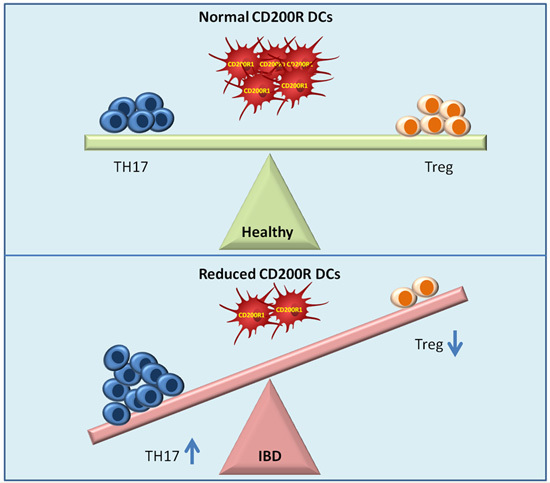
Reduced Dendritic Cells Expressing CD200R1 in Children with Inflammatory Bowel Disease: Correlation with Th17 and Regulatory T Cells
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics
| Parameter | Ulcerative Colitis (n = 14) | Crohn’s Disease (n = 23) | Healthy Control (n = 14) |
|---|---|---|---|
| Age (years) | 13.25 ± 2.94 | 13.73 ± 3.10 | 15.21 ± 2.41 |
| Sex (M/F) | 4/10 | 9/14 | 5/9 |
| Weight (kg) | 21.43 ± 1.51 | 33.79 ± 3.89 | 47.9 ± 4.73 |
| Height (cm) | 114 ± 3.09 | 136.95 ± 5.07 | 152.8 ± 7.67 |
| Disease duration (months.) | 7 ± 3.16 | 9 ± 4.01 | |
| Paris classification | |||
| Location | Left-sided 4 (28.6%) | Terminal ileum 8 (34.8%) | |
| Extensive 2 (14.3%) | Colon 4 (17.4%) | ||
| Pancolitis 8 (57.1%) | Ileocolon in 11 (47.8%) | ||
| Behavior | N/A | Non-stricturing, non-penetrating 16 (69.6%) | |
| Stricturing 4 (17.4%) | |||
| Perforating 3 (13.04%) | |||
| Mayo score | Mild 0 | N/A | |
| Moderate 5 (35.7%) | |||
| Severe 9 (64.3%) | |||
| Rectal bleeding | 14 (100%) | 13 (56.5%) | |
| Diarrhea | 14 (100%) | 19 (82.6%) | |
| Therapy | |||
| Prednisone | 12 (85.7%) | 23 (100%) | |
| Mesalamine | 14 (100%) | 18 (78.3%) | |
| Azathioprine | 12 (85.7%) | 23 (100%) | |
| Sulfasalazine | 0 | 10 (43.5%) | |
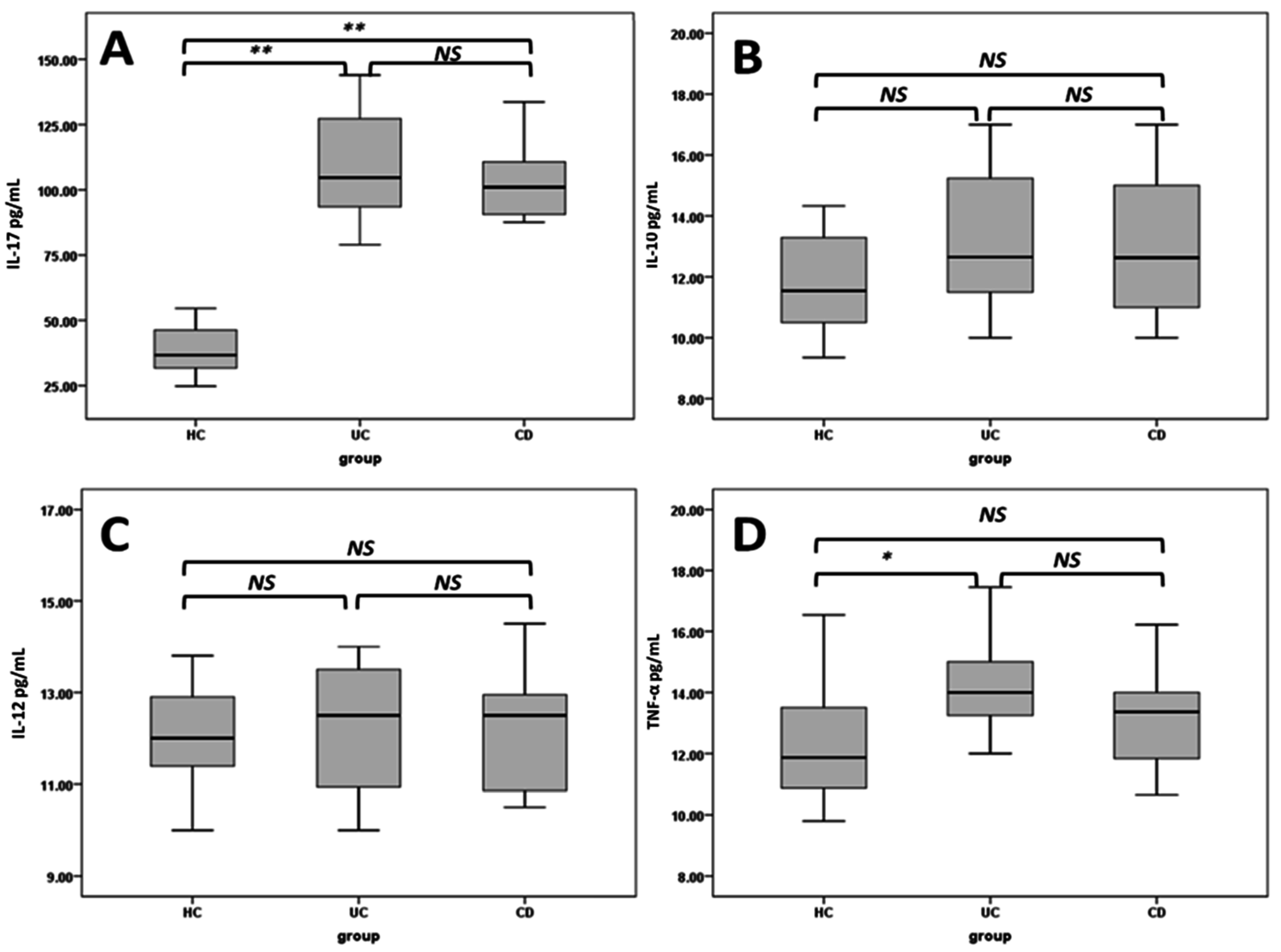
2.2. Biochemical and Serological Markers
| Marker | Ulcerative Colitis (n = 14) | Crohn’s Disease (n = 23) | Healthy Control (n = 14) |
|---|---|---|---|
| ALP (IU/L) | 175.29 ± 32.24 aa,b | 195.15 ± 27.05 aa | 67.2 ± 12.74 |
| CRP (mg/dL) | 13.57 ± 4.46 aa,b | 34.05 ± 8.56 aa | 3.42 ± 1.07 |
| ESR (mm/H) | 30 ± 4.08 a | 35.5 ± 6.23 a | 11.2 ± 2.1 |
| pANCA | 5 (35.7)% bb | 4 (17.4%) | 0 |
| ASCA-IgA | 2 (14.2%) bb | 6 (26.1)% | 0 |
| ASCA-IgG | 3 (21.4%) b | 7 (30.4%) | 0 |
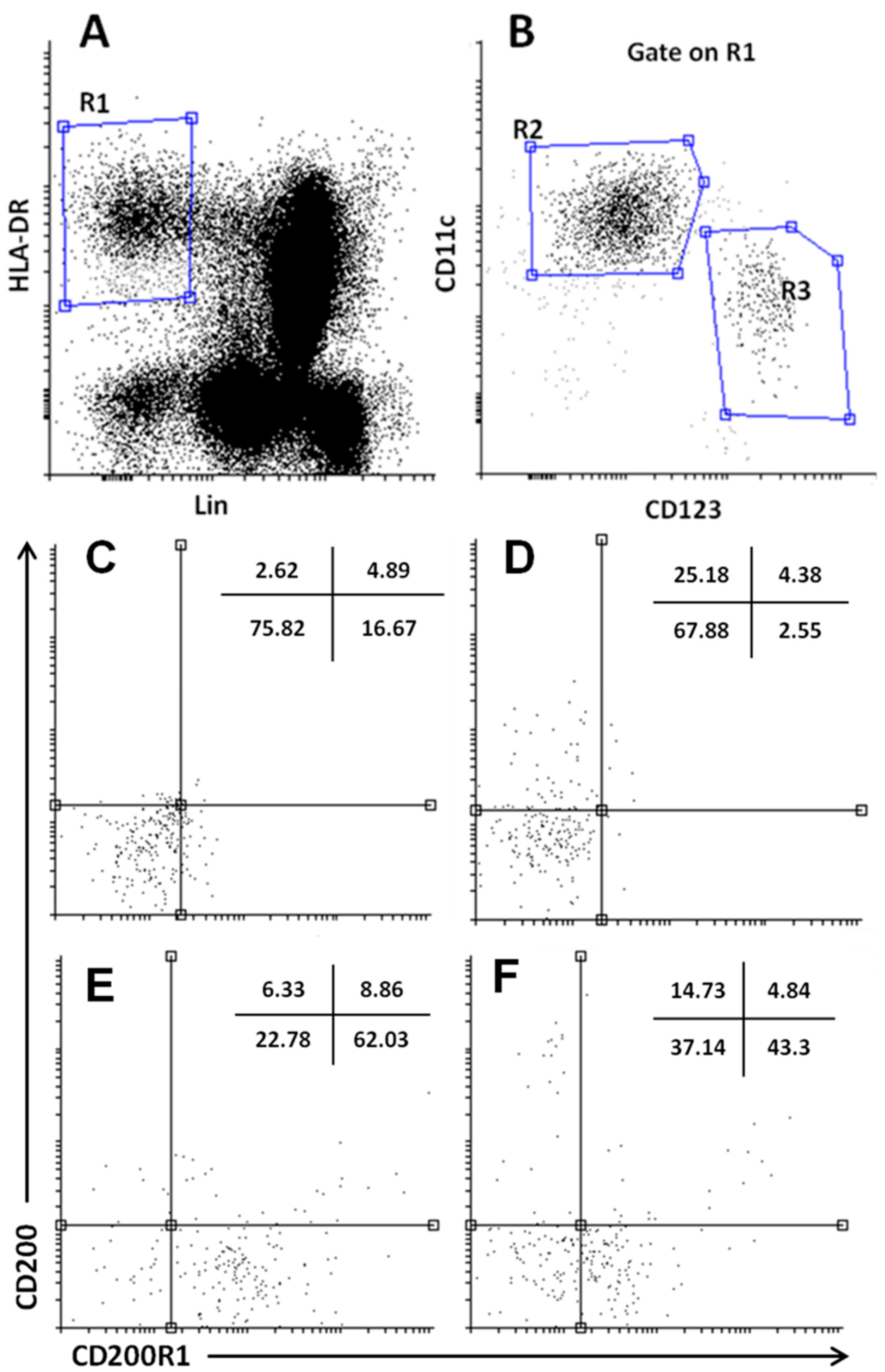
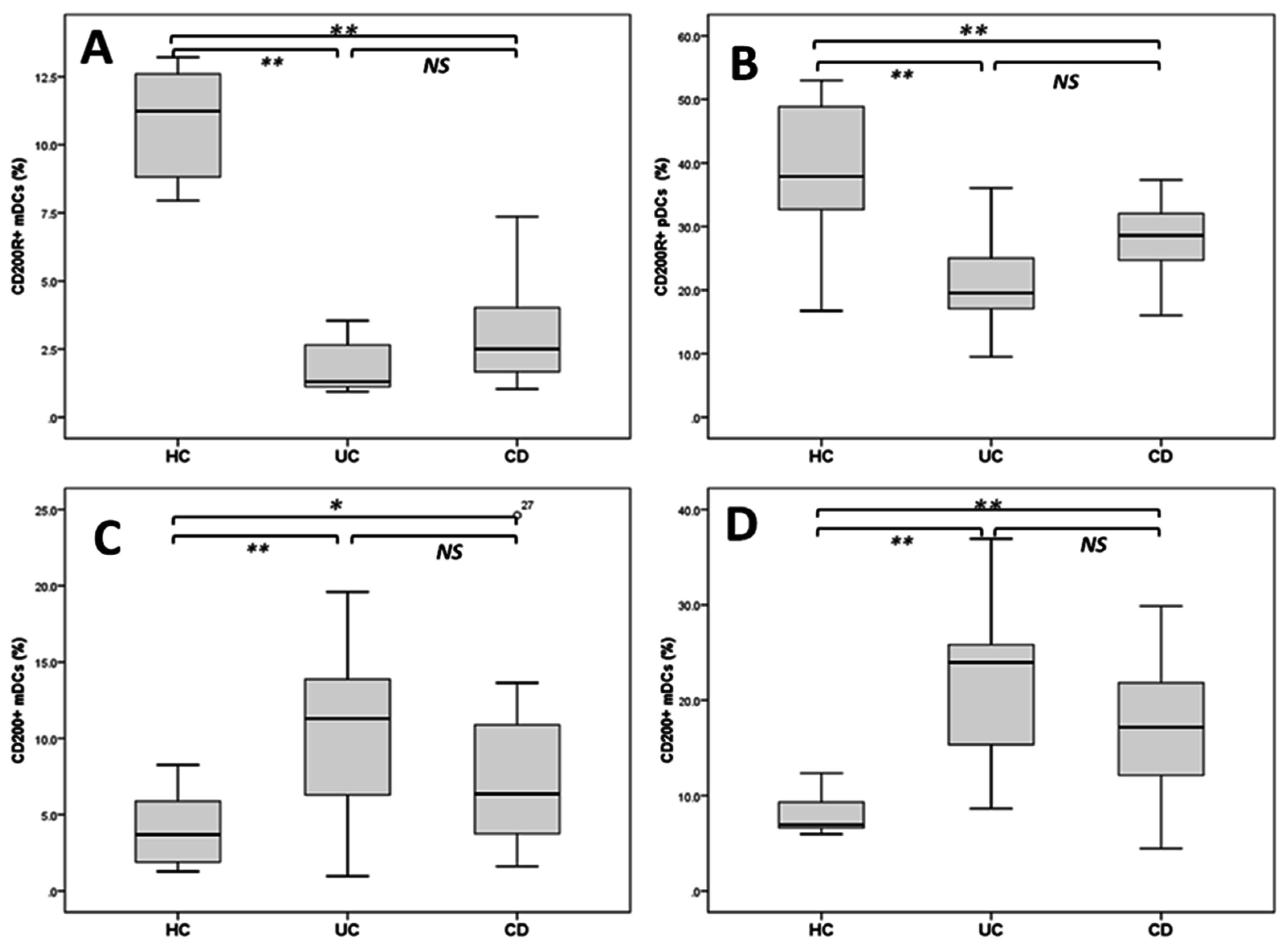
2.3. Reduced DCs Expressing CD200R1 and Increased CD200+DCs
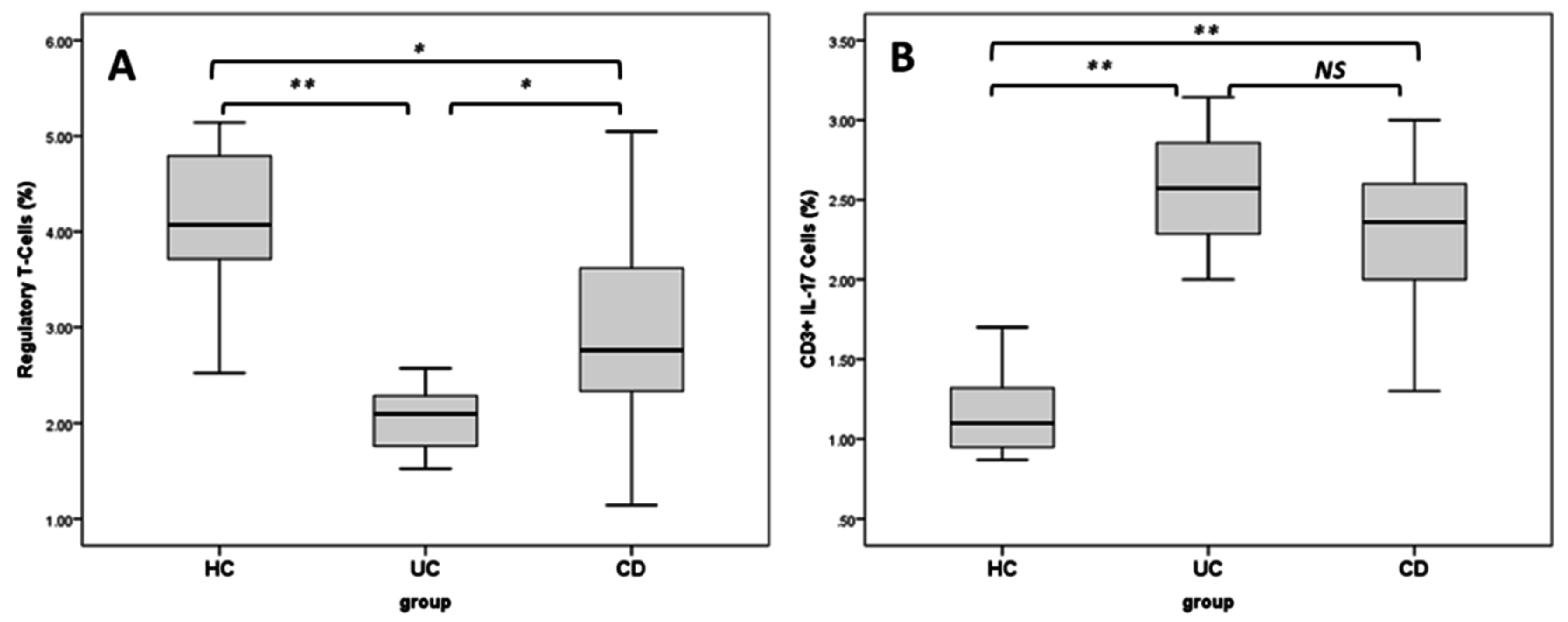
2.4. Regulatory T Cells and TH17 Cells
2.5. Correlation Analyses
| Parameter | Treg | Th17 |
|---|---|---|
| mDCs | −0.103 (0.568) | 0.441 (0.008) ** |
| pDCs | −0.245 (0.169) | 0.104 (0.552) |
| CD200R1+ mDCs | 0.383 (0.023) * | −0.412 (0.011) * |
| CD200+ mDCs | −0.420 (0.01) ** | 0.193 (0.251) |
| CD200R1+ pDCs | 0.406 (0.012) * | −0.319 (0.001) ** |
| CD200+ pDCs | −0.576 (0.001) ** | 0.416 (0.009) ** |
3. Discussion
4. Experimental Section
4.1. Study Population
4.2. Sample Preparations
4.3. Biochemical and Serological Investigations
4.4. Flow Cytometry Assays
4.4.1. Dendritic Cells Subsets Analysis
4.4.2. Regulatory T Cells Analysis
4.4.3. Th17 Cells’ Analysis
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lennard-Jones, J.E. Classification of inflammatory bowel disease. Scand. J. Gastroenterol. 1989, 170, 2–69. [Google Scholar] [CrossRef]
- Benchimol, E.I.; Manuel, D.G.; Guttmann, A.; Nguyen, G.C.; Mojaverian, N.; Quach, P.; Mack, D.R. Changing age demographics of inflammatory bowel disease in Ontario, Canada: A population-based cohort study of epidemiology trends. Inflamm. Bowel Dis. 2014, 20, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Malaty, H.M.; Fan, X.; Opekun, A.R.; Thibodeaux, C.; Ferry, G.D. Rising incidence of inflammatory bowel disease among children: A 12-year study. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Martin-de-Carpi, J.; Rodriguez, A.; Ramos, E.; Jimenez, S.; Martinez-Gomez, M.J.; Medina, E. SPIRIT-IBD Working Group of Sociedad Española de Gastroenterología, Hepatología y Nutricion Pediátrica. Increasing incidence of pediatric inflammatory bowel disease in Spain (1996–2009): The SPIRIT Registry. Inflamm. Bowel Dis. 2013, 19, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Hviid, A.; Svanstrom, H.; Frisch, M. Antibiotic use and inflammatory bowel diseases in childhood. Gut 2011, 60, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Sartor, R.B. Mechanisms of disease: Pathogenesis of Crohn’s disease and ulcerative colitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 390–407. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef] [PubMed]
- Rakoff-Nahoum, S.; Paglino, J.; Eslami-Varzaneh, F.; Edberg, S.; Medzhitov, R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 2004, 118, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Elson, C.O.; Cong, Y.; McCracken, V.J.; Dimmitt, R.A.; Lorenz, R.G.; Weaver, C.T. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol. Rev. 2005, 206, 260–276. [Google Scholar] [CrossRef] [PubMed]
- Hawiger, D.; Inaba, K.; Dorsett, Y.; Guo, M.; Mahnke, K.; Rivera, M.; Ravetch, J.V.; Steinman, R.M.; Nussenzweig, M.C. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 2001, 194, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Fallarino, F.; Asselin-Paturel, C.; Vacca, C.; Bianchi, R.; Gizzi, S.; Fioretti, M.C.; Trinchieri, G.; Grohmann, U.; Puccetti, P. Murine plasmacytoid dendritic cells initiate the immunosuppressive pathway of tryptophan catabolism in response to CD200 receptor engagement. J. Immunol. 2004, 173, 3748–3754. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.K.; Park, H.J.; Macleod, M.; Chandler, P.; Munn, D.H.; Mellor, A.L. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology 2002, 107, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Hwu, P.; Du, M.X.; Lapointe, R.; Do, M.; Taylor, M.W.; Young, H.A. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J. Immunol. 2000, 164, 3596–3599. [Google Scholar] [CrossRef] [PubMed]
- Holmannova, D.; Kolackova, M.; Kondelkova, K.; Kunes, P.; Krejsek, J.; Andrys, C. CD200/CD200R paired potent inhibitory molecules regulating immune and inflammatory responses; Part I: CD200/CD200R structure, activation, and function. Acta Med. 2012, 55, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Gorczynski, R.; Khatri, I.; Lee, L.; Boudakov, I. An interaction between CD200 and monoclonal antibody agonists to CD200R2 in development of dendritic cells that preferentially induce populations of CD4+CD25+ T regulatory cells. J. Immunol. 2008, 180, 5946–5955. [Google Scholar] [CrossRef] [PubMed]
- Boden, E.K.; Snapper, S.B. Regulatory T cells in inflammatory bowel disease. Curr. Opin. Gastroenterol. 2008, 24, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, X.P.; Zhao, Z.B.; Chen, J.H.; Yu, C.G. Expression of CD4+ forkhead box P3 (FOXP3)+ regulatory T cells in inflammatory bowel disease. J. Dig. Dis. 2011, 12, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Yang, B.; Yin, Y.; Leng, X.; Jiang, Y.; Zhang, L.; Li, Y.; Li, X.; Zhang, F.; He, W.; et al. Aberrant CD200/CD200R1 expression and its potential role in Th17 cell differentiation, chemotaxis and osteoclastogenesis in rheumatoid arthritis. Rheumatology 2015, 54, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Su, J.; Zhang, X.; Cheng, X.; Zhou, J.; Shi, R.; Zhang, H. Elevated levels of Th17 cells and Th17-related cytokines are associated with disease activity in patients with inflammatory bowel disease. Inflamm. Res. 2014, 63, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Gorczynski, R.M.; Chen, Z.; Lee, L.; Yu, K.; Hu, J. Anti-CD200R ameliorates collagen-induced arthritis in mice. Clin. Immunol. 2002, 104, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Meuth, S.G.; Simon, O.J.; Grimm, A.; Melzer, N.; Herrmann, A.M.; Spitzer, P.; Landgraf, P.; Wiendl, H. CNS inflammation and neuronal degeneration is aggravated by impaired CD200-CD200R-mediated macrophage silencing. J. Neuroimmunol. 2008, 194, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, X.J.; Tian, L.P.; Pan, J.; Lu, G.Q.; Zhang, Y.J.; Ding, J.Q.; Chen, S.D. CD200-CD200R dysfunction exacerbates microglial activation and dopaminergic neurodegeneration in a rat model of Parkinson’s disease. J. Neuroinflamm. 2011, 8. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.G.; Dalsing-Hernandez, J.E.; Campbell, N.A.; Lue, L.F. Decreased expression of CD200 and CD200 receptor in Alzheimer’s disease: A potential mechanism leading to chronic inflammation. Exp. Neurol. 2009, 215, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Chakera, A.; Bennett, S.C.; Morteau, O.; Bowness, P.; Luqmani, R.A.; Cornall, R.J. The phenotype of circulating follicular-helper T cells in patients with rheumatoid arthritis defines CD200 as a potential therapeutic target. Clin. Dev. Immunol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Broderick, C.; Hoek, R.M.; Forrester, J.V.; Liversidge, J.; Sedgwick, J.D.; Dick, A.D. Constitutive retinal CD200 expression regulates resident microglia and activation state of inflammatory cells during experimental autoimmune uveoretinitis. Am. J. Pathol. 2002, 161, 1669–1677. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, L.D.; Tong, L.S.; Qian, S.N.; Ren, Y.; Zhang, L.; Ding, X.; Chen, Y.; Wang, Y.X.; Zhang, W.; et al. Aberrant CD200/CD200R1 expression and function in systemic lupus erythematosus contributes to abnormal T-cell responsiveness and dendritic cell activity. Arthritis Res. Ther. 2012, 14. [Google Scholar] [CrossRef] [PubMed]
- Akman-Karakas, A.; Yalcin, A.D.; Koc, S.; Gumuslu, S.; Senol, Y.Y.; Ozkesici, B.; Genc, G.E.; Ergun, E.; Ongut, G.; Yilmaz, E.; et al. There might be a role for CD200 in the pathogenesis of autoimmune and inflammatory skin disorders. Med. Sci. Monit. 2013, 19, 888–891. [Google Scholar] [PubMed]
- Darmochwal-Kolarz, D.A.; Kludka-Sternik, M.; Chmielewski, T.; Kolarz, B.; Rolinski, J.; Leszczynska-Gorzelak, B.; Oleszczuk, J. The expressions of CD200 and CD200R molecules on myeloid and lymphoid dendritic cells in pre-eclampsia and normal pregnancy. Am. J. Reprod. Immunol. 2012, 67, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Hubo, M.; Trinschek, B.; Kryczanowsky, F.; Tuettenberg, A.; Steinbrink, K.; Jonuleit, H. Costimulatory molecules on immunogenic versus tolerogenic human dendritic cells. Front. Immunol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Jenmalm, M.C.; Cherwinski, H.; Bowman, E.P.; Phillips, J.H.; Sedgwick, J.D. Regulation of myeloid cell function through the CD200 receptor. J. Immunol. 2006, 176, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liu, Y.J. Development of dendritic-cell lineages. Immunity 2007, 26, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Goubier, A.; Dubois, B.; Gheit, H.; Joubert, G.; Villard-Truc, F.; Asselin-Paturel, C.; Trinchieri, G.; Kaiserlian, D. Plasmacytoid dendritic cells mediate oral tolerance. Immunity 2008, 29, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Joubert, G.; Gomez de Aguero, M.; Gouanvic, M.; Goubier, A.; Kaiserlian, D. Sequential role of plasmacytoid dendritic cells and regulatory T cells in oral tolerance. Gastroenterology 2009, 137, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Shan, M.; Cheng, H.F.; Song, L.Z.; Roberts, L.; Green, L.; Hacken-Bitar, J.; Huh, J.; Bakaeen, F.; Coxson, H.O.; Storness-Bliss, C.; et al. Lung myeloid dendritic cells coordinately induce TH1 and TH17 responses in human emphysema. Sci. Transl. Med. 2009, 1. [Google Scholar] [CrossRef] [PubMed]
- Chappell, C.P.; Giltiay, N.V.; Draves, K.E.; Chen, C.; Hayden-Ledbetter, M.S.; Shlomchik, M.J.; Kaplan, D.H.; Clark, E.A. Targeting antigens through blood dendritic cell antigen 2 on plasmacytoid dendritic cells promotes immunologic tolerance. J. Immunol. 2014, 192, 5789–5801. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Yang, M.; Wang, Y.H.; Lande, R.; Gregorio, J.; Perng, O.A.; Qin, X.F.; Liu, Y.J.; Gilliet, M. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J. Exp. Med. 2007, 204, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Thomas, S.; Przesdzing, I.; Metzke, D.; Bielecki, C.; Lehmann, S.M.; Lehnardt, S.; Dorffel, Y.; Sturm, A.; Scheffold, A.; et al. Exaggerated inflammatory response of primary human myeloid dendritic cells to lipopolysaccharide in patients with inflammatory bowel disease. Clin. Exp. Immunol. 2009, 157, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Ochando, J.C.; Homma, C.; Yang, Y.; Hidalgo, A.; Garin, A.; Tacke, F.; Angeli, V.; Li, Y.; Boros, P.; Ding, Y.; et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat. Immunol. 2006, 7, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Noack, M.; Miossec, P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun. Rev. 2014, 13, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Eastaff-Leung, N.; Mabarrack, N.; Barbour, A.; Cummins, A.; Barry, S. Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J. Clin. Immunol. 2010, 30, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Phillips, J.H. Identification of tyrosine residues crucial for CD200R-mediated inhibition of mast cell activation. J. Leukoc. Biol. 2006, 79, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, I.; Yamaguchi, M.; Suzukawa, M.; Yamamoto, K.; Lanier, L.L.; Saito, T.; Arase, H. Down-regulation of basophil function by human CD200 and human herpesvirus-8 CD200. J. Immunol. 2005, 175, 4441–4449. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Plüddemann, A.; Hoe, J.C.; Williams, K.J.; Varin, A.; Makepeace, K.; Aknin, M.-L.; Bowdish, D.M.E.; Smale, S.T.; Barclay, A.N.; et al. Immune inhibitory ligand CD200 induction by TLRs and NLRs limits macrophage activation to protect the host from meningococcal septicemia. Cell Host Microbe 2010, 8, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, M.D.; Olasz, E.; Woodliff, J.E.; Johnson, B.D.; Konkol, M.C.; Gerber, K.A.; Orentas, R.J.; Sandford, G.; Truitt, R.L. CD200 is a novel p53-target gene involved in apoptosis-associated immune tolerance. Blood 2004, 103, 2691–2698. [Google Scholar] [CrossRef] [PubMed]
- Jurgens, H.A.; Amancherla, K.; Johnson, R.W. Influenza infection induces neuroinflammation, alters hippocampal neuron morphology, and impairs cognition in adult mice. J. Neurosci. 2012, 32, 3958–3968. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Griffiths, A.; Markowitz, J.; Wilson, D.C.; Turner, D.; Russell, R.K.; Fell, J.; Ruemmele, F.M.; Walters, T.; Sherlock, M.; et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: The Paris classification. Inflamm. Bowel Dis. 2011, 17, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, K.W.; Tremaine, W.J.; Ilstrup, D.M. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N. Engl. J. Med. 1987, 317, 1625–1629. [Google Scholar] [CrossRef] [PubMed]
- Zholudev, A.; Zurakowski, D.; Young, W.; Leichtner, A.; Bousvaros, A. Serologic testing with ANCA, ASCA, and anti-OmpC in children and young adults with Crohn’s disease and ulcerative colitis: Diagnostic value and correlation with disease phenotype. Am. J. Gastroenterol. 2004, 99, 2235–2241. [Google Scholar] [CrossRef] [PubMed]
- Slukvin, I.I.; Vodyanik, M.A.; Thomson, J.A.; Gumenyuk, M.E.; Choi, K.D. Directed differentiation of human embryonic stem cells into functional dendritic cells through the myeloid pathway. J. Immunol. 2006, 176, 2924–2932. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elshal, M.F.; Aldahlawi, A.M.; Saadah, O.I.; McCoy, J.P. Reduced Dendritic Cells Expressing CD200R1 in Children with Inflammatory Bowel Disease: Correlation with Th17 and Regulatory T Cells. Int. J. Mol. Sci. 2015, 16, 28998-29010. https://doi.org/10.3390/ijms161226143
Elshal MF, Aldahlawi AM, Saadah OI, McCoy JP. Reduced Dendritic Cells Expressing CD200R1 in Children with Inflammatory Bowel Disease: Correlation with Th17 and Regulatory T Cells. International Journal of Molecular Sciences. 2015; 16(12):28998-29010. https://doi.org/10.3390/ijms161226143
Chicago/Turabian StyleElshal, Mohamed F., Alia M. Aldahlawi, Omar I. Saadah, and J. Philip McCoy. 2015. "Reduced Dendritic Cells Expressing CD200R1 in Children with Inflammatory Bowel Disease: Correlation with Th17 and Regulatory T Cells" International Journal of Molecular Sciences 16, no. 12: 28998-29010. https://doi.org/10.3390/ijms161226143