Bone-Remodeling Transcript Levels Are Independent of Perching in End-of-Lay White Leghorn Chickens
Abstract
:1. Introduction
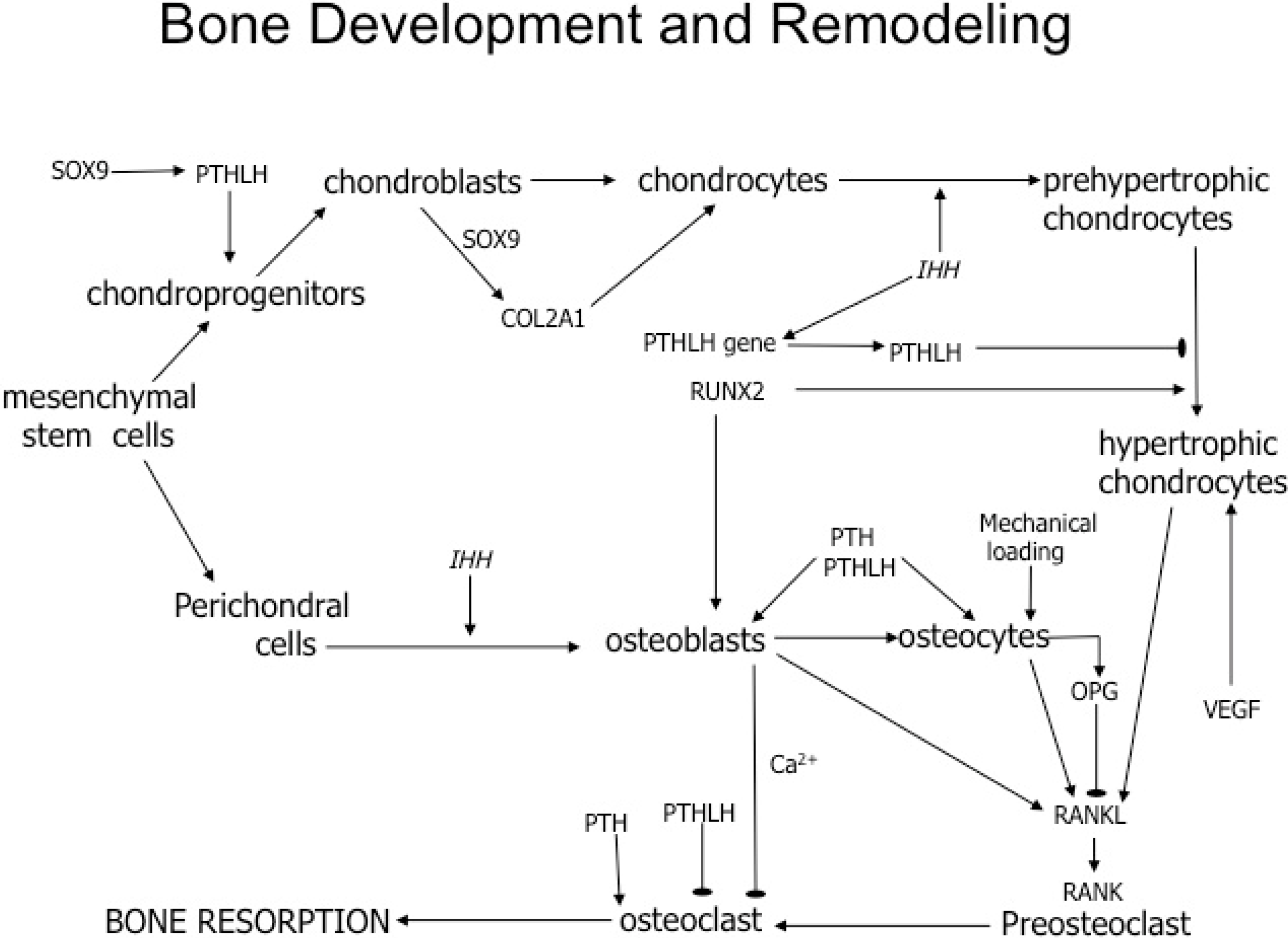
2. Results
| Transcript | Primer Pair (5' to 3') | Amplified NT Location (Amplicon Size) | Accession Number |
|---|---|---|---|
| GAPDH | F: GCACCACCAACTGCCTGGCACCCTTG | 425–634 (210) | ENSGALT00000023323 |
| R: GGATGACTTTCCCCACAGCCTTAGCAGC | |||
| COL2A1 | F: CATCCTCATCCAGGGATCCAAC | 3533–3733 (200) | AF452711 |
| R: ACTCCTGATCGGCTCCGCCAATGT | |||
| SOX9 | F: AAATGACAGAAGAACAGGACAAATG | 293–585 (293) | ENSGALT00000038513 |
| R: CTTCACGTGGGGTTTGTTCTTG | |||
| PTHLH | F: TCAGAGCACCAGCTACTGCATGAC | 136–286 (151) | NM_205338 |
| R: CCTAAGCCTGCTACCAACACAA | |||
| PTH1R | F: GCACTAGAAACTACATCCACATGC | 521–754 (234) | DQ914925.1 |
| R: AGTAGTAATTGGTTGCCAGGAAGT | |||
| PTH3R | F: ACCAGCTGCCTCCCAGAATGGGAT | 111–204 (94) | XM_425837 |
| R: TGAAGTCGTAGATGTAGTCAGGGC | |||
| OPG | F: AGACTGGAACAGCAACGACGAG | 272–470 (199) | ENSGALT00000030811 |
| R: GACAGACTGCTTTGGATGACGT | |||
| RANKL | F: AGGAGGTGAAGTTAATGCCAGAAT | 101–398 (298) | ENSGALT00000027412 |
| R: AGTTTCCCATCACTGAACGTCATA |
| Transcript | Treatment | Bone | Interaction |
|---|---|---|---|
| RANKL/OPG RATIO | F3,27 = 1.81, p = 0.17 | F2,64 = 1.48, p = 0.25 | F6,64 = 1.09, p = 0.39 |
| SOX9 | F3,32 = 0.24, p = 0.87 | F2,64 = 1.12, p = 0.34 | F6,64 = 0.76, p = 0.61 |
| PTH1R | F3,32 = 0.59, p = 0.63 | F2,64 = 2.47, p = 0.10 | F6,64 = 0.92, p = 0.50 |
| PTH3R | F3,32 = 0.49, p = 0.70 | F2,64 = 0.49, p = 0.61 | F6,64 = 1.13, p = 0.37 |
| PTHLH | F3,32 = 0.22, p = 0.88 | F2,64 = 0.07, p = 0.94 | F6,64 = 1.25, p = 0.30 |
| COL2A1 | F3,32 = 1.73, p = 0.18 | F2,64 = 4.25, p = 0.02 | F6,64 = 0.96, p = 0.46 |
| Phalange | Tibia | Femur |
|---|---|---|
| A 0.54 ± 0.06 | AB 0.68 ± 0.06 | B 0.79 ± 0.10 |
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Total RNA Isolation and cDNA Synthesis
4.3. Real-Time Quantitative PCR and Cloning cDNA for Sequence Validation
4.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Appendix
| Treatment Bone | Control | Perch during Laying | Perch during Pullet | Perch during Pullet and Laying | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P | T | F | P | T | F | P | T | F | P | T | |
| SOX9 | 0.45 ± 0.99 | 4.29 ± 3.13 | 0.90 ± 0.74 | 1.65 ± 0.99 | 5.96 ± 3.13 | 0.59 ± 0.74 | 0.77 ± 0.99 | 1.41 ± 3.13 | 1.68 ± 0.74 | 1.75 ± 0.99 | 0.58 ± 3.13 | 1.71 ± 0.74 |
| PTHLH | 0.75 ± 0.69 | 1.30 ± 0.58 | 0.38 ± 0.84 | 1.77 ± 0.69 | 0.46 ± 0.58 | 0.64 ± 0.84 | 0.25 ± 0.69 | 0.55 ± 0.58 | 0.53 ± 0.84 | 0.10 ± 0.69 | 0.25 ± 0.58 | 1.67 ± 0.84 |
| PTH1R | 0.43 ± 0.15 | 1.52 ± 0.64 | 0.30 ± 0.75 | 0.38 ± 0.15 | 0.80 ± 0.64 | 1.25 ± 0.75 | 0.18 ± 0.15 | 0.24 ± 0.64 | 1.89 ± 0.75 | 0.14 ± 0.15 | 0.16 ± 0.64 | 0.40 ± 0.75 |
| PTH3R | 1.76 ± 0.68 | 2.99 ± 1.20 | 0.91 ± 0.67 | 1.85 ± 0.68 | 2.23 ± 0.64 | 1.41 ± 0.67 | 0.68 ± 0.68 | 1.28 ± 1.20 | 1.41 ± 0.67 | 0.64 ± 0.68 | 0.56 ± 1.20 | 2.31 ± 0.67 |
| OPG | 0.76 ± 1.09 | 0.18 ± 0.33 | 0.39 ± 0.39 | 0.24 ± 1.16 | 0.70 ± 0.39 | 0.19 ± 0.41 | 1.82 ± 1.03 | 0.76 ± 0.33 | 0.94 ± 0.37 | 1.42 ± 1.03 | 0.08 ± 0.32 | 0.68 ± 0.36 |
| RANKL | 1.09 ± 3.85 | 0.07 ± 0.09 | 0.23 ± 0.24 | 7.75 ± 3.84 | 0.34 ± 0.11 | 0.26 ± 0.30 | −0.66 ± 4.50 | 0.25 ± 0.09 | 0.51 ± 0.25 | 0.45 ± 3.60 | 0.30 ± 0.09 | 0.53 ± 0.24 |
| RANKL/OPG RATIO | 0.23 ± 4.24 | −0.04 ± 0.42 | 0.02 ± 0.53 | 9.73 ± 4.15 | −0.62 ± 0.61 | 0.05 ± 0.73 | −2.67 ± 4.77 | −0.62 ± 0.40 | 0.36 ± 0.57 | −1.49 ± 3.79 | −0.19 ± 0.41 | 0.41 ± 0.51 |
| COL2A1 | 0.92 ± 0.19 | 0.59 ± 0.11 | 0.68 ± 0.13 | 1.02 ± 0.19 | 0.45 ± 0.11 | 0.69 ± 0.13 | 0.53 ± 0.19 | 0.39 ± 0.11 | 0.51 ± 0.13 | 0.68 ± 0.19 | 0.74 ± 0.11 | 0.84 ± 0.13 |
Conflicts of Interest
References
- Saini, V.; Marengi, D.; Barry, K.; Fulzele, K.; Heiden, E.; Liu, X.; Dedic, C.; Maeda, A.; Lotinun, S.; Baron, R.; et al. Parathyroid hormone (PTH)/PTH-related peptide type 1 reeptor (PPR) signaling in osteocytes regulates anabolic and catabolic skeletal responses to PTH. J. Biol. Chem. 2013, 288, 20122–20134. [Google Scholar] [CrossRef] [PubMed]
- Raggatt, L.; Partridge, N. Cellular and molecular mechanisms of bone remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, C. Overview of bone biology in the egg-laying hen. Poult. Sci. 2004, 83, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Dacke, C.; Arkle, S.; Cook, D.; Wormstone, I.; Jones, S.; Zaidi, M.; Bascal, Z. Medullary bone and avian calcium regulation. J. Exp. Biol. 1993, 184, 63–88. [Google Scholar]
- Khosla, S. Minireview: The OPG/RANKL/RANK system. Endocrinology 2001, 142, 5050–5055. [Google Scholar] [CrossRef] [PubMed]
- Raisz, L. Physiology and pathophysiology of bone remodeling. Clin. Chem. 1999, 45, 1353–1358. [Google Scholar] [PubMed]
- Webster, A. Welfare implications of avian osteoporosis. Poult. Sci. 2004, 83, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, C.; Fleming, R. Osteoporosis in cage layers. Poult. Sci. 2000, 79, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- McCoy, M.; Reilly, G.; Kilpatrick, D. Density and breaking strength of bones of mortalities among caged layers. Res. Vet. Sci. 1996, 60, 185–186. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K. Final report of the thirty fourth North Carolina layer performance and management test. Poult. Sci. 2002, 34, 15–20. [Google Scholar]
- Nasr, M.; Nicol, C.; Murrell, J. Do laying hens with keel bone fractures experience pain? PLoS One 2012, 7, 1–6. [Google Scholar] [CrossRef]
- Hughes, B.; Appleby, M. Increase in bone strength of spent laying hens housed in modified cages with perches. Vet. Rec. 1989, 124, 483–484. [Google Scholar] [CrossRef] [PubMed]
- Duncan, E.; Appleby, M.; Hughes, B. Effects of perches in laying cages on welfare and production of hens. Poult. Sci. 1992, 33, 25–35. [Google Scholar] [CrossRef]
- Hughes, B.; WIlson, S.; Appleby, M.; Smith, S. Comparison of bone volume and strength as measures of skeletcal integrity in caged laying hens with access to perches. Res. Vet. Sci. 1993, 54, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, P.; Tauson, R. Effects of perches at different positions in conventional cages for laying hens of two different strains. Acta Agric. Scand. A-Anim. Sci. 1993, 43, 228–235. [Google Scholar]
- Tauson, R.; Abrahamsson, P. Foot and skeletal disorders in laying hens: Effects of perch design, hybrid, housing system and stocking density. Acta Agric. Scand. A-Anim. Sci. 1994, 44, 110–119. [Google Scholar]
- Abrahamsson, P.; Tauson, R.; Appleby, M. Behavior, health and integument of four hybrids of laying hens in modified and conventional cages. Br. Poult. Sci. 1996, 37, 521–540. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, P.; Tauson, R. Effects of group size on performance, health and birds’ use of facilities in furnished cages for laying hens. Acta Agric. Scand. A-Anim. Sci. 1997, 47, 254–260. [Google Scholar]
- Barnett, J.; Glatz, P.; Newman, E.; Cronin, G. Effects of modifying layer cages with perches on stress physiology, plumage, pecking and bone strength of hens. Aust. J. Exp. Agric. 1997, 37, 523–529. [Google Scholar] [CrossRef]
- Jendral, M.; Korver, D.; Church, J.; Feddes, J. Bone mineral density and breaking strength of White Leghorns. Poult. Sci. 2008, 87, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Tactacan, G.; Guenter, W.; Lewis, N.; Rodriguez-Lecompte, J.; House, J. Performance and welfare of laying hens in conventional and enriched cages. Poult. Sci. 2009, 88, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.-L.; Bhattacharya, P.; He, X.; Ponugoti, B.; Marquardt, B.; Layman, J.; Grunloh, M.; Postlethwait, J.; Rubin, D. Duplicated zebrafish co-orthologs of parathyroid hormone-related peptide (PTHrP, PTHLH) play different roles in craniofacial skeletogenesis. J. Endocrinol. 2012, 214, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Healy, C.; Uwanogho, D.; Sharpe, P. Regulation and role of Sox9 in cartilage formation. Dev. Dyn. 1999, 215, 69–78. [Google Scholar] [CrossRef] [PubMed]
- St-Jacques, B.; Hammerschmidt, M.; McMahon, A. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999, 13, 2072–2086. [Google Scholar] [CrossRef] [PubMed]
- Usui, M.; Xing, L.; Drissi, H.; Zuscik, M.; O’Keefe, R.; Chen, D.; Boyce, B. Murine and chicken chondrocytes regulate osteoclastogenesis by producing RANKL in response to BMP2. J. Bone Miner. Res. 2008, 23, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Hou, J.; Yao, J.; Zhou, Z. Effects of osteoprotegrin from transfection of pcDNA3.1(+)/chOPG on bioactivity of chicken osteoclasts. Acta Vet. Scand. 2011, 53, 21. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.; Jüppner, H. Zebrafish express the common PTH/PTHrP receptor (PTH1R) and a novel receptor (PTH3R) that is preferentially activated by mammalian and fugufish parathyroid hormone-related peptide (PTHrP). J. Biol. Chem. 1999, 274, 28185–28190. [Google Scholar]
- Gay, C. Avian bone turnover and the role of bone cells. In The Comparative Endocrinology of Calcium Regulation; Dacke, C., Danks, J., Caple, I., Flik, G., Eds.; Journal of Endocrinolgy, Ltd.: Bristol, England, 1996; pp. 113–121. [Google Scholar]
- Pinheiro, P.; Cardoso, J.; Power, D.; Canario, A. Functional characterization and evolution of PTH/PTHrP receptors: Insights from the chicken. BMC Evol. Biol. 2012, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Aoki, K.; Masuda, W.; Alles, N.; Nagano, K.; Fukushima, H.; Osawa, K.; Yasuda, H.; Nakamura, I.; Mikuni-Takagaki, Y.; et al. Disruption of NK-κB1 prevents bone loss caused by mechanical unloading. J. Bone Miner. Res. 2013, 28, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Hester, P.; Garner, J.; Enneking, S.; Cheng, H.; Einstein, M. The effect of perch availability during pullet rearing and egg laying on the behavior of caged White Leghorn hens. Poult. Sci. 2014, 93, 2423–2431. [Google Scholar] [CrossRef] [PubMed]
- Hester, P.; Enneking, S.; Haley, B.; Cheng, H.; Einstein, M.; Rubin, D. The effect of perch availability during pullet rearing and egg laying on musculoskeletal health of caged White Leghorn hens. Poult. Sci. 2013, 92, 1972–1980. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Dallas, M.; Qiu, N.; Nicolella, D.; Cao, L.; Johnson, M.; Bonewald, L.; Quarles, L. Conditional deletion of Pkd1 in osteocytes disrupts skeletal mechanosensing in mice. FASEB J. 2011, 25, 2418–2432. [Google Scholar] [CrossRef] [PubMed]
- Herron, J.; Freeman, S. Evolutionary Analysis, 5th ed.; Pearson: Boston, MA, USA, 2014. [Google Scholar]
- Hester, P.; Enneking, S.; Jefferson-Moore, K.; Einstein, M.; Cheng, H.; Rubin, D. The effect of perches in cages during pullet rearing and egg laying on hen performance, foot health, and plumage. Poult. Sci. 2013, 92, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, J.; Riley, L.; Hirano, T.; Grau, E.; Rubin, D. Differential expression of tuberoinfundibular peptide 38 and glucose-6-phosphatase in tilapia. Gen. Comp. Endocrinol. 2006, 146, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M. Quantification strategies in real-time PCR. In A–Z of Quantitative PCR; Bustin, S., Ed.; International University Line: La Jolla, CA, USA, 2004; pp. 87–112. [Google Scholar]
- Schmittgen, T.; Livak, K. Analyzing real-time PCR data by the comparative Ct method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Sokal, R.; Rohlf, F. Biometry, Fourth ed.; W.H. Freeman: San Francisco, CA, USA, 2012; p. 937. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dale, M.D.; Mortimer, E.M.; Kolli, S.; Achramowicz, E.; Borchert, G.; Juliano, S.A.; Halkyard, S.; Sietz, N.; Gatto, C.; Hester, P.Y.; et al. Bone-Remodeling Transcript Levels Are Independent of Perching in End-of-Lay White Leghorn Chickens. Int. J. Mol. Sci. 2015, 16, 2663-2677. https://doi.org/10.3390/ijms16022663
Dale MD, Mortimer EM, Kolli S, Achramowicz E, Borchert G, Juliano SA, Halkyard S, Sietz N, Gatto C, Hester PY, et al. Bone-Remodeling Transcript Levels Are Independent of Perching in End-of-Lay White Leghorn Chickens. International Journal of Molecular Sciences. 2015; 16(2):2663-2677. https://doi.org/10.3390/ijms16022663
Chicago/Turabian StyleDale, Maurice D., Erin M. Mortimer, Santharam Kolli, Erik Achramowicz, Glenn Borchert, Steven A. Juliano, Scott Halkyard, Nick Sietz, Craig Gatto, Patricia Y. Hester, and et al. 2015. "Bone-Remodeling Transcript Levels Are Independent of Perching in End-of-Lay White Leghorn Chickens" International Journal of Molecular Sciences 16, no. 2: 2663-2677. https://doi.org/10.3390/ijms16022663







