EGCG Inhibits Proliferation, Invasiveness and Tumor Growth by Up-Regulation of Adhesion Molecules, Suppression of Gelatinases Activity, and Induction of Apoptosis in Nasopharyngeal Carcinoma Cells
Abstract
:1. Introduction
2. Results
2.1. (−)-Epigallocatechin-3-gallate (EGCG) Inhibits the Proliferation of Nasopharyngeal Carcinoma (NPC) Cells but not Immortalized Nasopharyngeal Epithelial Cells
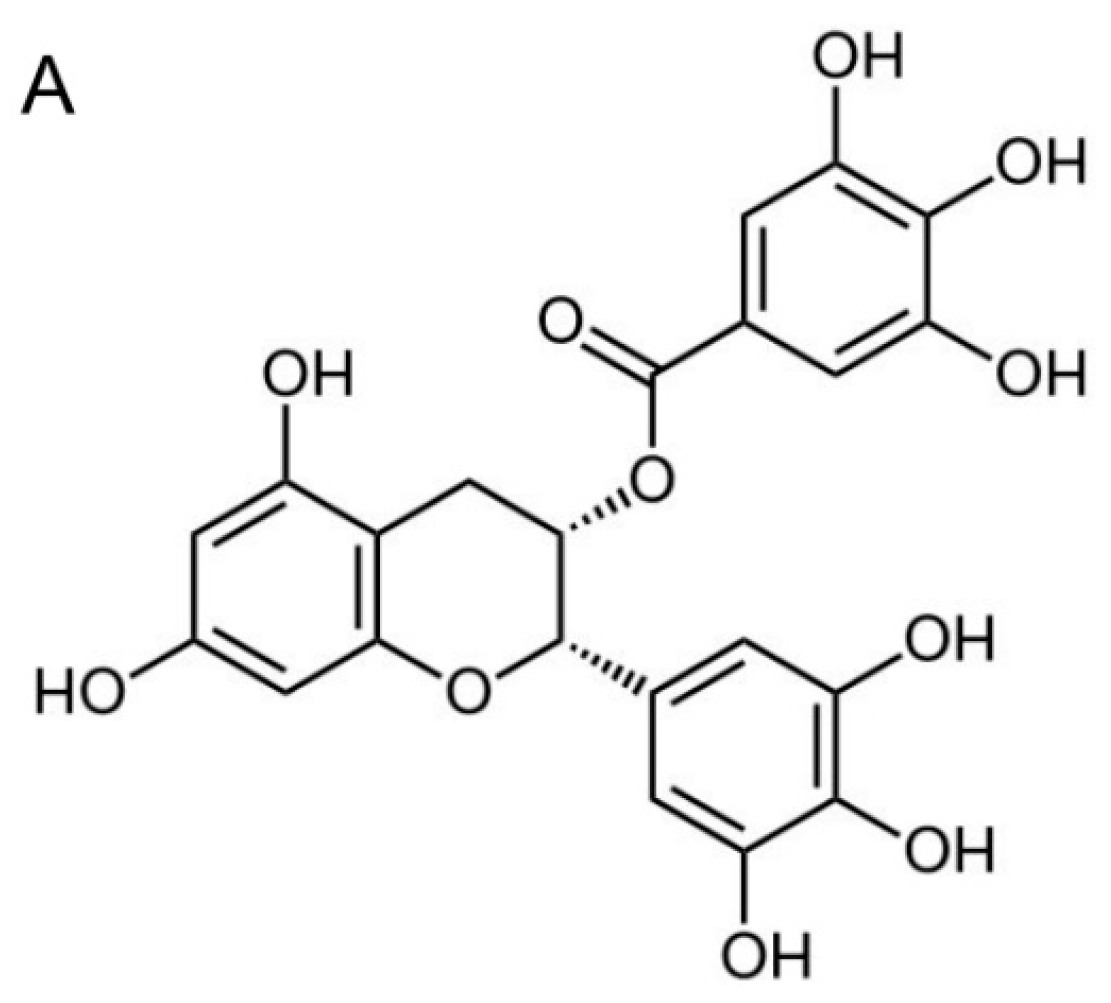

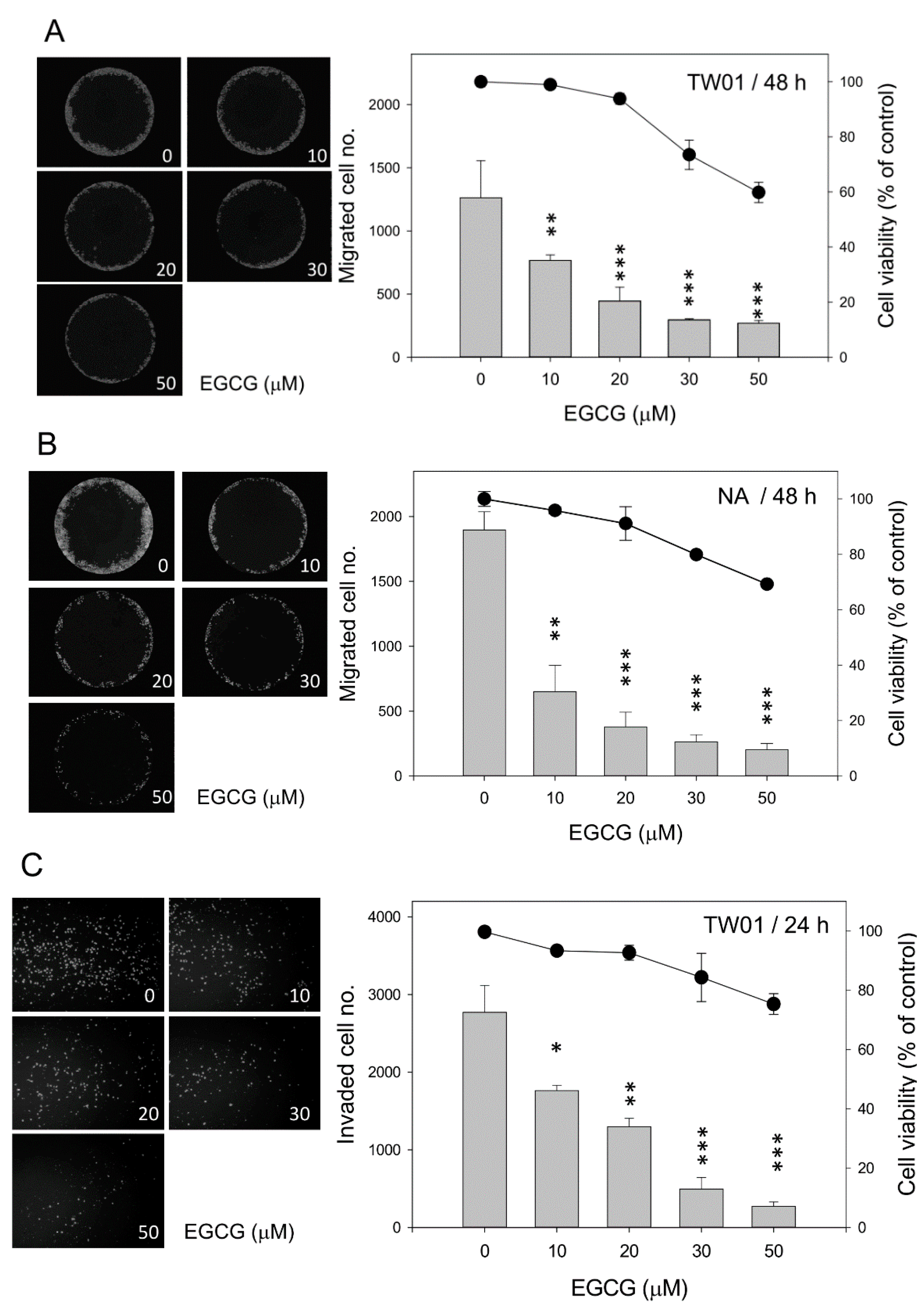
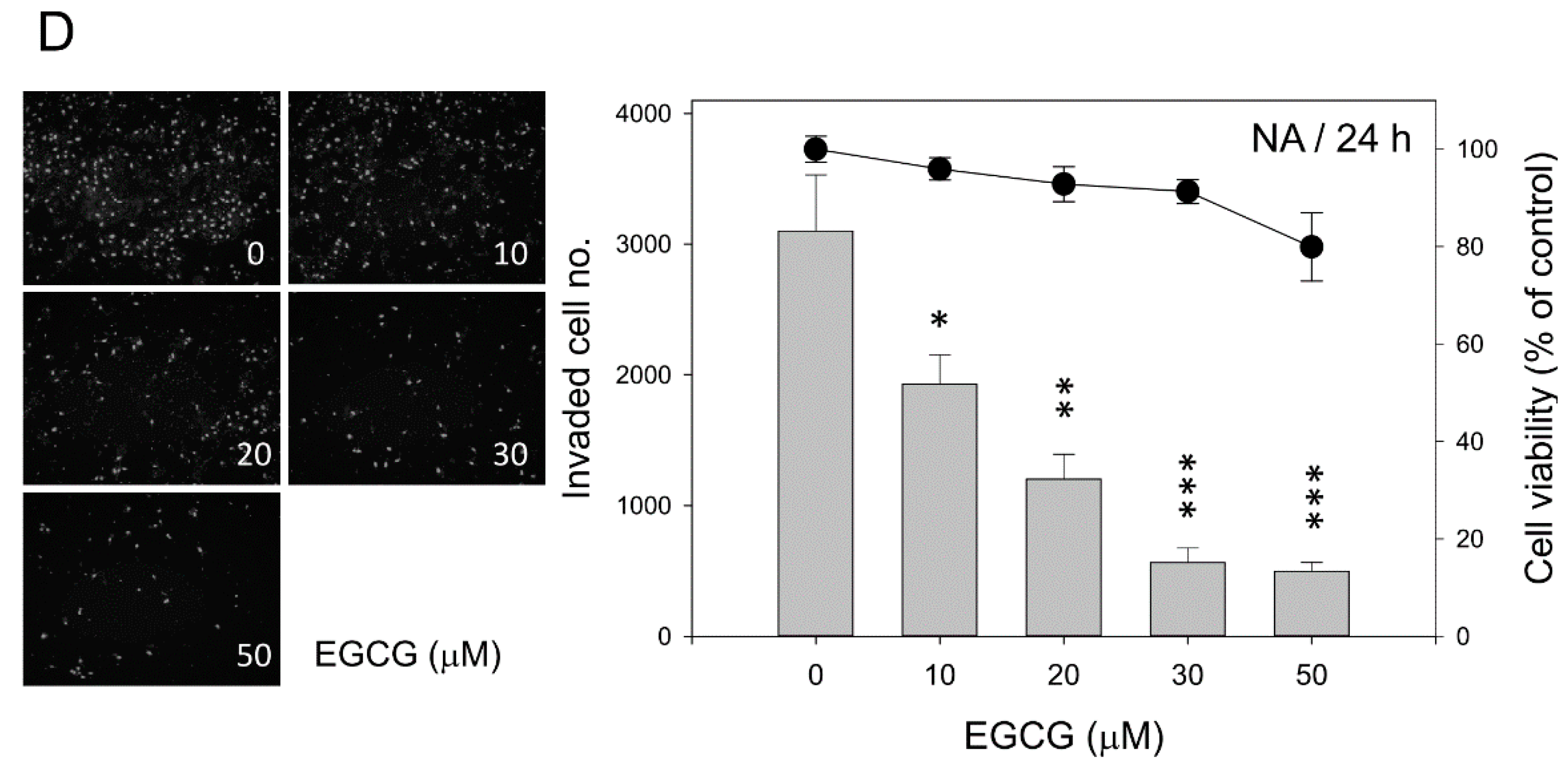
2.2. EGCG Inhibits Nasopharyngeal Carcinoma (NPC) Cell Migration and Invasion
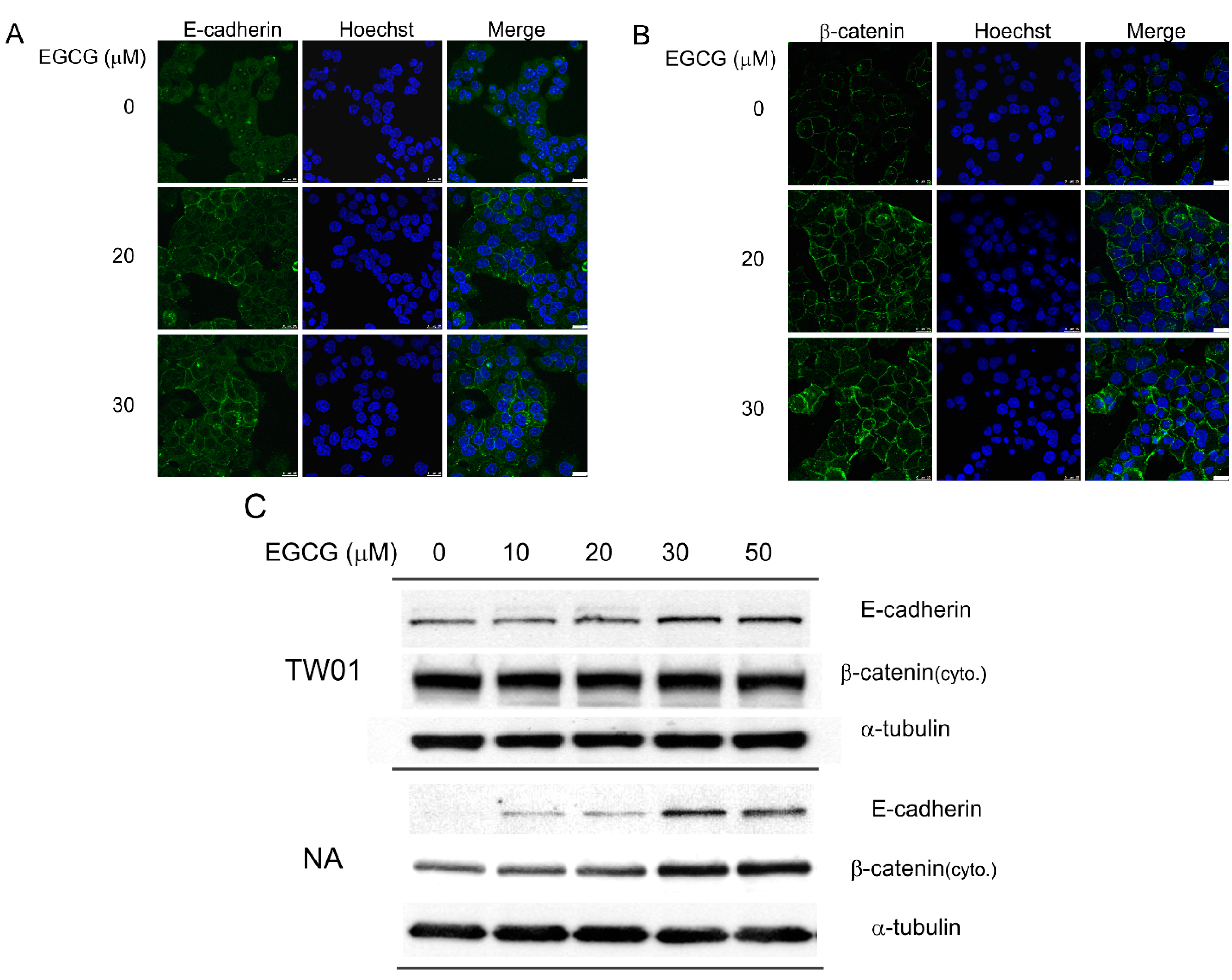
2.3. EGCG Up-Regulates the Expression of Cell Adhesion Molecules E-Cadherin and β-Catenin
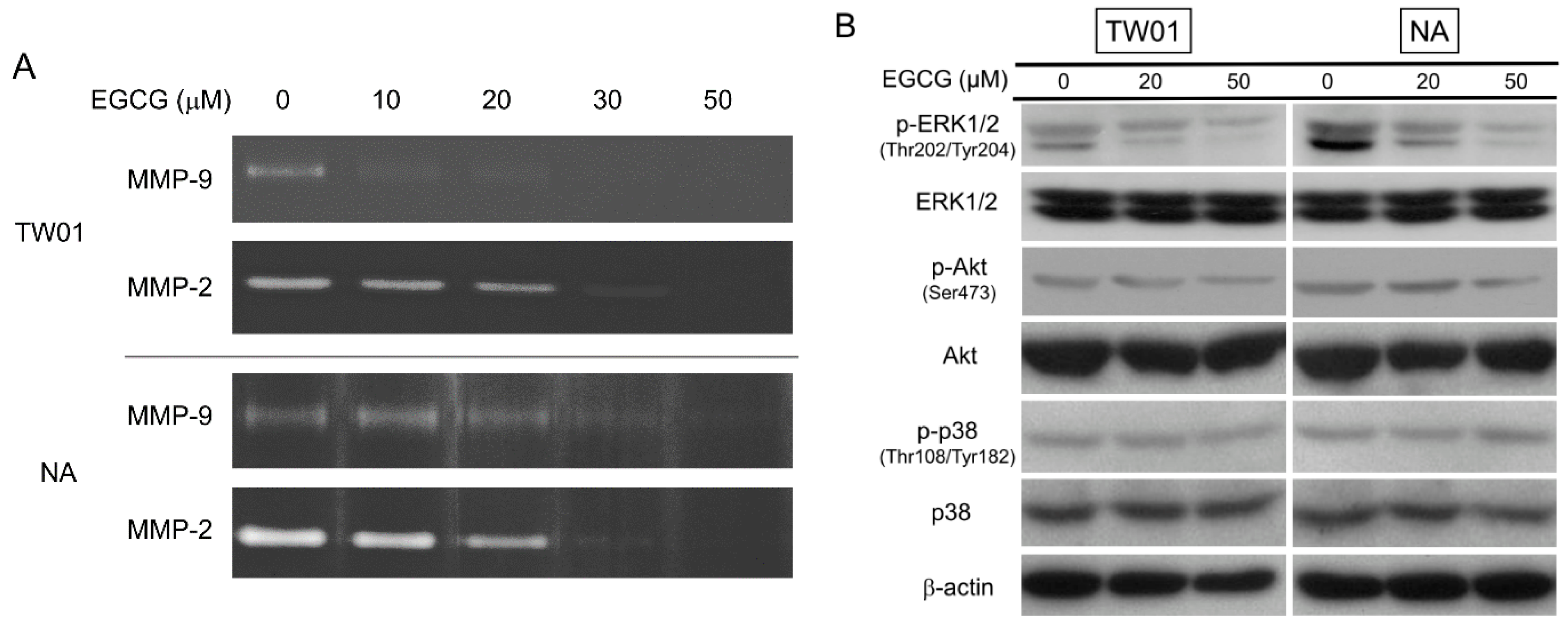
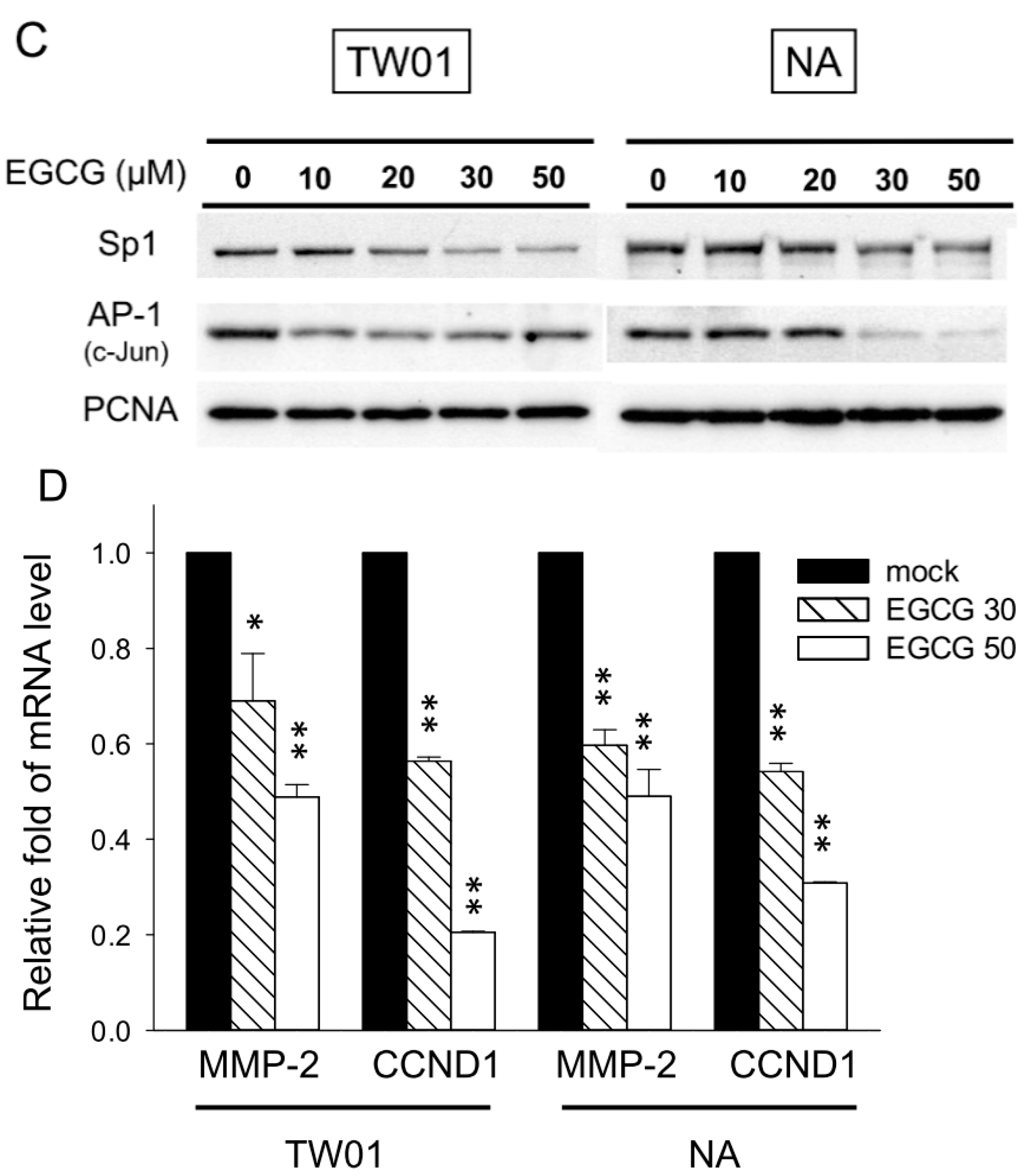
2.4. EGCG Suppressed the Activity of Matrix Metalloproteinase 2 (MMP-2) and MMP-9, Supposedly through Regulating the Extracellular Signal-Regulated Kinase (ERK) Signaling Pathways
2.5. EGCG Reduces Spheroid Formation by NPC Cells in Culture

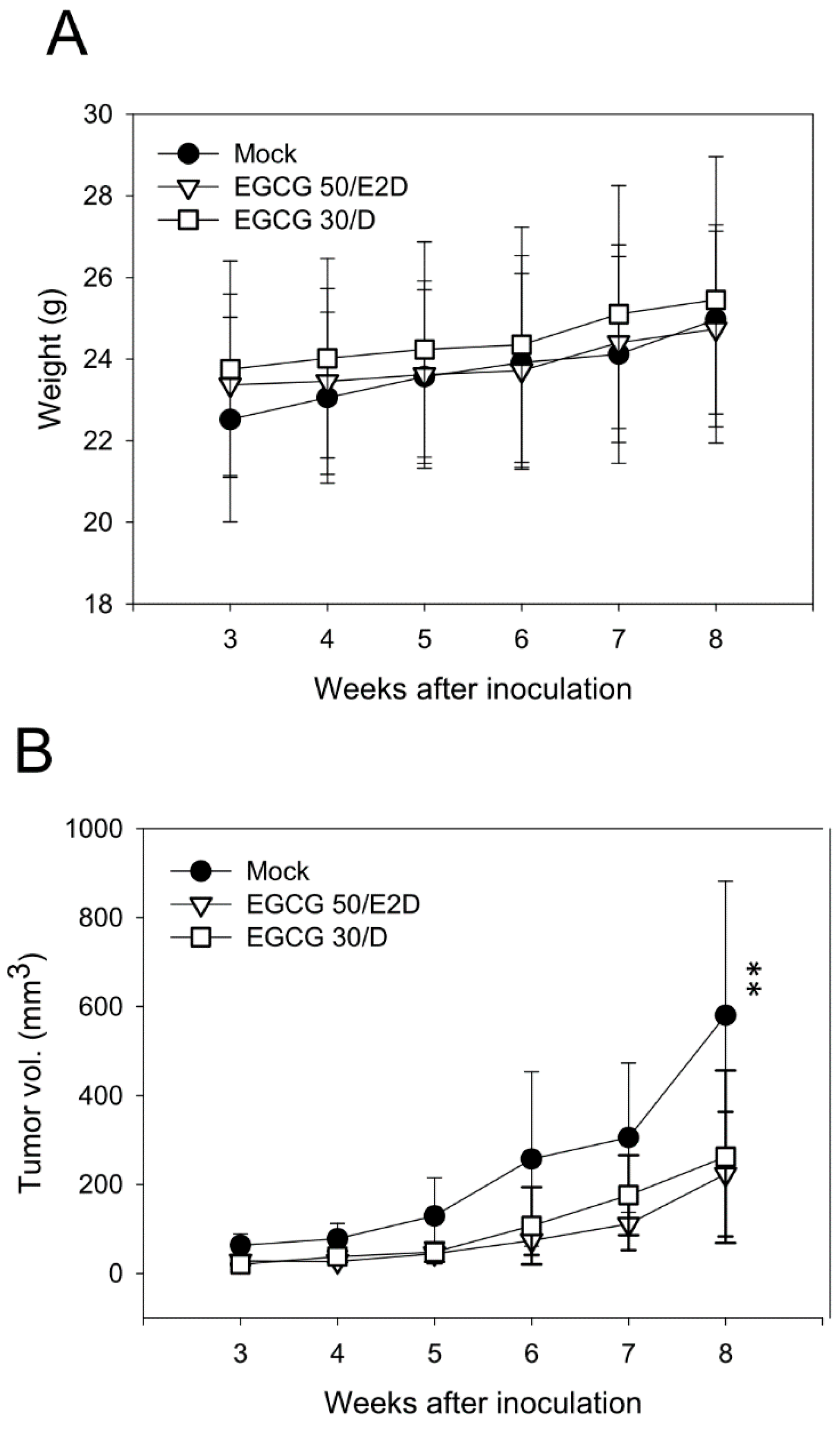
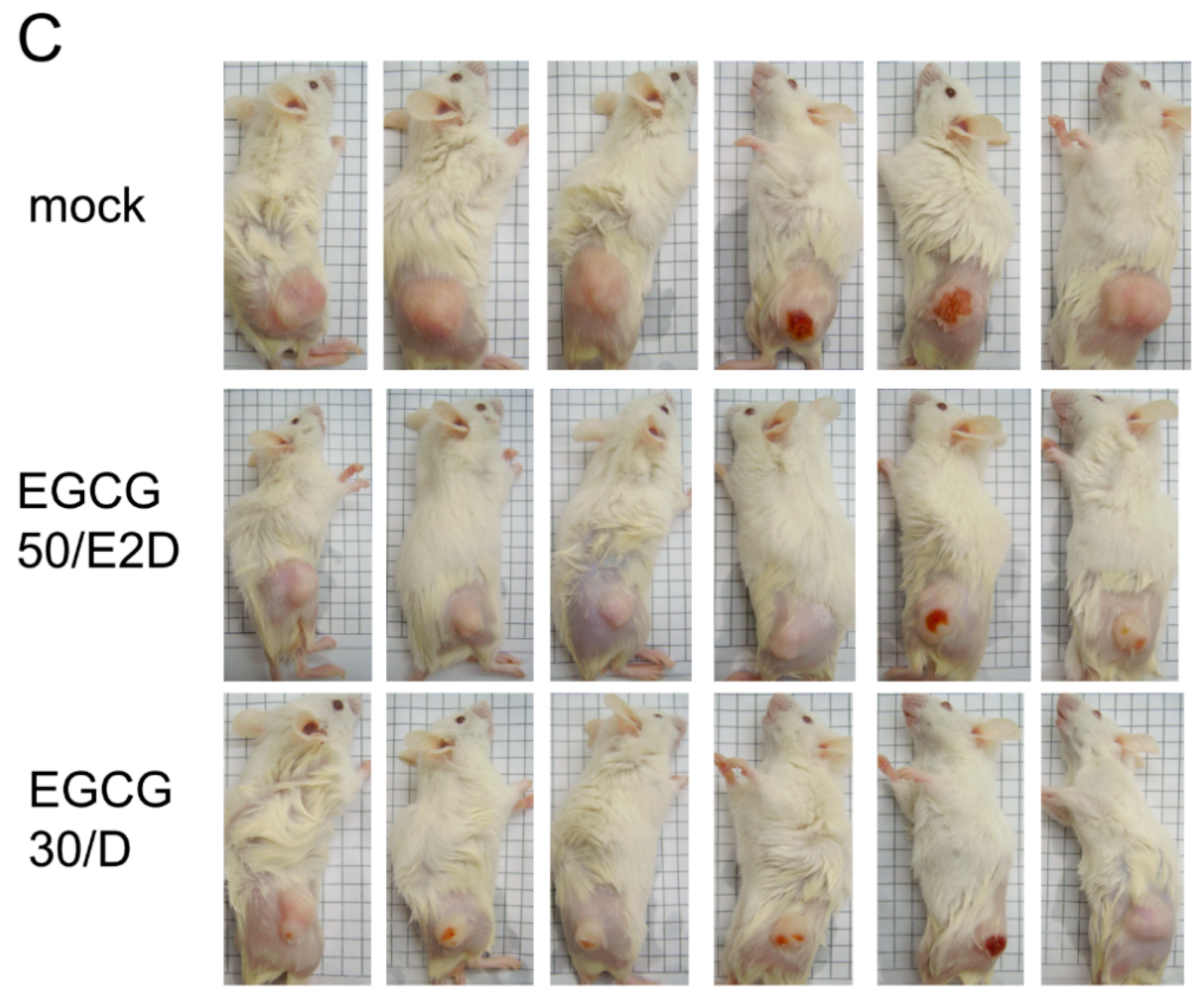
2.6. EGCG Inhibits NPC Tumor Growth in Vivo
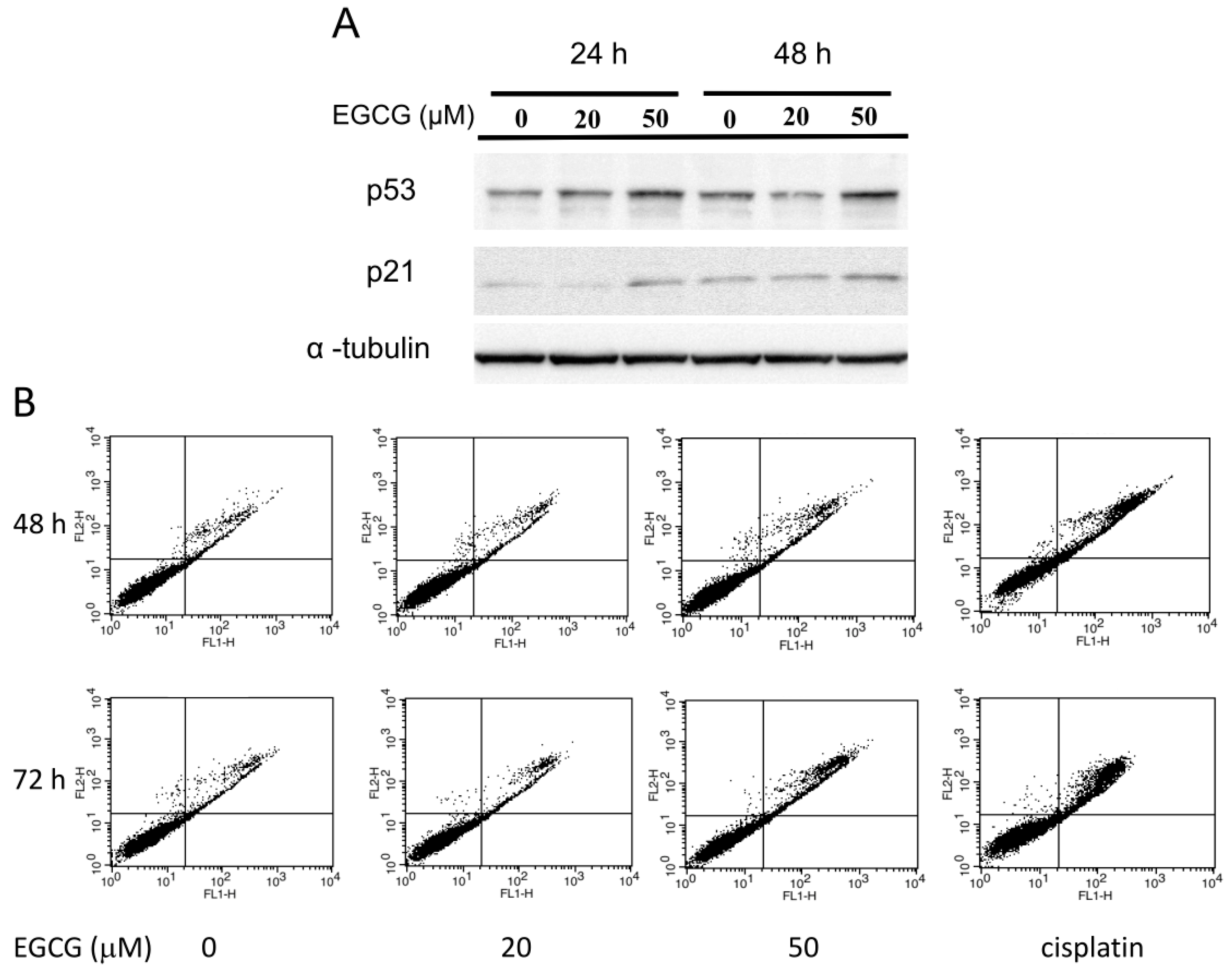
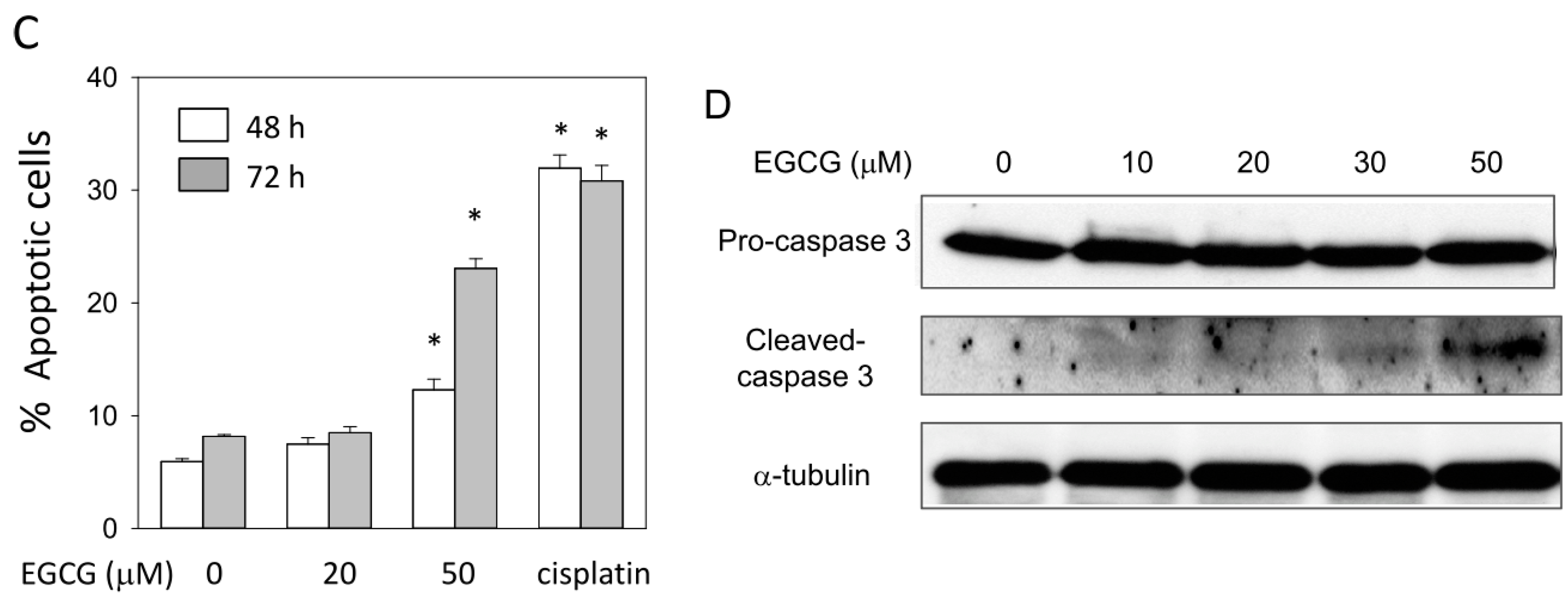
2.7. EGCG Up-Regulates the Expression of p53/p21 and Induces Apoptosis of NA Cells via Caspase 3 Activation
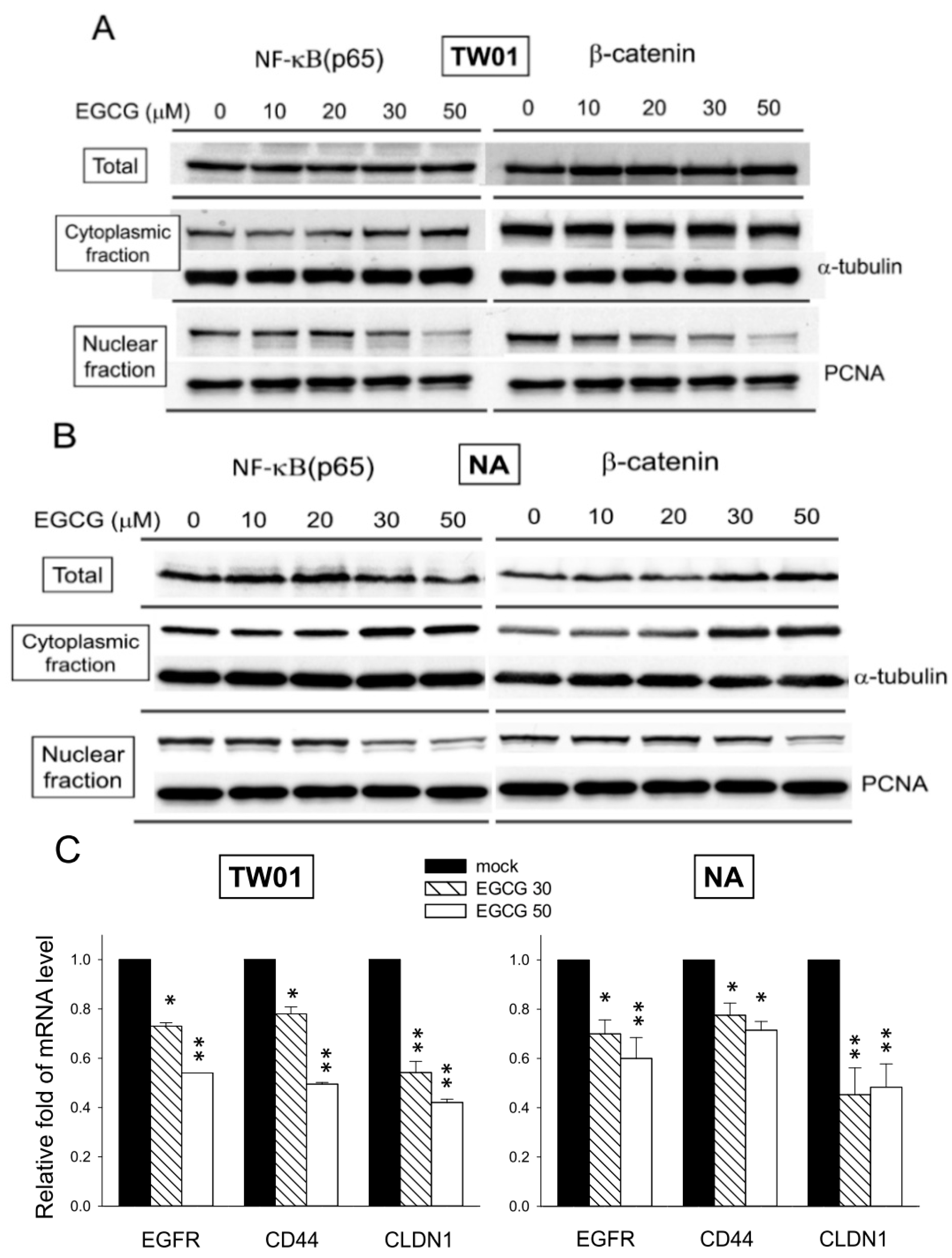
2.8. EGCG Suppresses the Nuclear Translocation of NF-κB and β-Catenin
3. Discussion
4. Experimental Section
4.1. Cell Lines and Chemicals
4.2. Proliferation and Cytotoxicity Assay
4.3. Cell Migration Assay
4.4. Cell Invasion Assay
4.5. Immunofluorescence Staining
4.6. Western Blot Assay
4.7. Gelatin Zymography
4.8. Spheriod Formation Assay
4.9. Tumor Growth in Severe Combined Immunodeficiency Mice
4.10. Apoptosis Assay by Annexin-V and Propidium Iodide Staining
4.11. RNA Extraction and Semi-Quantitative Real-Time PCR
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De-The, G. Epidemiology of Epstein-Barr virus and associated diseases in man. In The Herpesviruses; Roizman, B., Ed.; Plenum Press: New York, NY, USA, 1982; pp. 25–103. [Google Scholar]
- Hildesheim, A.; Levine, P.H. Etiology of nasopharyngeal carcinoma: A review. Epidemiol. Rev. 1993, 15, 466–485. [Google Scholar]
- McDermott, A.L.; Dutt, S.N.; Watkinson, J.C. The aetiology of nasopharyngeal carcinoma. Clin. Otolaryngol. Allied Sci. 2001, 26, 82–92. [Google Scholar]
- Young, L.S.; Rickinson, A.B. Epstein-Barr virus: 40 years on. Nat. Rev. Cancer 2004, 4, 757–768. [Google Scholar]
- Ho, J.H. Nasopharyngeal carcinoma (NPC). Adv. Cancer Res. 1972, 15, 57–92. [Google Scholar]
- Zou, X.N.; Lu, S.H.; Liu, B. Volatile N-nitrosamines and their precursors in Chinese salted fish—A possible etological factor for NPC in china. Int. J. Cancer 1994, 59, 155–158. [Google Scholar]
- Zur Hausen, H.; O’Neill, F.J.; Freese, U.K.; Hecker, E. Persisting oncogenic herpesvirus induced by the tumour promotor TPA. Nature 1978, 272, 373–375. [Google Scholar]
- Luka, J.; Kallin, B.; Klein, G. Induction of the Epstein-Barr virus (EBV) cycle in latently infected cells by n-butyrate. Virology 1979, 94, 228–231. [Google Scholar]
- Rickinson, A.B.; Kieff, E. Epstein-Barr Virus. In Fields’ Virology, 4th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001; pp. 2575–2627. [Google Scholar]
- Hirayama, T.; Ito, Y. A new view of the etiology of nasopharyngeal carcinoma. Prev. Med. 1981, 10, 614–622. [Google Scholar]
- Fang, C.Y.; Huang, S.Y.; Wu, C.C.; Hsu, H.Y.; Chou, S.P.; Tsai, C.H.; Chang, Y.; Takada, K.; Chen, J.Y. The synergistic effect of chemical carcinogens enhances Epstein-Barr virus reactivation and tumor progression of nasopharyngeal carcinoma cells. PLoS One 2012, 7, e44810. [Google Scholar]
- Gallicchio, L.; Matanoski, G.; Tao, X.G.; Chen, L.; Lam, T.K.; Boyd, K.; Robinson, K.A.; Balick, L.; Mickelson, S.; Caulfield, L.E.; et al. Adulthood consumption of preserved and nonpreserved vegetables and the risk of nasopharyngeal carcinoma: A systematic review. Int. J. Cancer 2006, 119, 1125–1135. [Google Scholar]
- Hsu, W.L.; Pan, W.H.; Chien, Y.C.; Yu, K.J.; Cheng, Y.J.; Chen, J.Y.; Liu, M.Y.; Hsu, M.M.; Lou, P.J.; Chen, I.H.; et al. Lowered risk of nasopharyngeal carcinoma and intake of plant vitamin, fresh fish, green tea and coffee: A case-control study in Taiwan. PLoS One 2012, 7, e41779. [Google Scholar]
- Zheng, Y.M.; Tuppin, P.; Hubert, A.; Jeannel, D.; Pan, Y.J.; Zeng, Y.; de The, G. Environmental and dietary risk factors for nasopharyngeal carcinoma: A case-control study in Zangwu County, Guangxi, China. Br. J. Cancer 1994, 69, 508–514. [Google Scholar]
- Feng, B.J.; Jalbout, M.; Ayoub, W.B.; Khyatti, M.; Dahmoul, S.; Ayad, M.; Maachi, F.; Bedadra, W.; Abdoun, M.; Mesli, S.; et al. Dietary risk factors for nasopharyngeal carcinoma in Maghrebian countries. Int. J. Cancer 2007, 121, 1550–1555. [Google Scholar]
- Ruan, H.L.; Xu, F.H.; Liu, W.S.; Feng, Q.S.; Chen, L.Z.; Zeng, Y.X.; Jia, W.H. Alcohol and tea consumption in relation to the risk of nasopharyngeal carcinoma in Guangdong, China. Front. Med. China 2010, 4, 448–456. [Google Scholar]
- Katiyar, S.K.; Mukhtar, H. Tea consumption and cancer. World Rev. Nutr. Diet. 1996, 79, 154–184. [Google Scholar]
- Yang, C.S.; Maliakal, P.; Meng, X. Inhibition of carcinogenesis by tea. Annual Rev. Pharmacol. Toxicol. 2002, 42, 25–54. [Google Scholar]
- Khan, N.; Mukhtar, H. Multitargeted therapy of cancer by green tea polyphenols. Cancer Lett. 2008, 269, 269–280. [Google Scholar]
- Ji, B.T.; Chow, W.H.; Hsing, A.W.; McLaughlin, J.K.; Dai, Q.; Gao, Y.T.; Blot, W.J.; Fraumeni, J.F., Jr. Green tea consumption and the risk of pancreatic and colorectal cancers. Int. J. Cancer 1997, 70, 255–258. [Google Scholar]
- Yang, C.S.; Wang, Z.Y. Tea and cancer. J. Natl. Cancer Inst. 1993, 85, 1038–1049. [Google Scholar]
- Chen, Z.P.; Schell, J.B.; Ho, C.T.; Chen, K.Y. Green tea epigallocatechin gallate shows a pronounced growth inhibitory effect on cancerous cells but not on their normal counterparts. Cancer Lett. 1998, 129, 173–179. [Google Scholar]
- Brusselmans, K.; de Schrijver, E.; Heyns, W.; Verhoeven, G.; Swinnen, J.V. Epigallocatechin-3-gallate is a potent natural inhibitor of fatty acid synthase in intact cells and selectively induces apoptosis in prostate cancer cells. Int. J. Cancer 2003, 106, 856–862. [Google Scholar]
- Lambert, J.D.; Yang, C.S. Mechanisms of cancer prevention by tea constituents. J. Nutr. 2003, 133, 3262S–3267S. [Google Scholar]
- Zhao, Y.; Yang, L.F.; Ye, M.; Gu, H.H.; Cao, Y. Induction of apoptosis by epigallocatechin-3-gallate via mitochondrial signal transduction pathway. Prev. Med. 2004, 39, 1172–1179. [Google Scholar]
- Zhao, Y.; Tao, Y.-G.; Luo, F.-J.; Tang, F.-Q.; Tang, M.; Cao, Y. Interference effect of epigallocatechin-3-gallate on targets of nuclear factor κB signal transduction pathways activated by EB virus encoded latent membrane protein 1. Int. J. Biochem. Cell Biol. 2004, 36, 1473–1481. [Google Scholar]
- Lin, C.H.; Shen, Y.A.; Hung, P.H.; Yu, Y.B.; Chen, Y.J. Epigallocathechin gallate, polyphenol present in green tea, inhibits stem-like characteristics and epithelial-mesenchymal transition in nasopharyngeal cancer cell lines. BMC Complement. Altern. Med. 2012, 12, 201. [Google Scholar]
- Lin, C.T.; Wong, C.I.; Chan, W.Y.; Tzung, K.W.; Ho, J.K.; Hsu, M.M.; Chuang, S.M. Establishment and characterization of two nasopharyngeal carcinoma cell lines. Lab. Investig. 1990, 62, 713–724. [Google Scholar]
- Glaser, R.; Zhang, H.Y.; Yao, K.T.; Zhu, H.C.; Wang, F.X.; Li, G.Y.; Wen, D.S.; Li, Y.P. Two epithelial tumor cell lines (HNE-1 and HONE-1) latently infected with Epstein-Barr virus that were derived from nasopharyngeal carcinomas. Proc. Natl. Acad. Sci. USA 1989, 86, 9524–9528. [Google Scholar]
- Chang, Y.; Tung, C.H.; Huang, Y.T.; Lu, J.; Chen, J.Y.; Tsai, C.H. Requirement for cell-to-cell contact in Epstein-Barr virus infection of nasopharyngeal carcinoma cells and keratinocytes. J. Virol. 1999, 73, 8857–8866. [Google Scholar]
- Fang, C.Y.; Lee, C.H.; Wu, C.C.; Chang, Y.T.; Yu, S.L.; Chou, S.P.; Huang, P.T.; Chen, C.L.; Hou, J.W.; Chang, Y.; et al. Recurrent chemical reactivations of EBV promotes genome instability and enhances tumor progression of nasopharyngeal carcinoma cells. Int. J. Cancer 2009, 124, 2016–2025. [Google Scholar]
- Li, H.M.; Man, C.; Jin, Y.; Deng, W.; Yip, Y.L.; Feng, H.C.; Cheung, Y.C.; Lo, K.W.; Meltzer, P.S.; Wu, Z.G.; et al. Molecular and cytogenetic changes involved in the immortalization of nasopharyngeal epithelial cells by telomerase. Int. J. Cancer 2006, 119, 1567–1576. [Google Scholar]
- Liotta, L.A.; Tryggvason, K.; Garbisa, S.; Hart, I.; Foltz, C.M.; Shafie, S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature 1980, 284, 67–68. [Google Scholar]
- Wang, L.; Zhang, Z.G.; Zhang, R.L.; Gregg, S.R.; Hozeska-Solgot, A.; LeTourneau, Y.; Wang, Y.; Chopp, M. Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration. J. Neurosci. 2006, 26, 5996–6003. [Google Scholar]
- Klein, E.A.; Assoian, R.K. Transcriptional regulation of the cyclin D1 gene at a glance. J. Cell Sci. 2008, 121, 3853–3857. [Google Scholar]
- Hirschhaeuser, F.; Menne, H.; Dittfeld, C.; West, J.; Mueller-Klieser, W.; Kunz-Schughart, L.A. Multicellular tumor spheroids: An underestimated tool is catching up again. J. Biotechnol. 2010, 148, 3–15. [Google Scholar]
- Liang, Y.C.; Lin-Shiau, S.Y.; Chen, C.F.; Lin, J.K. Inhibition of cyclin-dependent kinases 2 and 4 activities as well as induction of Cdk inhibitors p21 and p27 during growth arrest of human breast carcinoma cells by (−)-epigallocatechin-3-gallate. J. Cell Biochem. 1999, 75, 1–12. [Google Scholar]
- Gupta, S.; Ahmad, N.; Nieminen, A.L.; Mukhtar, H. Growth inhibition, cell-cycle dysregulation, and induction of apoptosis by green tea constituent (−)-epigallocatechin-3-gallate in androgen-sensitive and androgen-insensitive human prostate carcinoma cells. Toxicol. Appl. Pharmacol. 2000, 164, 82–90. [Google Scholar]
- Gupta, S.; Hussain, T.; Mukhtar, H. Molecular pathway for (−)-epigallocatechin-3-gallate-induced cell cycle arrest and apoptosis of human prostate carcinoma cells. Arch. Biochem. Biophys. 2003, 410, 177–185. [Google Scholar]
- Earnshaw, W.C.; Martins, L.M.; Kaufmann, S.H. Mammalian caspases: Structure, activation, substrates, and functions during apoptosis. Annu. Rev. Biochem. 1999, 68, 383–424. [Google Scholar]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-κB in development and progression of human cancer. Virchows Arch. 2005, 446, 475–482. [Google Scholar]
- Wang, C.Y.; Mayo, M.W.; Korneluk, R.G.; Goeddel, D.V.; Baldwin, A.S., Jr. NF-κB antiapoptosis: Induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 1998, 281, 1680–1683. [Google Scholar]
- Kolligs, F.T.; Bommer, G.; Goke, B. Wnt/β-catenin/Tcf signaling: A critical pathway in gastrointestinal tumorigenesis. Digestion 2002, 66, 131–144. [Google Scholar]
- Nishi, H.; Neta, G.; Nishi, K.H.; Akers, L.M.; Rikiyama, T.; Proctor, K.N.; Murphy, B.A.; Johnson, A.C. Analysis of the epidermal growth factor receptor promoter: The effect of nuclear factor-κB. Int. J. Mol. Med. 2003, 11, 49–55. [Google Scholar]
- Miwa, N.; Furuse, M.; Tsukita, S.; Niikawa, N.; Nakamura, Y.; Furukawa, Y. Involvement of claudin-1 in the β-catenin/Tcf signaling pathway and its frequent up-regulation in human colorectal cancers. Oncol. Res. 2001, 12, 469–476. [Google Scholar]
- Hinz, M.; Lemke, P.; Anagnostopoulos, I.; Hacker, C.; Krappmann, D.; Mathas, S.; Dorken, B.; Zenke, M.; Stein, H.; Scheidereit, C. Nuclear factor κB-dependent gene expression profiling of Hodgkin’s disease tumor cells, pathogenetic significance, and link to constitutive signal transducer and activator of transcription 5a activity. J. Exp. Med. 2002, 196, 605–617. [Google Scholar]
- Wielenga, V.J.; Smits, R.; Korinek, V.; Smit, L.; Kielman, M.; Fodde, R.; Clevers, H.; Pals, S.T. Expression of CD44 in Apc and Tcf mutant mice implies regulation by the WNT pathway. Am. J. Pathol. 1999, 154, 515–523. [Google Scholar]
- Ito, Y.; Kawanishi, M.; Harayama, T.; Takabayashi, S. Combined effect of the extracts from Croton tiglium, Euphorbia lathyris or Euphorbia tirucalli and n-butyrate on Epstein-Barr virus expression in human lymphoblastoid P3HR-1 and Raji cells. Cancer Lett. 1981, 12, 175–180. [Google Scholar]
- Huang, S.Y.; Fang, C.Y.; Tsai, C.H.; Chang, Y.; Takada, K.; Hsu, T.Y.; Chen, J.Y. N-Methyl-N'-nitro-N-nitrosoguanidine induces and cooperates with 12-O-tetradecanoylphorbol-1,3-acetate/sodium butyrate to enhance Epstein-Barr virus reactivation and genome instability in nasopharyngeal carcinoma cells. Chem. Biol. Interact. 2010, 188, 623–634. [Google Scholar]
- Huang, S.Y.; Fang, C.Y.; Wu, C.C.; Tsai, C.H.; Lin, S.F.; Chen, J.Y. Reactive oxygen species mediate Epstein-Barr virus reactivation by N-methyl-N'-nitro-N-nitrosoguanidine. PLoS One 2013, 8, e84919. [Google Scholar]
- Farrow, D.C.; Vaughan, T.L.; Berwick, M.; Lynch, C.F.; Swanson, G.M.; Lyon, J.L. Diet and nasopharyngeal cancer in a low-risk population. Int. J. Cancer 1998, 78, 675–679. [Google Scholar]
- Ahmad, N.; Feyes, D.K.; Nieminen, A.L.; Agarwal, R.; Mukhtar, H. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J. Natl. Cancer Inst. 1997, 89, 1881–1886. [Google Scholar]
- Gupta, S.; Hastak, K.; Afaq, F.; Ahmad, N.; Mukhtar, H. Essential role of caspases in epigallocatechin-3-gallate-mediated inhibition of nuclear factor κB and induction of apoptosis. Oncogene 2004, 23, 2507–2522. [Google Scholar]
- Zhang, X.; Guo, Y.; Ye, Q.; Yang, Z.; Dong, Z. Study of the relation between MMP2, MMP9 and nasopharyngeal carcinoma. J. Clin. Otorhinolaryngol. 1999, 13, 356–358. [Google Scholar]
- Takahra, T.; Smart, D.E.; Oakley, F.; Mann, D.A. Induction of myofibroblast MMP-9 transcription in three-dimensional collagen I gel cultures: regulation by NF-κB, AP-1 and Sp1. Int. J. Biochem. Cell Biol. 2004, 36, 353–363. [Google Scholar]
- Yan, C.; Boyd, D.D. Regulation of matrix metalloproteinase gene expression. J. Cell Physiol. 2007, 211, 19–26. [Google Scholar]
- Byun, H.J.; Hong, I.K.; Kim, E.; Jin, Y.J.; Jeoung, D.I.; Hahn, J.H.; Kim, Y.M.; Park, S.H.; Lee, H. A splice variant of CD99 increases motility and MMP-9 expression of human breast cancer cells through the AKT-, ERK-, and JNK-dependent AP-1 activation signaling pathways. J. Biol. Chem. 2006, 281, 34833–34847. [Google Scholar]
- Kuo, L.; Chang, H.C.; Leu, T.H.; Maa, M.C.; Hung, W.C. Src oncogene activates MMP-2 expression via the ERK/Sp1 pathway. J. Cell Physiol. 2006, 207, 729–734. [Google Scholar]
- Joyce, D.; Albanese, C.; Steer, J.; Fu, M.; Bouzahzah, B.; Pestell, R.G. NF-κB and cell-cycle regulation: The cyclin connection. Cytokine Growth Factor Rev. 2001, 12, 73–90. [Google Scholar]
- Korinek, V.; Barker, N.; Morin, P.J.; van Wichen, D.; de Weger, R.; Kinzler, K.W.; Vogelstein, B.; Clevers, H. Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science 1997, 275, 1784–1787. [Google Scholar]
- Song, Y.; Yang, Q.X.; Zhang, F.; Meng, F.; Li, H.; Dong, Y.; Han, A. Suppression of nasopharyngeal carcinoma cell by targeting β-catenin signaling pathway. Cancer Epidemiol. 2012, 36, e116–e121. [Google Scholar]
- Mills, C.N.; Nowsheen, S.; Bonner, J.A.; Yang, E.S. Emerging roles of glycogen synthase kinase 3 in the treatment of brain tumors. Front. Mol. Neurosci. 2011, 4, 47. [Google Scholar]
- Yuhas, J.M.; Li, A.P.; Martinez, A.O.; Ladman, A.J. A simplified method for production and growth of multicellular tumor spheroids. Cancer Res. 1977, 37, 3639–3643. [Google Scholar]
- Nicolis, S.K. Cancer stem cells and “stemness” genes in neuro-oncology. Neurobiol. Dis. 2007, 25, 217–229. [Google Scholar]
- Isbrucker, R.A.; Edwards, J.A.; Wolz, E.; Davidovich, A.; Bausch, J. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 2: Dermal, acute and short-term toxicity studies. Food Chem. Toxicol. 2006, 44, 636–650. [Google Scholar]
- Cao, Y.; Cao, R. Angiogenesis inhibited by drinking tea. Nature 1999, 398, 381. [Google Scholar]
- Jung, Y.D.; Ellis, L.M. Inhibition of tumour invasion and angiogenesis by epigallocatechin gallate (EGCG), a major component of green tea. Int. J. Exp. Pathol. 2001, 82, 309–316. [Google Scholar]
- Fidler, I.J.; Ellis, L.M. The implications of angiogenesis for the biology and therapy of cancer metastasis. Cell 1994, 79, 185–188. [Google Scholar]
- Plate, K.H.; Breier, G.; Weich, H.A.; Risau, W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature 1992, 359, 845–848. [Google Scholar]
- Jung, Y.D.; Nakano, K.; Liu, W.; Gallick, G.E.; Ellis, L.M. Extracellular signal-regulated kinase activation is required for up-regulation of vascular endothelial growth factor by serum starvation in human colon carcinoma cells. Cancer Res. 1999, 59, 4804–4807. [Google Scholar]
- Bancroft, C.C.; Chen, Z.; Yeh, J.; Sunwoo, J.B.; Yeh, N.T.; Jackson, S.; Jackson, C.; van Waes, C. Effects of pharmacologic antagonists of epidermal growth factor receptor, PI3K and MEK signal kinases on NF-κB and AP-1 activation and IL-8 and VEGF expression in human head and neck squamous cell carcinoma lines. Int. J. Cancer 2002, 99, 538–548. [Google Scholar]
- Lengyel, E.; Gum, R.; Juarez, J.; Clayman, G.; Seiki, M.; Sato, H.; Boyd, D. Induction of Mr 92,000 type IV collagenase expression in a squamous cell carcinoma cell line by fibroblasts. Cancer Res. 1995, 55, 963–967. [Google Scholar]
- Bieche, I.; Laurendeau, I.; Tozlu, S.; Olivi, M.; Vidaud, D.; Lidereau, R.; Vidaud, M. Quantitation of MYC gene expression in sporadic breast tumors with a real-time reverse transcription-PCR assay. Cancer Res. 1999, 59, 2759–2765. [Google Scholar]
- Arvidsson, S.; Kwasniewski, M.; Riano-Pachon, D.M.; Mueller-Roeber, B. QuantPrime—A flexible tool for reliable high-throughput primer design for quantitative PCR. BMC Bioinform. 2008, 9, 465. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, C.-Y.; Wu, C.-C.; Hsu, H.-Y.; Chuang, H.-Y.; Huang, S.-Y.; Tsai, C.-H.; Chang, Y.; Tsao, G.S.-W.; Chen, C.-L.; Chen, J.-Y. EGCG Inhibits Proliferation, Invasiveness and Tumor Growth by Up-Regulation of Adhesion Molecules, Suppression of Gelatinases Activity, and Induction of Apoptosis in Nasopharyngeal Carcinoma Cells. Int. J. Mol. Sci. 2015, 16, 2530-2558. https://doi.org/10.3390/ijms16022530
Fang C-Y, Wu C-C, Hsu H-Y, Chuang H-Y, Huang S-Y, Tsai C-H, Chang Y, Tsao GS-W, Chen C-L, Chen J-Y. EGCG Inhibits Proliferation, Invasiveness and Tumor Growth by Up-Regulation of Adhesion Molecules, Suppression of Gelatinases Activity, and Induction of Apoptosis in Nasopharyngeal Carcinoma Cells. International Journal of Molecular Sciences. 2015; 16(2):2530-2558. https://doi.org/10.3390/ijms16022530
Chicago/Turabian StyleFang, Chih-Yeu, Chung-Chun Wu, Hui-Yu Hsu, Hsin-Ying Chuang, Sheng-Yen Huang, Ching-Hwa Tsai, Yao Chang, George Sai-Wah Tsao, Chi-Long Chen, and Jen-Yang Chen. 2015. "EGCG Inhibits Proliferation, Invasiveness and Tumor Growth by Up-Regulation of Adhesion Molecules, Suppression of Gelatinases Activity, and Induction of Apoptosis in Nasopharyngeal Carcinoma Cells" International Journal of Molecular Sciences 16, no. 2: 2530-2558. https://doi.org/10.3390/ijms16022530





