Combined Toxic Effects of Heavy Metals and Antibiotics on a Pseudomonas fluorescens Strain ZY2 Isolated from Swine Wastewater
Abstract
:1. Introduction
2. Results and Discussion
2.1. Heavy Metal Resistance and Antibiotic Resistance
| Antibiotic (µg/disc) | CLSI Category | P. fluorescens ZY2 | |||
|---|---|---|---|---|---|
| Sensitive (mm) | Intermediary (mm) | Resistant (mm) | Mean of Inhibitory Zones (mm) | Sensitive Case | |
| Cefradine (30) | ≥18 | 15~17 | ≤14 | 15.28 ± 0.26 | Intermediate |
| Norfloxacin (10) | ≥17 | 13~16 | ≤12 | 14.62 ± 0.19 | Intermediate |
| Amoxicillin (10) | ≥18 | 14~17 | ≤13 | 12.70 ± 0.63 | Resistant |
| Tetracycline (30) | ≥15 | 12~14 | ≤11 | 0.00 ± 0.00 | Resistant |
2.2. Results of the Binary Exposure
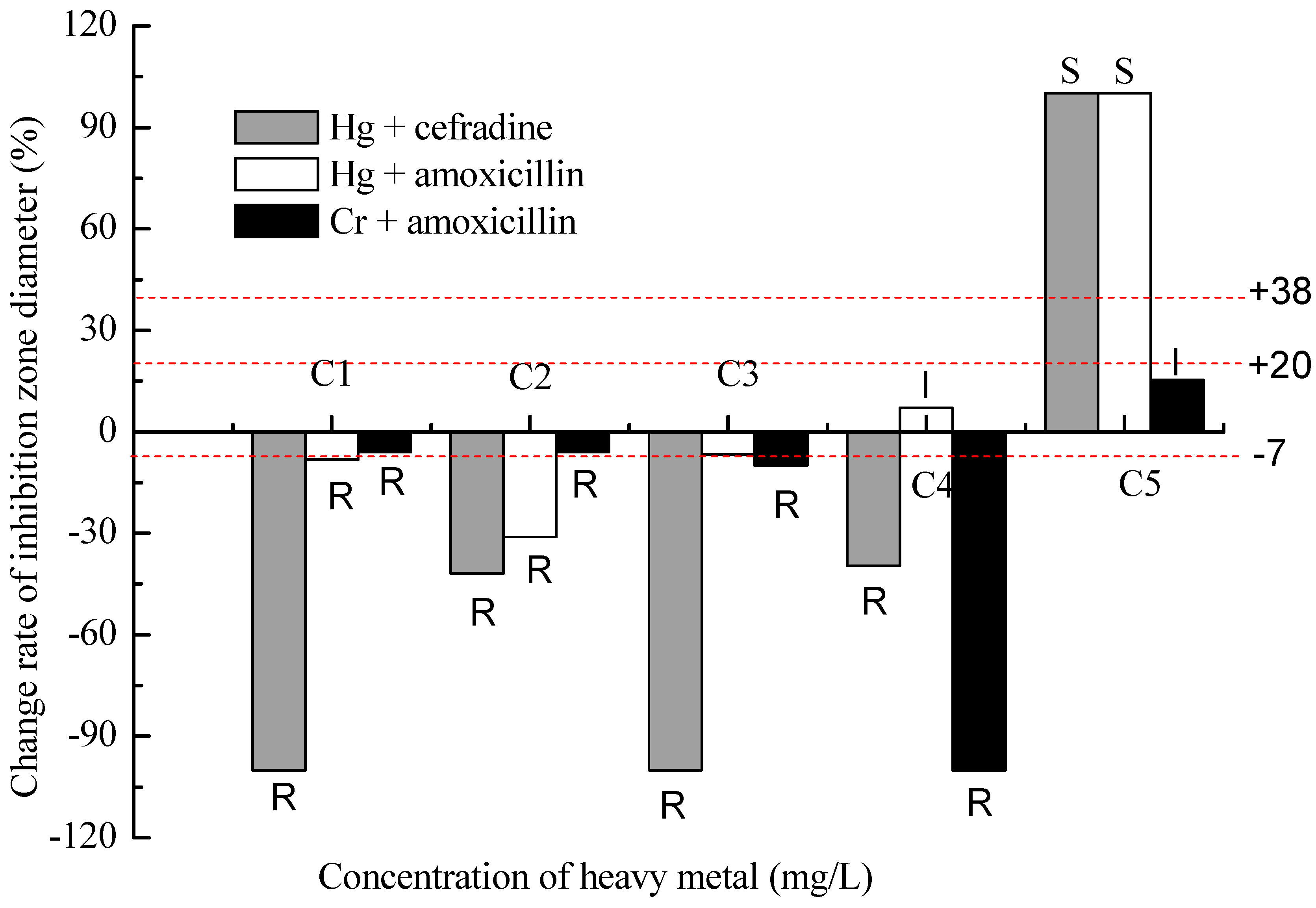

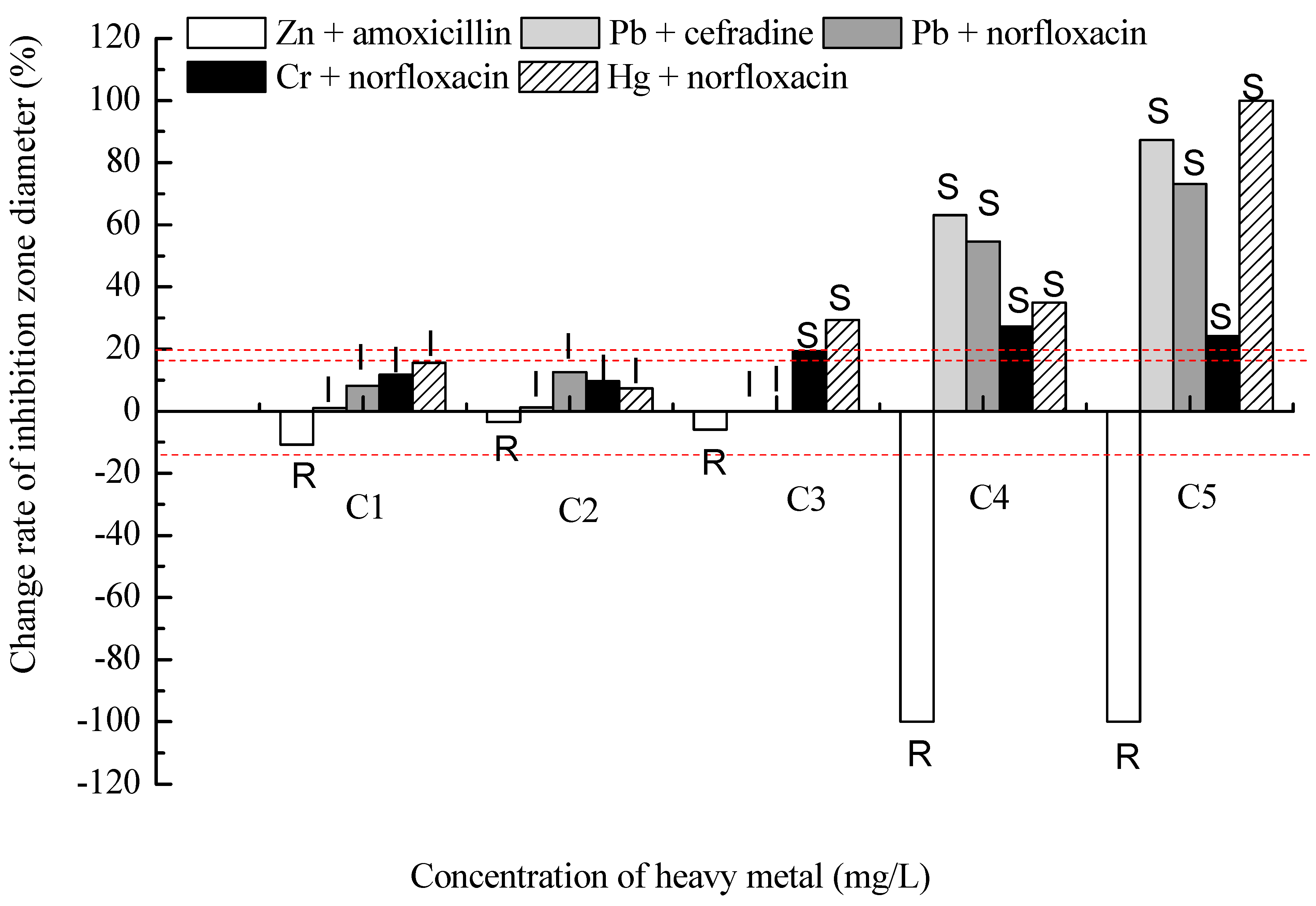

3. Experimental Section
3.1. Characteristics of the Strain
3.2. Determination of the Resistance to Heavy Metals
3.3. Antimicrobial Susceptibility Testing
3.4. Binary Exposure Experiment
3.5. Statistics
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhu, Y.G.; Johnson, T.A.; Su, J.; Qiao, M.; Guo, G.X.; Stedtfeld, R.D.; Hashsham, S.A.; Tiedje, J.M. Diverse and abundant antibiotic resistance genes in Chinese swine farms. Proc. Natl. Acad. Sci. USA 2013, 110, 3435–3440. [Google Scholar]
- Chiang, Y.W.; Santos, R.M.; Ghyselbrecht, K.; Cappuyns, V.; Martens, J.A.; Swennen, R.; van Gerven, T.; Meesschaert, B. Strategic selection of an optimal sorbent mixture for in situ remediation of heavy metal contaminated sediments: Framework and case study. J. Environ. Manag. 2012, 105, 1–11. [Google Scholar]
- Grosa, M.; Rodríguez-Mozaz, S.; Barceló, D. Rapid analysis of multiclass antibiotic residues and some of their metabolites in hospital, urban wastewater and river water by ultra-high-performance liquid chromatography coupled to quadrupole-linear ion trap tandem mass spectrometry. J. Chromatogr. A 2013, 1292, 173–188. [Google Scholar]
- Filali, B.K.; Taoufik, J.; Zeroual, Y.; Dzairi, F.Z.; Talbi, M.; Blaghen, M. Waste water bacterial isolates resistant to heavy metals and antibiotics. Curr. Microbiol. 2000, 41, 151–156. [Google Scholar]
- Uĝur, A.; Ceylan, Ö. Occurrence of resistance to antibiotics, metals, and plasmids in clinical strains of Staphylococcus spp. Arch. Med. Res. 2003, 34, 130–136. [Google Scholar]
- Wang, Y.; Leung, P.C.; Qian, P.; Gu, J.D. Antibiotic resistance and plasmid profile of environmental isolates of Vibrio species from Mai Po Nature Reserve, Hong Kong. Ecotoxicology 2006, 15, 371–378. [Google Scholar]
- Akinbowale, O.L.; Peng, H.; Grant, P.; Barton, M.D. Antibiotic and heavy metal resistance in motile aeromonads and pseudomonads from rainbow trout (Oncorhynchus mykiss) farms in Australia. Int. J. Antimicrob. Agents 2007, 30, 177–182. [Google Scholar]
- Guillemot, D. Antibiotic use in humans and bacterial resistance. Curr. Opin. Microbiol. 1999, 2, 494–498. [Google Scholar]
- Hazen, T.H.; Pan, L.; Gu, J.D.; Sobecky, P.A. The contribution of mobile genetic elements to the evolution and ecology of Vibrios. FEMS Microbiol. Ecol. 2010, 74, 485–499. [Google Scholar]
- Pan, L.; Leung, P.C.; Gu, J.D. A new ColE1-like plasmid group revealed by comparative analysis of the replication proficient fragments of Vibrionaceae plasmids. J. Microbiol. Biotechnol. 2010, 20, 1163–1178. [Google Scholar]
- Martinez, J.L. Antibiotics and antibiotic resistance genes in natural environments. Science 2008, 321, 365–367. [Google Scholar]
- Cheung, K.H.; Gu, J.D. Mechanisms of hexavalent chromium detoxification by bacteria and bioremediation applications. Int. Biodeterior. Biodegrad. 2007, 59, 8–15. [Google Scholar]
- Cheung, K.H.; Gu, J.D. Reduction of chromate (CrO42−) by an enrichment consortium and an isolate of marine sulfate-reducing bacteria. Chemosphere 2003, 52, 1523–1529. [Google Scholar]
- Cheung, K.H.; Gu, J.D. Reduction of chromate (CrO42−) by a Bacillus magnetarium isolated from marine. World J. Microbiol. Biotechnol. 2005, 21, 213–219. [Google Scholar]
- Cheung, K.H.; Lai, H.Y.; Gu, J.D. Membrane-associated hexavalent chromium reductase of Bacillus megaterium TKW3 with induced expression. World J. Microbiol. Biotechnol. 2006, 16, 855–862. [Google Scholar]
- Xu, X.R.; Li, H.B.; Gu, J.D. Reduction of hexavalent chromium by ascorbic acid in aqueous solutions. Chemosphere 2004, 57, 609–613. [Google Scholar]
- Xu, X.R.; Li, H.B.; Gu, J.D. Kinetics of the reduction of chromium (VI) by vitamin C. Environ. Toxicol. Chem. 2005, 24, 1310–1314. [Google Scholar]
- Xu, X.R.; Li, H.B.; Gu, J.D. Simultaneous decontamination of hexavalent chromium and methyl tert-butyl ether by UV/TiO2 process. Chemosphere 2006, 63, 254–260. [Google Scholar]
- Yu, X.; Gu, J.D. Metabolic responses of weeping willows to selenate and selenite. Environ. Sci. Pollut. Res. 2007, 14, 510–517. [Google Scholar]
- Yu, X.Z.; Gu, J.D.; Huang, S.Z. Hexavalent chromium induced stress and metabolic responses in hybrid willows. Ecotoxicology 2007, 16, 299–309. [Google Scholar]
- Yu, X.; Gu, J.D. The role of EDTA in phytoextraction of hexavalent and trivalent chromium by two willow trees. Ecotoxicology 2008, 17, 143–152. [Google Scholar]
- Yu, X.; Gu, J.D.; Xing, L.Q. Differences in uptake and translocation of hexavalent and trivalent chromium by two species of willows. Ecotoxicology 2008, 17, 747–755. [Google Scholar]
- Martinez, J.L. Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ. Pollut. 2009, 157, 2893–2902. [Google Scholar]
- Chudobova, D.; Dostalova, S.; Blazkova, I.; Michalek, P.; Ruttkay-Nedecky, B.; Sklenar, M.; Nejdl, L.; Kudr, J.; Gumulec, J.; Tmejova, K.; et al. Effect of ampicillin, streptomycin, penicillin and tetracycline on metal resistant and non-resistant Staphylococcus aureus. Int. J. Environ. Res. Public Health 2014, 11, 3233–3255. [Google Scholar]
- Kiliç, N.K.; Stensballe, A.; Otzen, D.E.; Dönmez, G. Proteomic changes in response to chromium(VI) toxicity in Pseudomonas aeruginosa. Bioresour. Technol. 2010, 101, 2134–2140. [Google Scholar]
- Abskharon, R.N.N.; Hassan, S.H.A.; Kabir, M.H.; Qadir, S.A.; EI-Rab, S.M.F.G.; Wang, M.H. The role of antioxidants enzymes of E. coli ASU3, a tolerant strain to heavy metals toxicity, in combating oxidative stress of copper. World J. Microbiol. Biotechnol. 2010, 26, 241–247. [Google Scholar]
- Matyar, F.; Akkan, T.; Uçak, Y.; Eraslan, B. Aeromonas and Pseudomonas: Antibiotic and heavy metal resistance species from Iskenderun Bay, Turkey (northeast Mediterranean Sea). Environ. Monit. Assess. 2010, 167, 309–320. [Google Scholar]
- Hölzel, C.S.; Müller, C.; Harms, K.S.; Mikolajewski, S.; Schäfer, S.; Schwaiger, K.; Bauer, J. Heavy metals in liquid pig manure in light of bacterial antimicrobial resistance. Environ. Res. 2012, 113, 21–27. [Google Scholar]
- Franklin, R.C.; Matthew, A.W.; Jeff, A.; Michael, N.D.; George, M.E. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2012. [Google Scholar]
- Máthé, I.; Benedek, T.; Táncsics, A.; Palatinszky, M.; Lányi, S.; Márialigeti, K. Diversity, activity, antibiotic and heavy metal resistance of bacteria from petroleum hydrocarbon contaminated soils located in Harghita County (Romania). Int. Biodeterior. Biodegrad. 2012, 73, 41–49. [Google Scholar]
- Serkan, K.; Kabatas, B.; Icgen, B. Multidrug and heavy metal-resistant Raoultella planticola isolated from surface water. Bull. Environ. Contam. Toxicol. 2013, 91, 177–183. [Google Scholar]
- Giudice, A.L.; Casella, P.; Bruni, V.; Michaud, L. Response of bacterial isolates from Antarctic shallow sediments towards heavy metals, antibiotics and polychlorinated biphenyls. Ecotoxicology 2013, 22, 240–250. [Google Scholar]
- Wu, Y.; Wang, W.X. Accumulation, subcellular distribution and toxicity of inorganic Hg2+ and methyl Hg2+ in marine phytoplankton. Environ. Pollut. 2011, 159, 3097–3105. [Google Scholar]
- Singh, S.K.; Tripathi, V.R.; Jain, R.K.; Vikram, S.; Garg, S.K. An antibiotic, heavy metal resistant and halotolerant Bacillus cereus SIU1 and its thermoalkaline protease. Microbiol. Cell Fact. 2010, 9, 59–65. [Google Scholar]
- Seiler, C.; Berendonk, T.U. Heavy metal driven co-selection of antibiotic resistance in soil and water bodies impacted by agriculture and aquaculture. Front. Microbiol. 2012, 3, 1–10. [Google Scholar]
- Zhang, Y.; Cai, X.Y.; Lang, X.M.; Qiao, X.L.; Li, X.H.; Chen, J.W. Insights into aquatic toxicities of the antibiotics oxytetracycline and ciprofloxacin in the presence of metal: Complexation versus mixture. Environ. Pollut. 2012, 166, 48–56. [Google Scholar]
- Tamilselvi, A.; Mugesh, G. Zinc and antibiotic resistance: Metallo-β-lactamases and their synthetic analogues. J. Biol. Inorg. Chem. 2008, 13, 1039–1053. [Google Scholar]
- Caille, O.; Rossier, C.; Perron, K. A Cu2+-activated two-component system interacts with Zinc and imipenem resistance in Pseudomonas aeruginosa. J. Bacteriol. 2007, 189, 4561–4568. [Google Scholar]
- Liu, Y.; Guan, Y.T.; Gao, B.Y.; Yue, Q.Y. Antioxidant responses and degradation of two antibiotic contaminants. Ecotoxicol. Environ. Saf. 2012, 86, 23–30. [Google Scholar]
- Sharma, S.; Sundaram, C.S.; Luthra, P.M.; Singh, Y.; Sirdeshmukh, R.; Gade, W.N. Role of proteins in resistance mechanism of Pseudomonas fluorescens against heavy metal induced stress with proteomics approach. J. Biotechnol. 2006, 126, 374–382. [Google Scholar]
- Petrova, M.; Gorlenko, Z.; Mindlin, S. Tn5045, a novel integron-containing antibiotic and chromate resistance. Res. Microbiol. 2011, 162, 337–345. [Google Scholar]
- Zhang, R.; Wang, Y.; Gu, J.D. Identification of environmental plasmid-bearing Vibrio species isolated from polluted and pristine marine reserves of Hong Kong and resistance to antibiotics and mercury. Antonie van Leeuwenhoek 2006, 89, 307–315. [Google Scholar]
- Strouhal, M.; Kizek, R.; Vacek, J.; Trnková, L.; Němeca, M. Electrochemical study of heavy metals and metallothionein in yeast Yarrowia lipolytica. Bioelectrochemistry 2003, 60, 29–36. [Google Scholar]
- Ministry of Environmental Protection of the People’s Republic of China. Available online: http://kjs.mep.gov.cn/hjbhbz/bzwb/shjbh/sjcgfffbz/ (accessed on 23 July 2014).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Xu, Y.-B.; Xu, J.-X.; Zhang, X.-H.; Xu, S.-H.; Du, Q.-P. Combined Toxic Effects of Heavy Metals and Antibiotics on a Pseudomonas fluorescens Strain ZY2 Isolated from Swine Wastewater. Int. J. Mol. Sci. 2015, 16, 2839-2850. https://doi.org/10.3390/ijms16022839
Zhou Y, Xu Y-B, Xu J-X, Zhang X-H, Xu S-H, Du Q-P. Combined Toxic Effects of Heavy Metals and Antibiotics on a Pseudomonas fluorescens Strain ZY2 Isolated from Swine Wastewater. International Journal of Molecular Sciences. 2015; 16(2):2839-2850. https://doi.org/10.3390/ijms16022839
Chicago/Turabian StyleZhou, Yan, Yan-Bin Xu, Jia-Xin Xu, Xiao-Hua Zhang, Shi-Hui Xu, and Qing-Ping Du. 2015. "Combined Toxic Effects of Heavy Metals and Antibiotics on a Pseudomonas fluorescens Strain ZY2 Isolated from Swine Wastewater" International Journal of Molecular Sciences 16, no. 2: 2839-2850. https://doi.org/10.3390/ijms16022839





