Hydrolysis of Oligosaccharides by a Thermostable α-Galactosidase from Termitomyces eurrhizus
Abstract
:1. Introduction
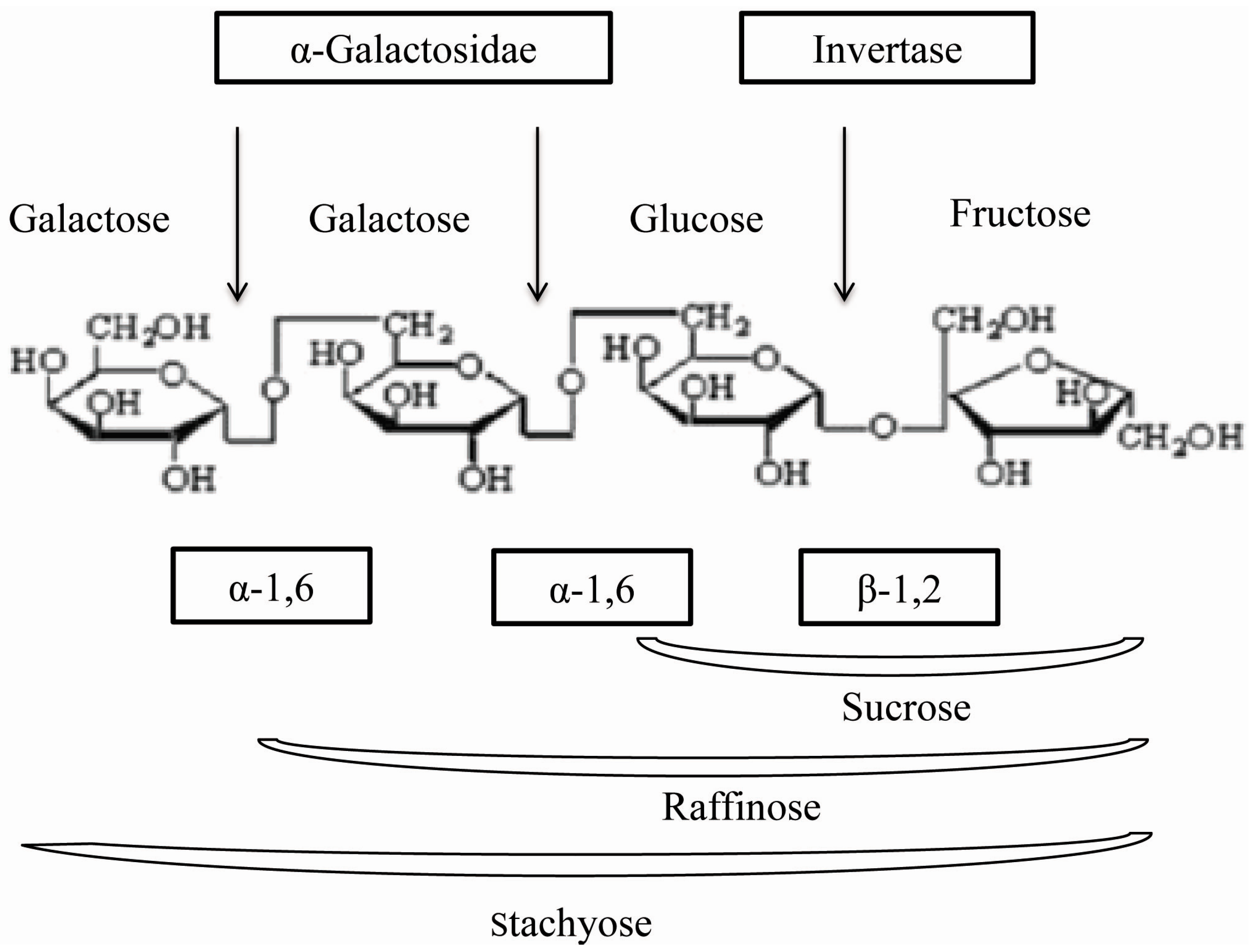
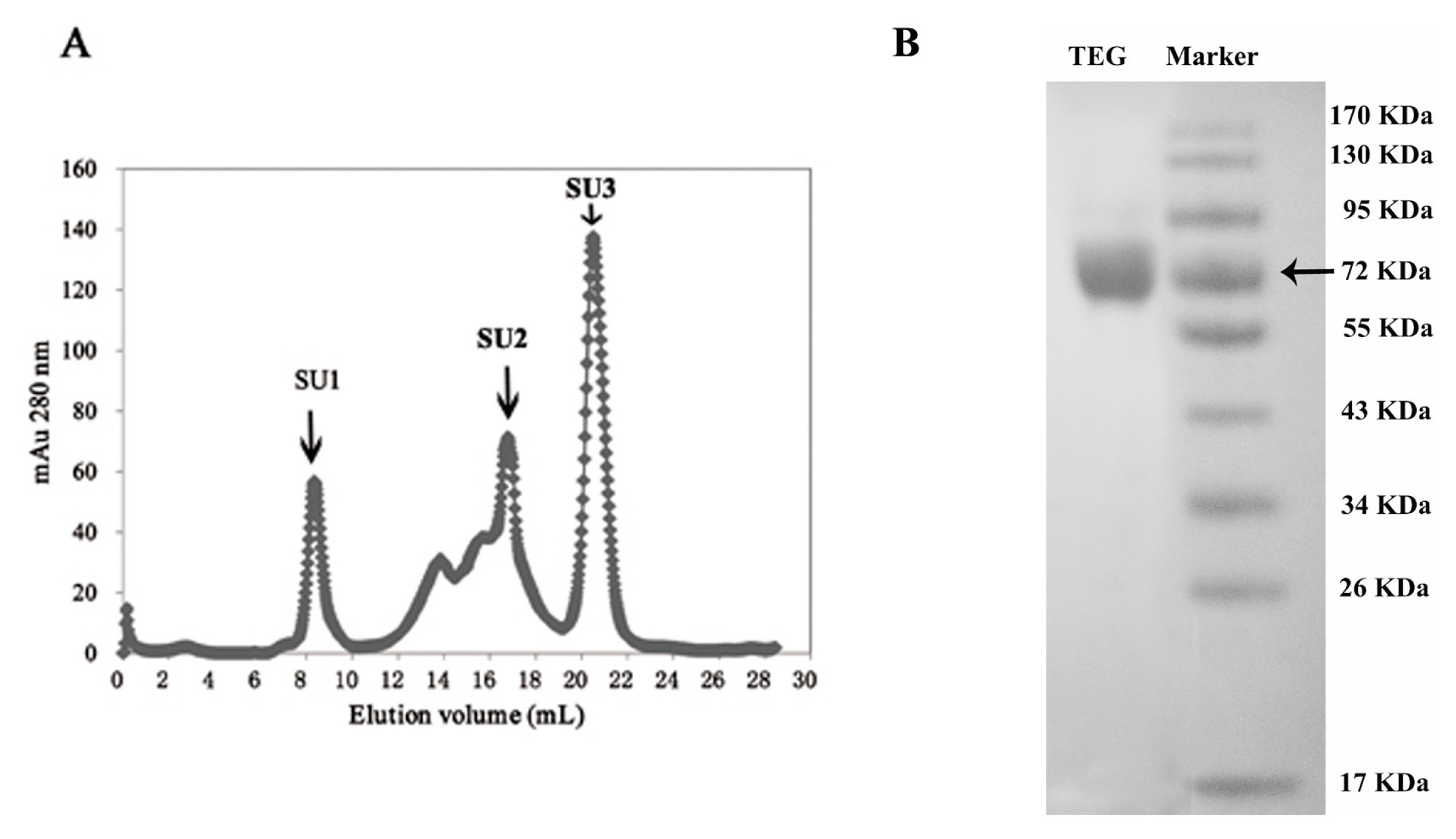
2. Results
| Chromatographic Fraction | Protein | Total Activity | Special Activity | Yield | Purification |
|---|---|---|---|---|---|
| (mg) | (U) a | (U/mg) b | (%) | Fold c | |
| Crude extract | 4384 | 93,032 | 21.39 | 100 | 1 |
| D2 | 1120 | 50,465 | 45 | 54.2 | 2.1 |
| CM2 | 40.25 | 26,744 | 664 | 28.7 | 31.1 |
| Q2 | 6.75 | 18,418 | 2728 | 19.8 | 127 |
| SU1 | 0.445 | 1395 | 3135 | 1.4 | 146 |

| Metalion | Relative Galactosidase Activity (%) | |||
|---|---|---|---|---|
| Concentration | 10 mM | 5 mM | 2.5 mM | 1.25 mM |
| Fe2+ | 109.77 ± 0.49 | 113.14 ± 0.25 | 109.12 ± 2.25 | 109.77 ± 0.49 |
| K+ | 138.74 ± 2.93 | 133.07 ± 1.73 | 128.02 ± 2.91 | 129.14 ± 0.25 |
| Ca2+ | 148.85 ± 2.14 | 137.22 ± 1.74 | 133.25 ± 0.81 | 123.04 ± 0.64 |
| Cd2+ | 10.07 ± 0.49 | 9.71 ± 0.36 | 8.47 ± 0.15 | 10.38 ± 0.26 |
| Cu2+ | 7.91 ± 0.75 | 95.03 ± 2.42 | 107.57 ± 2.22 | 98.05 ± 0.60 |
| Hg2+ | 11.67 ± 0.35 | 8.47 ± 0.32 | 11.02 ± 0.36 | 14.01 ± 0.87 |
| Mg2+ | 91.31 ± 0.79 | 89.8 ± 0.55 | 93.43 ± 2.40 | 95.68 ± 1.53 |
| Mn2+ | 35.67 ± 2.00 | 46.91 ± 2.29 | 63.99 ± 1.84 | 88.59 ± 2.19 |
| Pb2+ | 116.04 ± 1.02 | 103.89 ± 0.87 | 101.99 ± 1.96 | 97.92 ± 2.07 |
| Zn2+ | 95.11 ± 1.63 | 116.34 ± 0.66 | 107.52 ± 2.38 | 105.53 ± 2.11 |
| Al3+ | 21.49 ± 0.50 | 61.31 ± 1.78 | 103.63 ± 1.85 | 115.48 ± 2.75 |
| Fe3+ | 3.72 ± 0.25 | 16.99 ± 0.70 | 20.62 ± 1.82 | 21.57 ± 1.19 |
| Substrate | Concentration (mM) | Relative Activity a (%) |
|---|---|---|
| pNPGal | 10 | 100 |
| oNPGal | 10 | 54.38 ± 0.13 |
| 4-Nitrophenyl β-d-glucuronide | 10 | 4.95 ± 0.24 |
| Raffinose | 50 | 40.24 ± 0.21 |
| Melibiose | 50 | 20.89 ± 0.20 |
| Stachyose | 50 | 15.11 ± 0.16 |
| Locust bean gum | 5% | - |
| Guar gum | 5% | - |
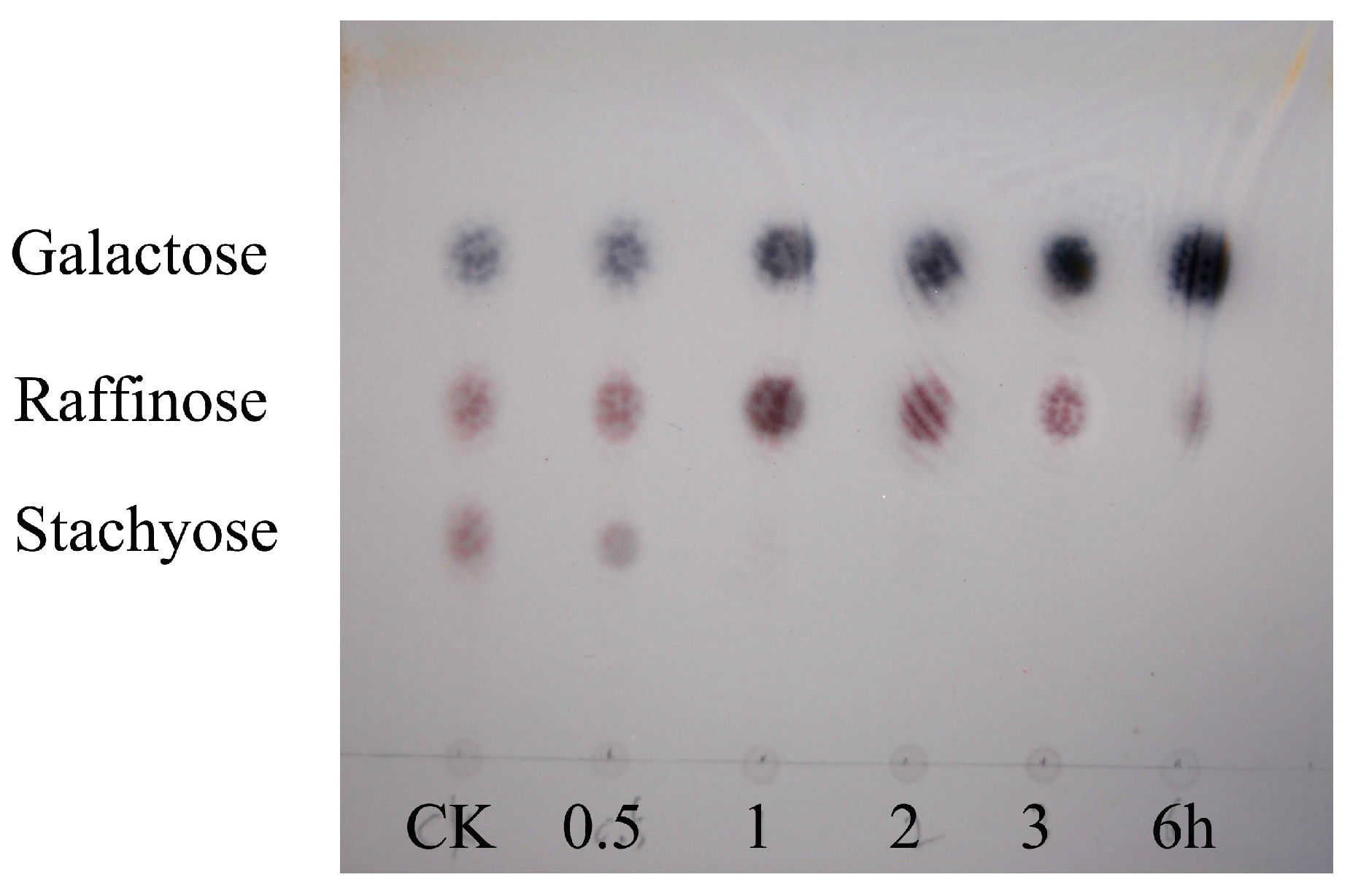
3. Discussion
4. Experimental Section
4.1. The Identification on the Genus of Termitomyces
4.2. Enzyme Activity Assay
4.3. Purification of TEG
4.4. The Molecular Mass of TEG
4.5. Internal Peptide Sequence Analysis of TEG
4.6. Biochemical Properties of TEG
4.7. Substrate Specificity of TEG
4.8. Analysis of Hydrolysis Products by TLC
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chakraborty, I.; Mondal, S.; Rout, D.; Islam, S.S. A water-insoluble (1→3)-β-d-glucan from the alkaline extract of an edible mushroom Termitomyces eurhizus. Carbohydr. Res. 2006, 341, 2990–2993. [Google Scholar] [CrossRef] [PubMed]
- Chandra, K.; Ghosh, K.; Roy, S.K.; Mondal, S.; Maiti, D.; Ojha, A.K.; Das, D.; Mondal, S.; Islam, S.S. A water-soluble glucan isolated from an edible mushroom Termitomyces microcapus. Carbohydr. Res. 2007, 342, 2484–2489. [Google Scholar] [CrossRef] [PubMed]
- Johjima, T.; Ohkuma, M.; Kudo, T. Isolation and cDNA cloning of novel hydrogen peroxide-dependent phenol oxidase from the basidiomycete Termitomyces albuminosus. Appl. Microbiol. Biotechnol. 2003, 61, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Ng, T.B. Isolation of a ribonuclease from fruiting bodies of the wild mushroom Termitomyces globulus. Peptides 2003, 24, 973–977. [Google Scholar] [CrossRef]
- Zheng, S.Y.; Wang, H.X.; Zhang, G.Q. A novel alkaline protease from wild edible mushroom Termitomyces albuminosus. Acta Biochim. Pol. 2011, 58, 269–273. [Google Scholar] [PubMed]
- Viana, P.A.; de Rezende, S.T.; Marques, V.M.; Trevizano, L.M.; Passos, F.M. L.; Oliveira, M.G.A.; Bemquerer, M.P.; Oliveira, J.S.; Guimaraes, V.M. Extracellular α-galactosidase from Debaryomyces hansenii UFV-1 and its use in the hydrolysis of raffinose oligosaccharides. J. Agric. Food Chem. 2006, 54, 2385–2391. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, N.; Saraswathy, N.; Sadasivam, S.; Subha, K.; Poorani, N. Purification and properties of α-galactosidase from white-rot fungus Pleurotus florida. Indian J. Biochem. Biophys. 2007, 44, 76–81. [Google Scholar] [PubMed]
- Shankar, S.K.; Dhananjay, S.K.; Mulimani, V.H. Purification and characterization of thermostable α-galactosidase from Aspergillus terreus (GR). Appl. Biochem. Biotechnol. 2009, 152, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Kayastha, A.M. A novel application of Cicer α-galactosidase in reduction of raffinose family oligosaccharides in soybean flour. J. Plant Biochem. Biotechnol. 2013, 22, 353–356. [Google Scholar] [CrossRef]
- Suzuki, H.; Ozawa, Y.; Oota, H.; Kobayashi, H. Studies on the decomposition of raffinose by α galactosidase of mold part 1-α galactosidase formation and hydrolysis of raffinose by the enzyme preparation. Agric. Biol. Chem. Tokyo 1969, 33, 506–513. [Google Scholar] [CrossRef]
- Ghazi, S.; Rooke, J.A.; Galbraith, H. Improvement of the nutritive value of soybean meal by protease and α-galactosidase treatment in broiler cockerels and broiler chicks. Br. Poult. Sci. 2003, 44, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Kotiguda, G.; Kapnoor, S.S.; Kulkarni, D.; Mulimani, V.H. Degradation of raffinose oligosaccharides in soymilk by immobilized α-galactosidase of Aspergillus oryzae. J. Microbiol. Biotechnol. 2007, 17, 1430–1436. [Google Scholar] [PubMed]
- Weignerova, L.; Simerska, P.; Kren, V. α-Galactosidases and their applications in biotransformations. Biocatal. Biotransform. 2009, 27, 79–89. [Google Scholar] [CrossRef]
- Gote, M.M.; Khan, M.I.; Khire, J.M. Active site directed chemical modification of α-galactosidase from Bacillus stearothermophilus (NCIM 5146): Involvement of lysine, tryptophan and carboxylate residues in catalytic site. Enzym. Microb. Technol. 2007, 40, 1312–1320. [Google Scholar] [CrossRef]
- Comfort, D.A.; Bobrov, K.S.; Ivanen, D.R.; Shabalin, K.A.; Harris, J.M.; Kulminskaya, A.A.; Brumer, H.; Kelly, R.M. Biochemical analysis of Thermotoga maritima GH36 α-galactosidase (TmGalA) confirms the mechanistic commonality of clan GH-D glycoside hydrolases. Biochemistry 2007, 46, 3319–3330. [Google Scholar] [CrossRef] [PubMed]
- Sampietro, D.; Quiroga, E.; Sgariglia, M.; Soberon, J.; Vattuone, M.A. A thermostable α-galactosidase from Lenzites elegans (Spreng.) ex Pat. MB445947: Purification and properties. Antonie Van Leeuwenhoek 2012, 102, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Dotsenko, G.S.; Semenova, M.V.; Sinitsyna, O.A.; Hinz, S.W.A.; Wery, J.; Zorov, I.N.; Kondratieva, E.G.; Sinitsyn, A.P. Cloning, purification, and characterization of galactomannan-degrading enzymes from Myceliophthora thermophila. Biochemistry 2012, 77, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Kayastha, A.M. Purification and characterization of α-galactosidase from white chickpea (Cicer arietinum). J. Agric. Food Chem. 2012, 60, 3253–3259. [Google Scholar] [CrossRef] [PubMed]
- Sripuan, T.; Aoki, K.; Yamamoto, K.; Tongkao, D.; Kumagi, H. Purification and characterization of thermostable α-galactosidase from Ganoderma lucidum. Biosci. Biotechnol. Biochem. 2003, 67, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Borzova, N.V.; Varbanets, L.D. Thermal inactivation of α-galactosidase from Penicillium canescens. Ukr. Biokhim. Zh. 2010, 82, 24–30. [Google Scholar]
- Patil, A.G.; Praveen Kumar, S.K.; Mulimani, V.H.; Veeranagouda, Y.; Lee, K. α-Galactosidase from Bacillus megaterium VHM1 and its application in removal of flatulence-causing factors from soymilk. J. Microbiol. Biotechnol. 2010, 20, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Shi, P.; Huang, H.; Cao, Y.; Meng, K.; Yang, P.; Zhang, R.; Chen, X.; Yao, B. A new α-galactosidase from symbiotic Flavobacterium sp. TN17 reveals four residues essential for α-galactosidase activity of gastrointestinal bacteria. Appl. Microbiol. Biotechnol. 2010, 88, 1297–1309. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, J.; Bao, Y.; An, L. Purification and characterization of Ulva pertusa Kjellm alkaline phosphatase. Prep. Biochem. Biotechnol. 2003, 33, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Vernekar, J.V.; Tanksale, A.M.; Ghatge, M.S.; Deshpande, V.V. Novel bifunctional alkaline protease inhibitor: Protease inhibitory activity as the biochemical basis of antifungal activity. Biochem. Biophys. Res. Commun. 2001, 285, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Q.; Cui, F.M.; Yang, X.Q.; Qian, S.J. Purification and properties of endoinulinase from Chaetomium sp. Wei Sheng Wu Xue Bao 2004, 44, 785–788. [Google Scholar] [PubMed]
- Ferreira, J.G.; Reis, A.P.; Guimaraes, V.M.; Falkoski, D.L.; Fialho, L.D.; de Rezende, S.T. Purification and Characterization of Aspergillus terreus α-galactosidases and their use for hydrolysis of soymilk oligosaccharides. Appl. Biochem. Biotechnol. 2011, 164, 1111–1125. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.M.; Patel, S.; Brownleader, M.D. Induction of α-galactosidase in penicillium-ochrochloron by guar (Cyamopsis-Tetragonobola) gum. Biotechnol. Appl. Biochem. 1993, 17, 361–371. [Google Scholar] [PubMed]
- MargollesClark, E.; Tenkanen, M.; Luonteri, E.; Penttila, M. Three α-galactosidase genes of Trichoderma reesei cloned by expression in yeast. Eur. J. Biochem. 1996, 240, 104–111. [Google Scholar] [CrossRef]
- Jindou, S.; Karita, S.; Fujino, E.; Fujino, T.; Hayashi, H.; Kimura, T.; Sakka, K.; Ohmiya, K. α-galactosidase Aga27A, an enzymatic component of the Clostridium josui cellulosome. J. Bacteriol. 2002, 184, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Luo, H.Y.; Li, J.A.; Bai, Y.G.; Huang, H.G.; Shi, P.J.; Fan, Y.L.; Yao, B. An α-galactosidase from an acidophilic Bispora sp MEY-1 strain acts synergistically with β-mannanase. Bioresour. Technol. 2010, 101, 8376–8382. [Google Scholar] [CrossRef] [PubMed]
- Kapnoor, S.; Mulimani, V.H. Production of α-Galactosidase by Aspergillus oryzae through solid-state fermentation and its application in soymilk galactooligosaccharide hydrolysis. Braz. Arch. Biol. Technol. 2010, 53, 211–218. [Google Scholar] [CrossRef]
- Laemmli, U.K.; Favre, M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J. Mol. Biol. 1973, 80, 575–599. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Katrolia, P.; Jia, H.Y.; Yan, Q.J.; Song, S.; Jiang, Z.Q.; Xu, H.B. Characterization of a protease-resistant α-galactosidase from the thermophilic fungus Rhizomucor miehei and its application in removal of raffinose family oligosaccharides. Bioresour. Technol. 2012, 110, 578–586. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Du, F.; Wang, L.; Zhao, L.; Wang, H.; Ng, T.B. Hydrolysis of Oligosaccharides by a Thermostable α-Galactosidase from Termitomyces eurrhizus. Int. J. Mol. Sci. 2015, 16, 29226-29235. https://doi.org/10.3390/ijms161226159
Zhang W, Du F, Wang L, Zhao L, Wang H, Ng TB. Hydrolysis of Oligosaccharides by a Thermostable α-Galactosidase from Termitomyces eurrhizus. International Journal of Molecular Sciences. 2015; 16(12):29226-29235. https://doi.org/10.3390/ijms161226159
Chicago/Turabian StyleZhang, Weiwei, Fang Du, Li Wang, Liyan Zhao, Hexiang Wang, and Tzi Bun Ng. 2015. "Hydrolysis of Oligosaccharides by a Thermostable α-Galactosidase from Termitomyces eurrhizus" International Journal of Molecular Sciences 16, no. 12: 29226-29235. https://doi.org/10.3390/ijms161226159





