Stem Cell Replacement Improves Expression of SMP30 in db/db Mice
Abstract
:1. Introduction
2. Results and Discussion
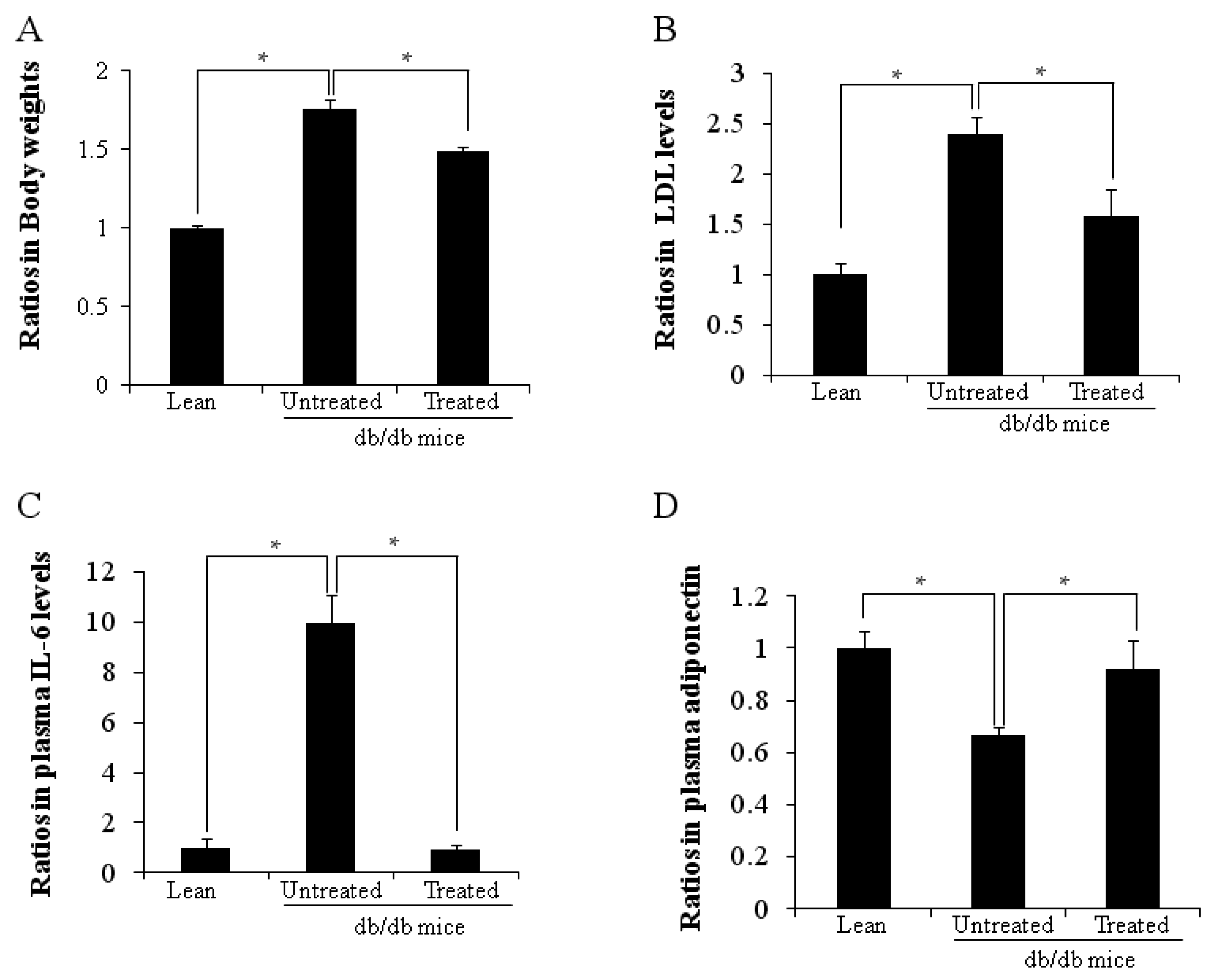
2.1. Ratios in Body Weights and Plasma Cytokine Levels
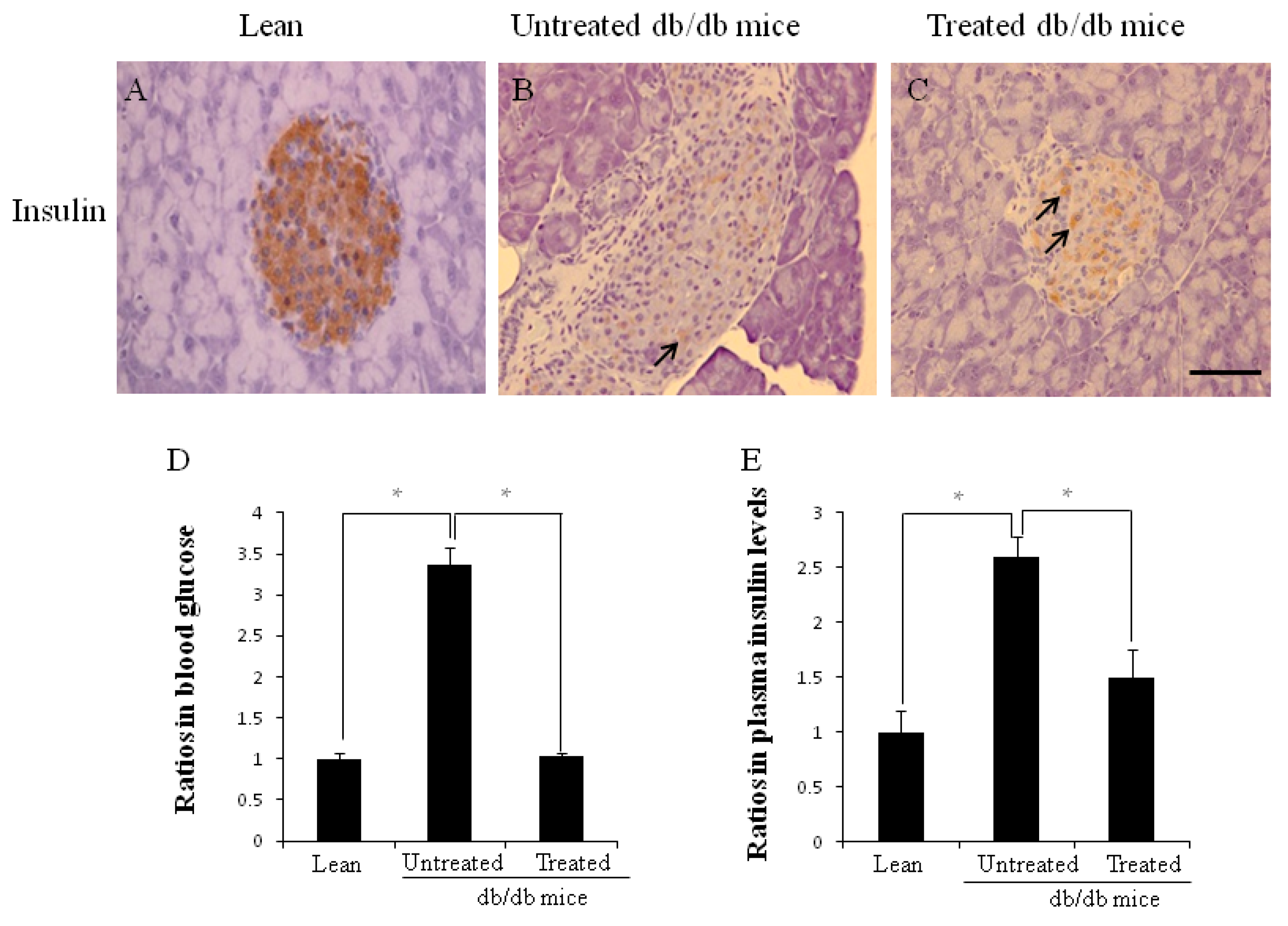
2.2. Morphology of Pancreata, Blood Glucose Levels, and Plasma Insulin Levels
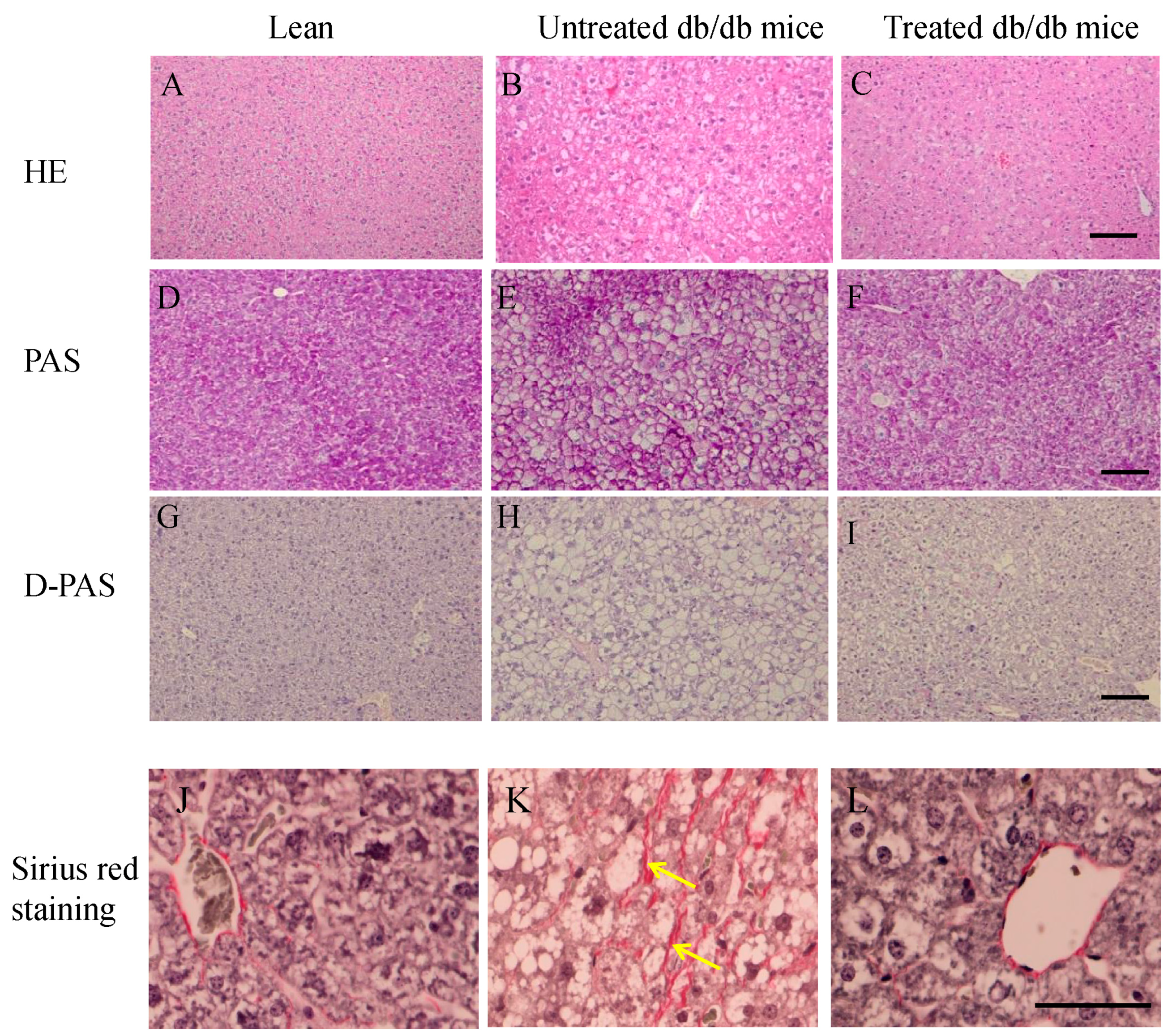
2.3. Morphology of Livers and Sirius Red Staining in the Liver
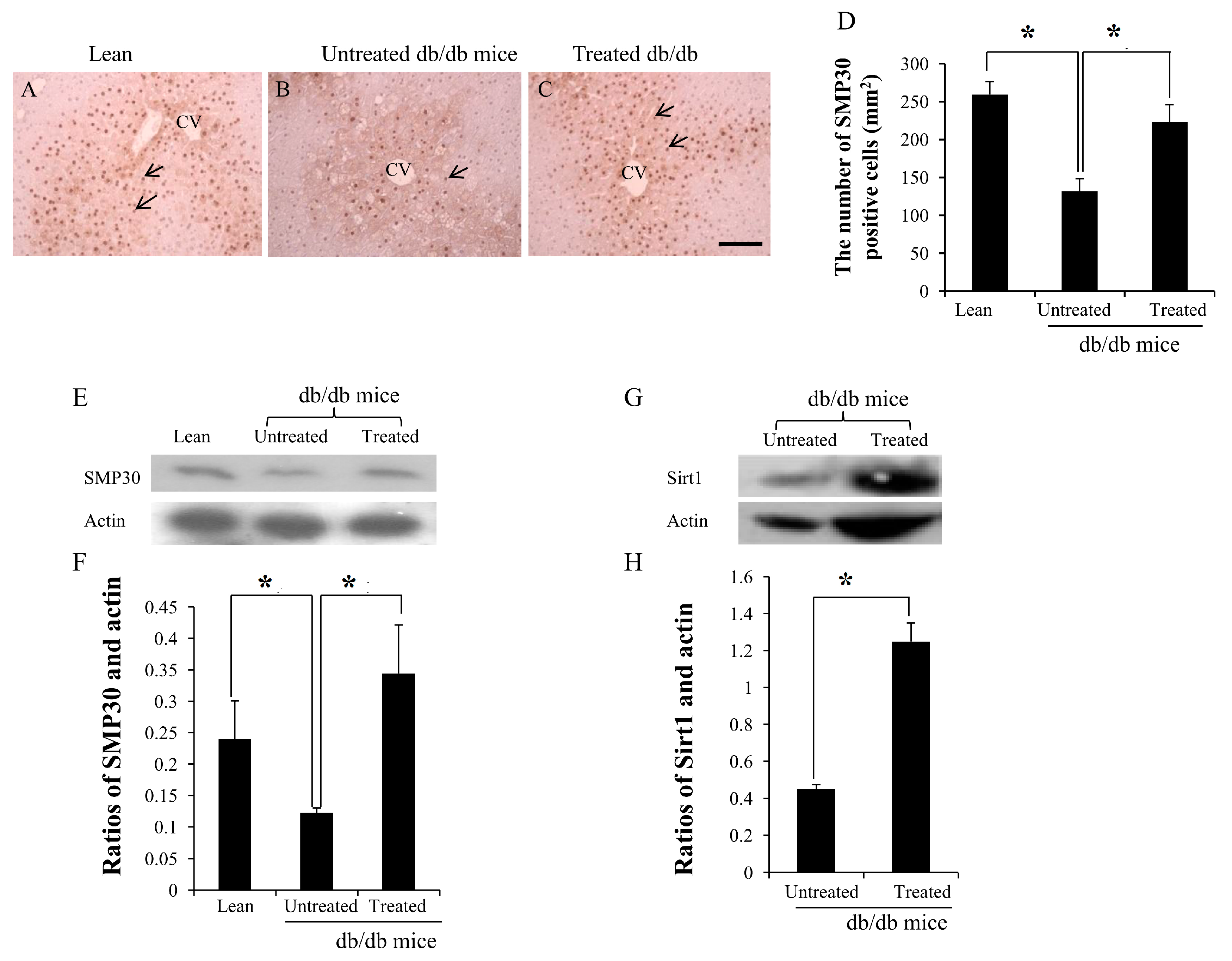
2.4. Expression of SMP30 and Sirt1 in the Livers
2.5. Discussion
3. Experimental Section
3.1. Animals
3.2. Stem Cell Replacement
3.3. Cytokines, Low-Density Lipoprotein (LDL) and Insulin Measurements
3.4. Immunochemistry
3.5. Western Blot Analysis of Sirt1 Expression
3.6. Statistical Analysis
4. Conclusion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Banks, A.S.E.D. McGlamry. Charcot foot. 1989. J. Am. Podiatr. Med. Assoc. 2007, 97, 325–348. [Google Scholar]
- Fujita, T.; Mandel, J.L.; Shirasawa, T.; Hino, O.; Shirai, T.; Maruyama, N. Isolation of cDNA clone encoding human homologue of senescence marker protein-30 (SMP30) and its location on the X chromosome. Biochim. Biophys. Acta 1995, 1263, 249–252. [Google Scholar] [CrossRef]
- Yamaguchi, M. Role of regucalcin in cell nuclear regulation: Involvement as a transcription factor. Cell Tissue Res. 2013, 354, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Murata, T. Involvement of regucalcin in lipid metabolism and diabetes. Metabolism 2013, 62, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M. The role of regucalcin in bone homeostasis: Involvement as a novel cytokine. Integr. Biol. 2014, 6, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M. The anti-apoptotic effect of regucalcin is mediated through multisignaling pathways. Apoptosis 2013, 18, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, A.; Kondo, Y.; Nanba, R.; Ohsawa, T.; Handa, S.; Kubo, S.; Akita, M.; Maruyama, N. SMP30 deficiency in mice causes an accumulation of neutral lipids and phospholipids in the liver and shortens the life span. Biochem. Biophys. Res. Commun. 2004, 315, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Iwama, M.; Amano, A.; Shimokado, K.; Maruyama, N.; Ishigami, A. Ascorbic acid levels in various tissues, plasma and urine of mice during aging. J. Nutr. Sci. Vitaminol. 2012, 58, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Inai, Y.; Sato, Y.; Handa, S.; Kubo, S.; Shimokado, K.; Goto, S.; Nishikimi, M.; Maruyama, N.; Ishigami, A. Senescence marker protein 30 functions as gluconolactonase in l-ascorbic acid biosynthesis, and its knockout mice are prone to scurvy. Proc. Natl. Acad. Sci. USA 2006, 103, 5723–5728. [Google Scholar] [CrossRef] [PubMed]
- Amano, A.; Sato, Y.; Kishimoto, Y.; Takahashi, K.; Handa, S.; Aigaki, T.; Maruyama, N.; Ishigami, A. Effects of ascorbic acid deficiency on protein and lipid oxidation in livers from SMP30/GNL knockout mice. J. Nutr. Sci. Vitaminol. 2013, 59, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Abraham, N.G.; Vanella, L.; Zhang, Y.; Inaba, M.; Hosaka, N.; Hoshino, S.; Shi, M.; Ambrosini, Y.M.; Gershwin, M.E.; et al. Successful modulation of type 2 diabetes in db/db mice with intra-bone marrow—Bone marrow transplantation plus concurrent thymic transplantation. J. Autoimmun. 2010, 35, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, A.; Masutomi, H.; Handa, S.; Maruyama, N. Age-associated decrease of senescence marker protein-30/gluconolactonase in individual mouse liver cells: Immunohistochemistry and immunofluorescence. Geriatr. Gerontol. Int. 2015, 15, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, K.; Terai, S.; Hirose, Y.; Takami, T.; Yamamoto, N.; Sakaida, I. Senescence marker protein 30 (SMP30)/regucalcin (RGN) expression decreases with aging, acute liver injuries and tumors in Zebrafish. Biochem. Biophys. Res. Commun. 2011, 414, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Guo, K.; Vanella, L.; Taketani, S.; Adachi, Y.; Ikehara, S. Stem cell transplantation upregulates Sirt1 and antioxidant expression, ameliorating fatty liver in type 2 diabetic mice. Int. J. Biol. Sci. 2015, 11, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, G.; Samuel, S.M.; Marei, I.; Ding, H.; Triggle, C.R. Metformin modulates hyperglycaemia-induced endothelial senescence and apoptosis through SIRT1. Br. J. Pharmacol. 2014, 171, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.J.; Lee, E.K.; Kim, S.J.; Song, C.W.; Maruyama, N.; Ishigami, A.; Kim, N.D.; Im, D.S.; Yu, B.P.; Chung, H.Y. Anti-inflammatory activity of SMP30 modulates NF-κB through protein tyrosine kinase/phosphatase balance. J. Mol. Med. 2015, 93, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, G.; Yamasaki, M.; Kadono, M.; Tanaka, M.; Asano, M.; Senmaru, T.; Kondo, Y.; Fukui, M.; Obayashi, H.; Maruyama, N.; et al. Senescence marker protein-30/gluconolactonase deletion worsens glucose tolerance through impairment of acute insulin secretion. Endocrinology 2010, 151, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Ishigami, A.; Shima, T.; Mizuno, M.; Maruyama, N.; Yamaguchi, K.; Mitsuyoshi, H.; Minami, M.; Yasui, K.; Itoh, Y.; et al. Hepatic senescence marker protein-30 is involved in the progression of nonalcoholic fatty liver disease. J. Gastroenterol. 2010, 45, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Ki, M.R.; Lee, H.R.; Hong, I.H.; Ji, A.R.; Ishigami, A.; Park, S.I.; Kim, J.M.; Chung, H.Y.; Yoo, S.E.; Jeong, K.S. Vitamin C deficiency attenuates liver fibrosis by way of up-regulated peroxisome proliferator-activated receptor-γ expression in senescence marker protein 30 knockout mice. Hepatology 2010, 51, 1766–1777. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Hasegawa, G.; Okada, H.; Senmaru, T.; Fukui, M.; Nakamura, N.; Sawada, M.; Kitawaki, J.; Okanoue, T.; Kishimoto, Y.; et al. Lepr(db/db) Mice with senescence marker protein-30 knockout (Lepr(db/db)SMP30(Y/‒)) exhibit increases in small dense-LDL and severe fatty liver despite being fed a standard diet. PLoS ONE 2013, 8, e65698. [Google Scholar] [CrossRef] [PubMed]
- Nejak-Bowen, K.N.; Zeng, G.; Tan, X.; Cieply, B.; Monga, S.P. β-catenin regulates vitamin C biosynthesis and cell survival in murine liver. J. Biol. Chem. 2009, 284, 28115–28127. [Google Scholar] [CrossRef] [PubMed]
- Kohli, R.; Pan, X.; Malladi, P.; Wainwright, M.S.; Whitington, P.F. Mitochondrial reactive oxygen species signal hepatocyte steatosis by regulating the phosphatidylinositol 3-kinase cell survival pathway. J. Biol. Chem. 2007, 282, 21327–21336. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, N.; Ishigami, A.; Kondo, Y. Pathophysiological significance of senescence marker protein-30. Geriatr. Gerontol. Int. 2010, 10, S88–S98. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Masutomi, H.; Noda, Y.; Ozawa, Y.; Takahashi, K.; Handa, S.; Maruyama, N.; Shimizu, T.; Ishigami, A. Senescence marker protein-30/superoxide dismutase 1 double knockout mice exhibit increased oxidative stress and hepatic steatosis. FEBS Open Biol. 2014, 4, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Vanella, L.; Zhang, Y.; Shi, M.; Takaki, T.; Shapiro, J.I.; Ikehara, S. Stem cell transplantation increases antioxidant effects in diabetic mice. Int. J. Biol. Sci. 2012, 8, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Guo, K.; Taketani, S.; Adachi, Y.; Ikehara, S. Stem Cell Replacement Improves Expression of SMP30 in db/db Mice. Int. J. Mol. Sci. 2015, 16, 29971-29979. https://doi.org/10.3390/ijms161226217
Li M, Guo K, Taketani S, Adachi Y, Ikehara S. Stem Cell Replacement Improves Expression of SMP30 in db/db Mice. International Journal of Molecular Sciences. 2015; 16(12):29971-29979. https://doi.org/10.3390/ijms161226217
Chicago/Turabian StyleLi, Ming, Kequan Guo, Shigeru Taketani, Yasushi Adachi, and Susumu Ikehara. 2015. "Stem Cell Replacement Improves Expression of SMP30 in db/db Mice" International Journal of Molecular Sciences 16, no. 12: 29971-29979. https://doi.org/10.3390/ijms161226217




