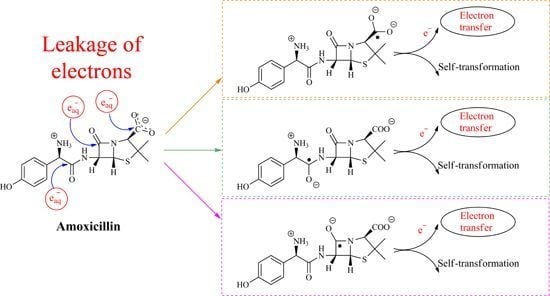
One-Electron Reduction of Penicillins in Relation to the Oxidative Stress Phenomenon
Abstract
:1. Introduction
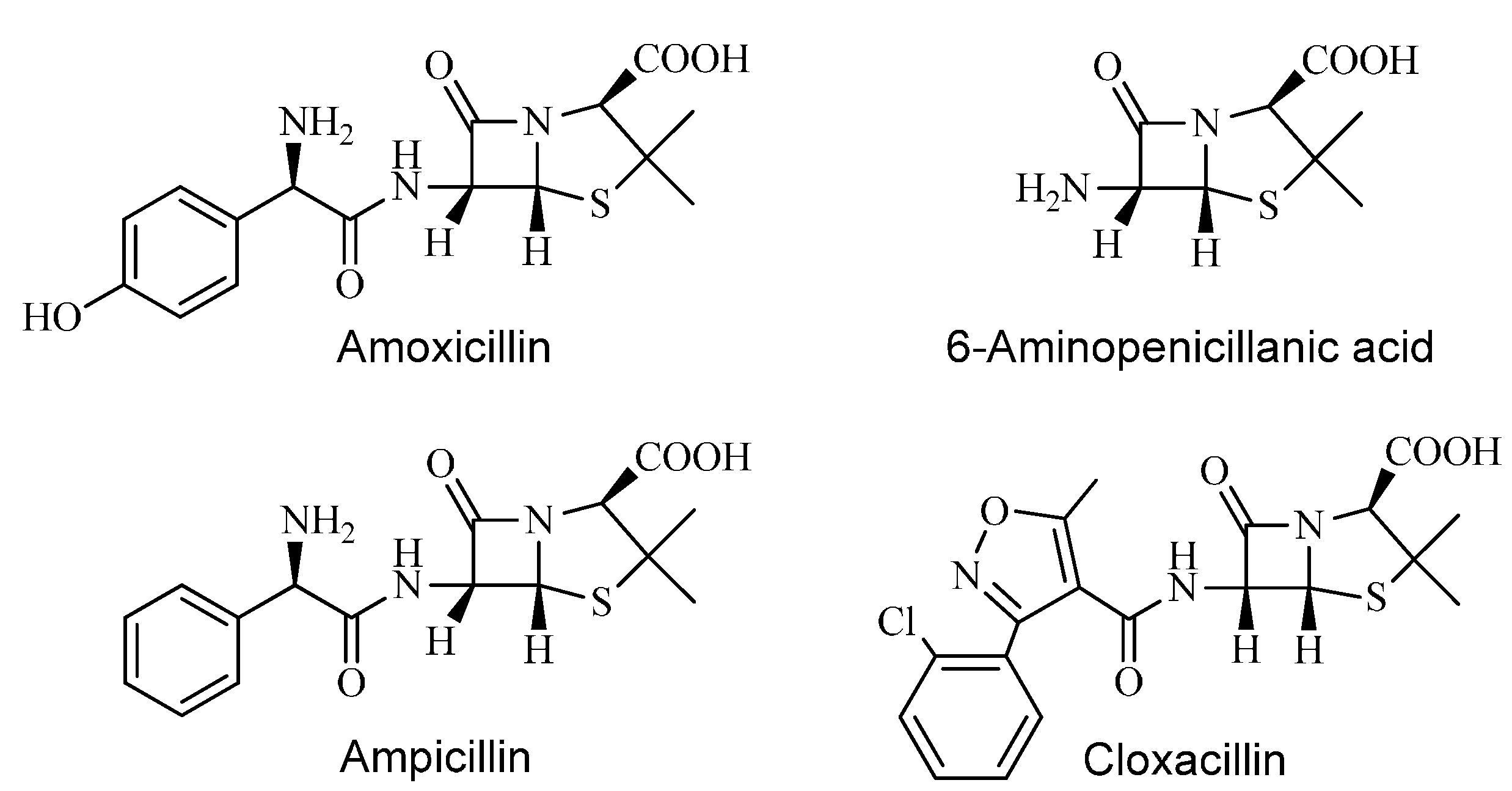
2. Results and Discussion
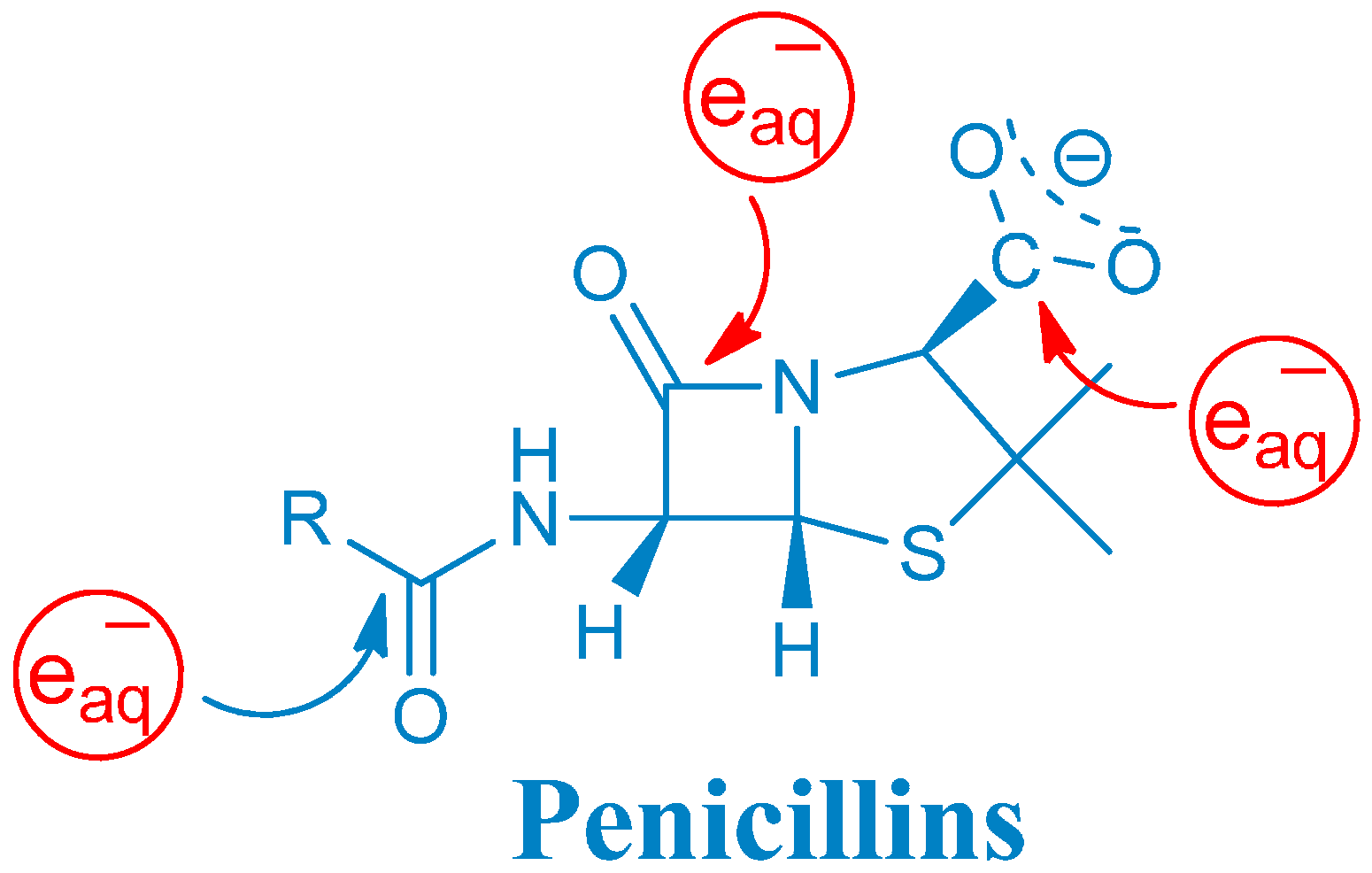
2.1. Reaction Mechanism of One-Electron Reduction
| 13C NMR Chemical Shifts of Penicillins | |||
|---|---|---|---|
| Lactam >C=O | Carboxyl –COOH | Peptidyl –C(O)NH– | |
| Amoxicillin | 172.93 | 169.9 | 169.56 |
| Ampicillin | 172.93 | 170.31 | 169.42 |
| Cloxacillin | 172.18/172.22 | 169.72 | 160.41 |
| 6-Aminopenicillanic acid | 169.86 | 179.2 | – |
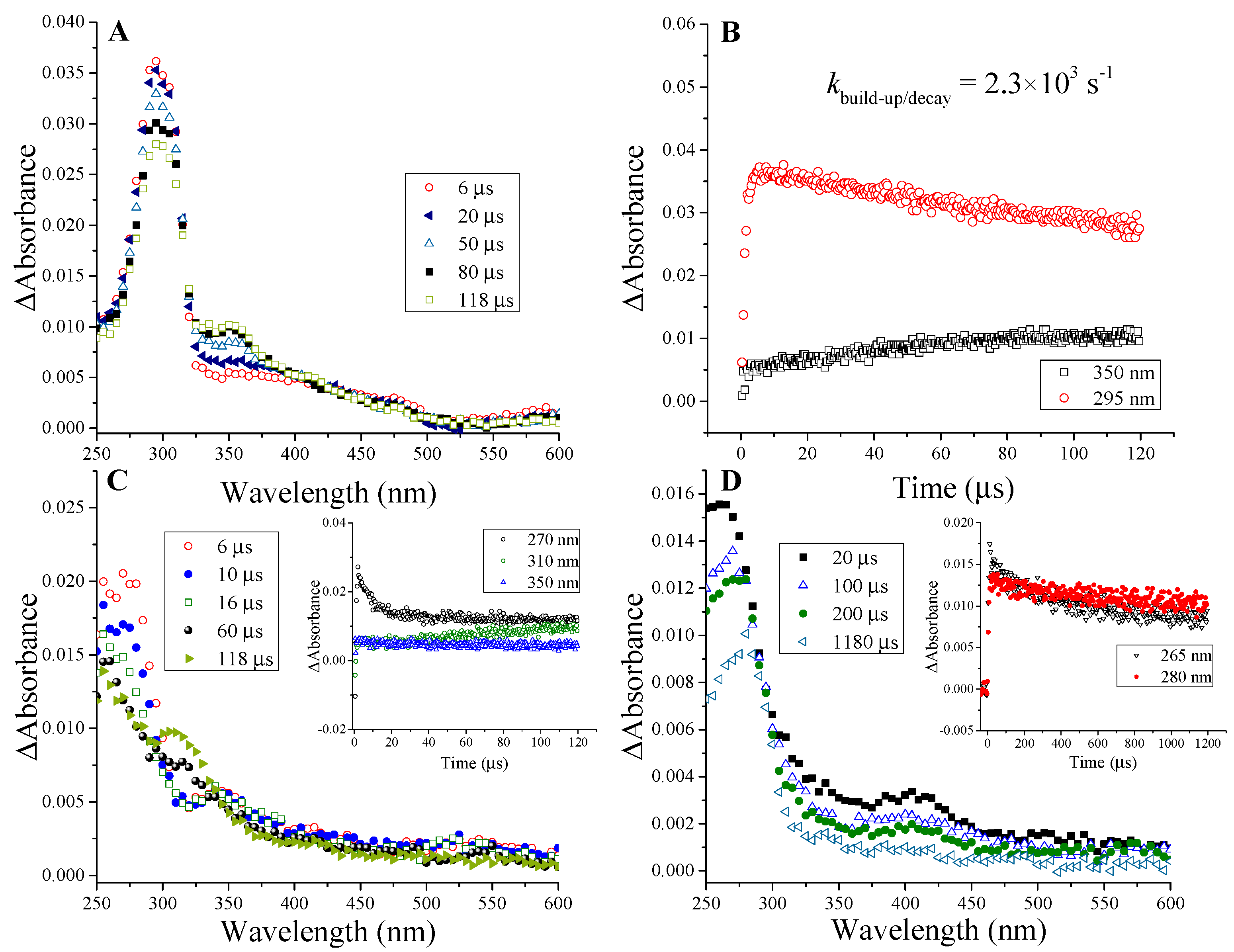
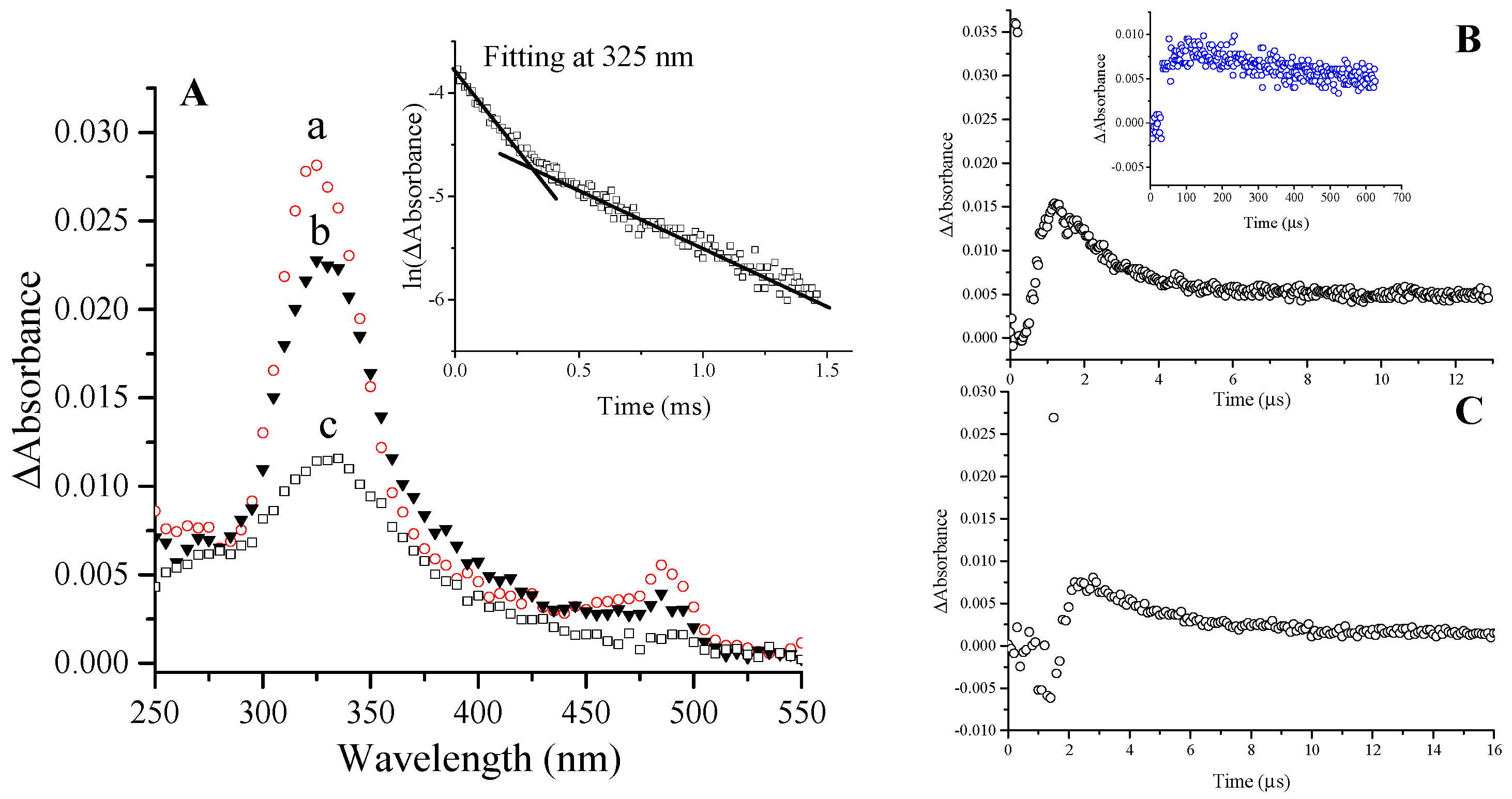
2.1.1. Ketyl Radicals of the β-Lactam Carbonyl
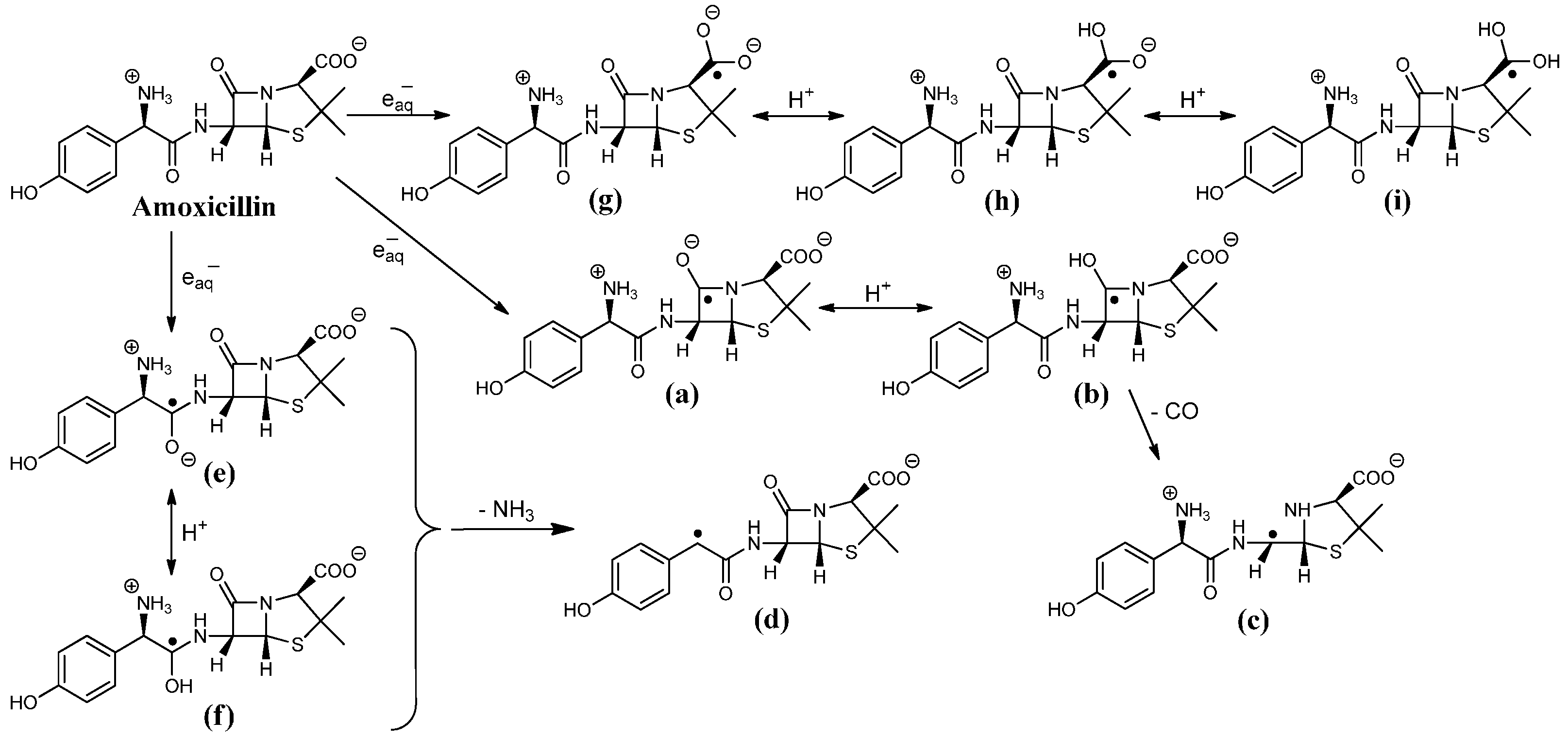
2.1.2. Benzyl Radicals of Ampicillin and Amoxicillin
2.1.3. Ketyl Radicals of the Peptidyl Carbonyl
2.1.4. Ketyl Radicals of the Carboxylate Group
2.1.5. General Mechanism of One-Electron Reduction of Penicillins
| keaq | α-Hydroxyalkyl Radicals | Benzyl Radicals | |||
|---|---|---|---|---|---|
| ×109 mol−1·dm3·s−1 | β-Lactam Carbonyl | Peptidyl Carbonyl | Carboxylate Carbonyl | ||
| 6-Aminopenicillanic acid | 8.8 [9] | 265 nm; k not determined | absorbs < 240 nm | 400 nm; kdecay ≈ 2.4 × 103 s−1 | no formation |
| Cloxacillin | 5.5 | 270 nm; k not determined | ~350 nm | no formation | |
| Ampicillin | 5.5 | 295 nm; kdecay = 2.3 × 103 s−1 | ~480 nm | 295 nm; kdecay = 1.1 × 103 s−1 | |
| Amoxicillin | 5.2 [9] | 325 nm; kdecay = 2.7 × 103s−1 | 485 nm; kdecay ≈ 2.9 × 103 s−1 | 325 nm; kdecay = 6 × 102 s−1 | |
3. Experimental Section
3.1. Materials
3.2. Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Keren, I.; Wu, Y.; Inocencio, J.; Mulcahy, L.R.; Lewis, K. Killing by bactericidal antibiotics does not depend on reactive oxygen species. Science 2013, 339, 1213–1216. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Imlay, J.A. Cell death from antibiotics without the involvement of reactive oxygen species. Science 2013, 339, 1210–1213. [Google Scholar] [CrossRef] [PubMed]
- Pochini, L.; Galluccio, M.; Scumaci, D.; Giangregorio, N.; Tonazzi, A.; Palmieri, F.; Indiveri, C. Interaction of β-lactam antibiotics with the mitochondrial carnitine/acylcarnitine transporter. Chem. Biol. Interact. 2008, 173, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Kalghatgi, S.; Spina, C.S.; Costello, J.C.; Liesa, M.; Morones-Ramirez, J.R.; Slomovic, S.; Molina, A.; Shirihai, O.S.; Collins, J.J. Bactericidal antibiotics induce mitochondrial dysfunction and oxidative damage in mammalian cells. Sci. Transl. Med. 2013, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Imlay, J.A. Diagnosing oxidative stress in bacteria: Not as easy as you might think. Curr. Opin. Microbiol. 2015, 24, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Philips, G.O.; Power, D.M.; Robinson, C.; Davies, J.V. Interactions of bovine serum albumin with penicillins and cephalosporins studied by pulse radiolysis. Biochim. Biophys. Acta 1973, 295, 8–17. [Google Scholar] [CrossRef]
- Song, W.; Chen, W.; Cooper, W.J.; Greaves, J.; Miller, G.E. Free-radical destruction of β-lactam antibiotics in aqueous solution. J. Phys. Chem. A 2008, 112, 7411–7417. [Google Scholar] [CrossRef] [PubMed]
- Szabó, L.; Tóth, T.; Rácz, G.; Takács, E.; Wojnárovits, L. OH and eaq− are yet good candidates for demolishing the β-lactam system of a penicillin eliminating the antimicrobial activity. Radiat. Phys. Chem. 2015, in press. [Google Scholar] [CrossRef]
- Szabó, L.; Tóth, T.; Rácz, G.; Takács, E.; Wojnárovits, L. Drugs with susceptible sites for free radical induced oxidative transformations: The case of a penicillin. Free Radic. Res. 2015, in press. [Google Scholar] [CrossRef]
- Hart, E.J.; Fielden, E.M.; Anbar, M. Reactions of carbonylic compounds with hydrated electrons. J. Phys. Chem. 1967, 71, 3993–3998. [Google Scholar] [CrossRef]
- Tal, Y.; Faraggi, M. The reaction of the hydrated electron with amino acids, peptides, and proteins in aqueous solution. I. Factors affecting the rate constants. Radiat. Res. 1975, 62, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S.; Hayon, E. Interaction of hydrated electrons with the peptide linkage. J. Phys. Chem. 1974, 78, 1193–1196. [Google Scholar] [CrossRef]
- Schwarz, H.A. Reaction of the hydrated electron with water. J. Phys. Chem. 1992, 96, 8937–8941. [Google Scholar] [CrossRef]
- Janata, E.; Schuler, R.H. Direct observation of the protonation of acetone ketyl radical by conductometric pulse radiolysis. J. Phys. Chem. 1980, 84, 3351–3353. [Google Scholar] [CrossRef]
- Simic, M.; Neta, P.; Hayon, E. Pulse radiolysis study of alcohols in aqueous solution. J. Phys. Chem. 1969, 73, 3794–3798. [Google Scholar] [CrossRef]
- Garrison, W.M. Reaction mechanisms in the radiolysis of peptides, polypeptides, and proteins. Chem. Rev. 1987, 87, 381–398. [Google Scholar] [CrossRef]
- Faraggi, M.; Tal, Y. The reactions of the hydrated electron with amino acids, peptides, and proteins in aqueous solution. II. Formation of radicals and electron transfer reactions. Radiat. Res. 1975, 62, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Strzelczak, G.; Bobrowski, K.; Holcman, J. Reduction of selected oligopeptides containing methionine induced by hydrated electrons. Radiat. Res. 1998, 150, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Chain, E.B. The chemical structure of the penicillins. In Nobel Lectures, Physiology or Medicine, 1942–1962; Elsevier: Amsterdam, The Netherlands, 1964; pp. 110–143. [Google Scholar]
- Robinson-Fuentes, V.A.; Jefferies, T.M.; Branch, S.K. Degradation pathways of ampicillin in alkaline solutions. J. Pharm. Pharmacol. 1997, 49, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Blanpain, P.C.; Nagy, J.B.; Laurent, G.H.; Durant, F.V. A multifaceted approach to the study of the side-chain conformation in β-lactam antibiotics. J. Med. Chem. 1980, 23, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.S.R.; Vasudevan, T.K. Conformation and activity of β-lactam antibiotics. Crit. Rev. Biochem. 1983, 14, 173–206. [Google Scholar] [CrossRef]
- Mittal, J.P.; Hayon, E. Interaction of hydrated electrons with phenylalanine and related compounds. J. Phys. Chem. 1974, 78, 1790–1794. [Google Scholar] [CrossRef]
- Simic, M.; Neta, P.; Hayon, E. Selectivity in the reactions of eaq− and OH radicals with simple peptides in aqueous solution. Optical absorption spectra of intermediates. J. Am. Chem. Soc. 1970, 92, 4763–4768. [Google Scholar] [CrossRef] [PubMed]
- Simic, M.; Hoffman, M.Z. Acid-base properties of the radicals produced in the pulse radiolysis of aqueous solutions of benzoic acid. J. Phys. Chem. 1972, 76, 1398–1404. [Google Scholar] [CrossRef]
- Buxton, G.V.; Stuart, C.R. Re-evaluation of the thiocyanate dosimeter for pulse radiolysis. J. Chem. Soc. Faraday Trans. 1995, 91, 279–281. [Google Scholar] [CrossRef]
- Földiák, G.; Hargittai, P.; Kaszanyiczki, L.; Wojnárovits, L. A computer controlled pulse radiolysis laboratory. J. Radioanal. Nucl. Chem. 1988, 125, 19–28. [Google Scholar] [CrossRef]
- Takács, E.; Wojnárovits, L.; Dajka, K. Kinetics of the early stages of high-energy radiation initiated polymerization. Macromol. Chem. Phys. 2000, 201, 2170–2175. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, L.; Tóth, T.; Takács, E.; Wojnárovits, L. One-Electron Reduction of Penicillins in Relation to the Oxidative Stress Phenomenon. Int. J. Mol. Sci. 2015, 16, 29673-29681. https://doi.org/10.3390/ijms161226130
Szabó L, Tóth T, Takács E, Wojnárovits L. One-Electron Reduction of Penicillins in Relation to the Oxidative Stress Phenomenon. International Journal of Molecular Sciences. 2015; 16(12):29673-29681. https://doi.org/10.3390/ijms161226130
Chicago/Turabian StyleSzabó, László, Tünde Tóth, Erzsébet Takács, and László Wojnárovits. 2015. "One-Electron Reduction of Penicillins in Relation to the Oxidative Stress Phenomenon" International Journal of Molecular Sciences 16, no. 12: 29673-29681. https://doi.org/10.3390/ijms161226130