechnol. 2007, 25, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Geraets, L.; Moonen, H.J.; Brauers, K.; Wouters, E.F.; Bast, A.; Hageman, G.J. Dietary flavones and flavonoles are inhibitors of poly(ADP-ribose)polymerase-1 in pulmonary epithelial cells. J. Nutr. 2007, 137, 2190–2195. [Google Scholar] [PubMed]
- Fang, X.K.; Gao, J.; Zhu, D.N. Kaempferol and quercetin isolated from Euonymus alatus improve glucose uptake of 3T3-L1 cells without adipogenesis activity. Life Sci. 2008, 82, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.P.; Akiyama, T.E.; Meinke, P.T. PPARs: Therapeutic targets for metabolic disease. Trends Pharmacol. Sci. 2005, 26, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Cuzzocrea, S. TNF-α as a therapeutic target in inflammatory diseases, ischemia-reperfusion injury and trauma. Curr. Med. Chem. 2009, 16, 3152–3167. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Boehm, J.; Lee, J.C. p38 MAP kinases: Key signalling molecules as therapeutic targets for inflammatory diseases. Nat. Rev. Drug Discov. 2003, 2, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Kobayashi, E.; Takamatsu, Y.; Li, S.H.; Hatano, T.; Sakagami, H.; Kusama, K.; Satoh, K.; Sugita, D.; Shimura, S.; Itoh, Y.; Yoshida, T. Polyphenols from Eriobotrya japonica and their cytotoxicity against human oral tumor cell lines. Chem. Pharm. Bull. 2000, 48, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Haegeman, G. Inhibition of signal transduction pathways involved in inflammation. Eur. Respir. J. Suppl. 2003, 44, 16s–19s. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S. NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Balough, K.; McCubbin, M.; Weinberger, M.; Smits, W.; Ahrens, R.; Fick, R. The relationship between infection and inflammation in the early stages of lung disease from cystic fibrosis. Pediatr. Pulmonol. 1995, 20, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Erdman, S.E.; Poutahidis, T. Cancer inflammation and regulatory T cells. Int. J. Cancer 2010, 127, 768–779. [Google Scholar] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Cha, D.S.; Shin, T.Y.; Eun, J.S.; Kim, D.K.; Jeon, H. Anti-metastatic properties of the leaves of Eriobotrya japonica. Arch. Pharm. Res. 2011, 34, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; You, M.; Rhuy, D.; Kim, Y.; Baek, H.; Kim, H. Loquat (Eriobotrya japonica) extracts suppress the adhesion, migration and invasion of human breast cancer cell line. Nutr. Res. Pract. 2009, 3, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Uto, T.; Sakamoto, A.; Tung, N.H.; Fujiki, T.; Kishihara, K.; Oiso, S.; Kariyazono, H.; Morinaga, O.; Shoyama, Y. Anti-proliferative activities and apoptosis induction by triterpenes derived from Eriobotrya japonica in human leukemia cell lines. Int. J. Mol. Sci. 2013, 14, 4106–4120. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Chen, J.; Li, W.L.; Ren, B.R.; Wu, J.L.; Kang, H.Y.; Zhang, H.Q.; Adams, A.; de Kimpe, N. Hypoglycemic and hypolipidemic effects of the total triterpene acid fraction from Folium Eriobotryae. J. Ethnopharmacol. 2009, 122, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Li, W.L.; Wu, J.L.; Ren, B.R.; Chen, J.; Lu, C.G. Pharmacological studies on anti-hyperglycemic effect of Folium Eriobotryae. Am. J. Chin. Med. 2007, 35, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, W.L.; Wu, J.L.; Ren, B.R.; Zhang, H.Q. Hypoglycemic effects of a sesquiterpene glycoside isolated from leaves of loquat (Eriobotrya japonica (Thunb.) Lindl.). Phytomedicine 2008, 15, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.F.; Wang, T.Y.; Zhao, B.; Lv, X.W.; Jin, Y.; Peng, L.; Yu, S.C.; Li, J. Anti-inflammatory effect of triterpenoic acids of Eriobotrya japonica (Thunb.) Lindl. leaf on rat model of chronic bronchitis. Am. J. Chin. Med. 2009, 37, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, J.; Cao, Q.; Yu, S.C.; Lv, X.W.; Jin, Y.; Zhang, L.; Zou, Y.H.; Ge, J.F. Anti-oxidative effect of triterpene acids of Eriobotrya japonica (Thunb.) Lindl. leaf in chronic bronchitis rats. Life Sci. 2006, 78, 2749–2757. [Google Scholar] [CrossRef] [PubMed]
- Uto, T.; Suangkaew, N.; Morinaga, O.; Kariyazono, H.; Oiso, S.; Shoyama, Y. Eriobotryae folium extract suppresses LPS-induced iNOS and COX-2 expression by inhibition of NF-kappaB and MAPK activation in murine macrophages. Am. J. Chin. Med. 2010, 38, 985–94. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Shin, T.Y. Anti-inflammatory effect of leaves of Eriobotrya japonica correlating with attenuation of p38 MAPK, ERK, and NF-kappaB activation in mast cells. Toxicol. In Vitro 2009, 23, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.G.; Seok, Y.H.; Yeo, S.; Jeong, M.Y.; Lim, S. Protective changes of inflammation-related gene expression by the leaves of Eriobotrya japonica in the LPS-stimulated human gingival fibroblast: microarray analysis. J. Ethnopharmacol. 2011, 135, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Cha, D.S.; Eun, J.S.; Jeon, H. Anti-inflammatory and antinociceptive properties of the leaves of Eriobotrya japonica. J. Ethnopharmacol. 2011, 134, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Kobayashi, E.; Li, S.H.; Hatano, T.; Sugita, D.; Kubo, N.; Shimura, S.; Itoh, Y.; Tokuda, H.; Nishino, H.; Yoshida, T. Antitumor activity of compounds isolated from leaves of Eriobotrya japonica. J. Agric. Food Chem. 2002, 50, 2400–2403. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kwon, Y.E.; Park, W.H.; Jeon, H.; Shin, T.Y. Effect of leaves of Eriobotrya japonica on anaphylactic allergic reaction and production of tumor necrosis factor-alpha. Immunopharmacol. Immunotoxicol. 2009, 31, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, W.L.; Wu, J.L.; Ren, B.R.; Zhang, H.Q. Euscaphic acid, a new hypoglycemic natural product from Folium Eriobotryae. Pharmazie 2008, 63, 765–767. [Google Scholar] [PubMed]
- Qa'dan, F.; Verspohl, E.J.; Nahrstedt, A.; Petereit, F.; Matalka, K.Z. Cinchonain Ib isolated from Eriobotrya japonica induces insulin secretion in vitro and in vivo. J. Ethnopharmacol. 2009, 124, 224–227. [Google Scholar] [CrossRef] [PubMed]
- De Tommasi, N.; De Simone, F.; Cirino, G.; Cicala, C.; Pizza, C. Hypoglycemic effects of sesquiterpene glycosides and polyhydroxylated triterpenoids of Eriobotrya japonica. Planta Med. 1991, 57, 414–416. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Nishizono, S.; Makino, N.; Tamaru, S.; Terai, O.; Ikeda, I. Hypoglycemic activity of Eriobotrya japonica seeds in type 2 diabetic rats and mice. Biosci. Biotechnol. Biochem. 2008, 72, 686–693. [Google Scholar] [CrossRef]
- Noreen, W.; Wadood, A.; Hidayat, H.K.; Wahid, S.A. Effect of Eriobotrya japonica on blood glucose levels of normal and alloxan-diabetic rabbits. Planta Med. 1988, 54, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Gumy, C.; Thurnbichler, C.; Aubry, E.M.; Balazs, Z.; Pfisterer, P.; Baumgartner, L.; Stuppner, H.; Odermatt, A.; Rollinger, J.M. Inhibition of 11beta-hydroxysteroid dehydrogenase type 1 by plant extracts used as traditional antidiabetic medicines. Fitoterapia 2009, 80, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Chen, J.; Li, W.L.; Ren, B.R.; Wu, J.L.; Zhang, H.Q. Hypoglycemic effect of the total flavonoid fraction from folium Eriobotryae. Phytomedicine 2009, 16, 967–971. [Google Scholar] [CrossRef] [PubMed]
- De Tommasi, N.; De Simone, F.; Pizza, C.; Mahmood, N.; Moore, P.S.; Conti, C.; Orsi, N.; Stein, M.L. Constituents of Eriobotrya japonica. A study of their antiviral properties. J. Nat. Prod. 1992, 55, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Lee, J.; Seong, A.R.; Lee, Y.H.; Kim, Y.J.; Baek, H.Y.; Jun, W.J.; Yoon, H.G. Neuroprotective effects of Eriobotrya japonica against beta-amyloid-induced oxidative stress and memory impairment. Food Chem. Toxicol. 2011, 49, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, X.; Wang, J.; Yu, H.; Wang, X.; Yang, H.; Xu, H.; Tang, S.; Li, Y.; Yang, L. A System-level investigation into the mechanisms of Chinese traditional medicine: Compound danshen formula for cardiovascular disease treatment. PLoS One 2012, 7, e43918. [Google Scholar] [CrossRef] [PubMed]
- Van de Waterbeemd, H.; Gifford, E. ADMET in silico modelling: Towards prediction paradise? Nat. Rev. Drug Discov. 2003, 2, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J.; Zhou, W.; Wang, Y.; Yang, L. Systems approaches and polypharmacology for drug discovery from herbal medicines: An example using licorice. J. Ethnopharmacol. 2013, 146, 773–793. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, W.; Huang, C.; Li, Y.; Yu, H.; Wang, Y.; Duan, J.; Ling, Y. A novel chemometric method for the prediction of human oral bioavailability. Int. J. Mol. Sci. 2012, 13, 6964–6982. [Google Scholar] [CrossRef] [PubMed]
- Artursson, P.; Karlsson, J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res. Commun. 1991, 175, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Ano, R.; Kimura, Y.; Shima, M.; Matsuno, R.; Ueno, T.; Akamatsu, M. Relationships between structure and high-throughput screening permeability of peptide derivatives and related compounds with artificial membranes: Application to prediction of Caco-2 cell permeability. Bioorg. Med. Chem. 2004, 12, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.X.; Zhang, C.H.; Zhao, W.N.; Yu, Q.S. QSPR study on the permeability of drugs across Caco-2 monolayer. J. Zhejiang Univ. 2009, 3, 304–308. [Google Scholar]
- Zhu, F.; Shi, Z.; Qin, C.; Tao, L.; Liu, X.; Xu, F.; Zhang, L.; Song, Y.; Liu, X.; Zhang, J. Therapeutic target database update 2012: A resource for facilitating target-oriented drug discovery. Nucleic Acids Res. 2012, 40, D1128–D1136. [Google Scholar] [CrossRef] [PubMed]
- Knox, C.; Law, V.; Jewison, T.; Liu, P.; Ly, S.; Frolkis, A.; Pon, A.; Banco, K.; Mak, C.; Neveu, V. DrugBank 3.0: A comprehensive resource for ‘omics’ research on drugs. Nucleic Acids Res. 2011, 39, D1035–D1041. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, J.; Xu, X.; Li, Y.; Zhao, H.; Fang, Y.; Li, X.; Zhou, W.; Wang, W.; Wang, Y. A systematic prediction of multiple drug-target interactions from chemical, genomic, and pharmacological data. PLoS One 2012, 7, e37608. [Google Scholar] [PubMed]
- Whirl-Carrillo, M.; McDonagh, E.; Hebert, J.; Gong, L.; Sangkuhl, K.; Thorn, C.; Altman, R.; Klein, T. Pharmacogenomics knowledge for personalized medicine. Clin. Pharmacol. Ther. 2012, 92, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Schwede, T.; Kopp, J.; Guex, N.; Peitsch, M.C. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003, 31, 3381–3385. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, Y.; Chen, S.-S.; Zhang, L.; Wang, J.; Yang, Y.; Zhang, S.; Pan, Y.; Wang, Y.; Yang, L. Systems Pharmacology Dissection of the Anti-Inflammatory Mechanism for the Medicinal Herb Folium Eriobotryae. Int. J. Mol. Sci. 2015, 16, 2913-2941. https://doi.org/10.3390/ijms16022913
Zhang J, Li Y, Chen S-S, Zhang L, Wang J, Yang Y, Zhang S, Pan Y, Wang Y, Yang L. Systems Pharmacology Dissection of the Anti-Inflammatory Mechanism for the Medicinal Herb Folium Eriobotryae. International Journal of Molecular Sciences. 2015; 16(2):2913-2941. https://doi.org/10.3390/ijms16022913
Chicago/Turabian StyleZhang, Jingxiao, Yan Li, Su-Shing Chen, Lilei Zhang, Jinghui Wang, Yinfeng Yang, Shuwei Zhang, Yanqiu Pan, Yonghua Wang, and Ling Yang. 2015. "Systems Pharmacology Dissection of the Anti-Inflammatory Mechanism for the Medicinal Herb Folium Eriobotryae" International Journal of Molecular Sciences 16, no. 2: 2913-2941. https://doi.org/10.3390/ijms16022913





