Effect of Guibi-Tang, a Traditional Herbal Formula, on Retinal Neovascularization in a Mouse Model of Proliferative Retinopathy
Abstract
:1. Introduction
2. Results
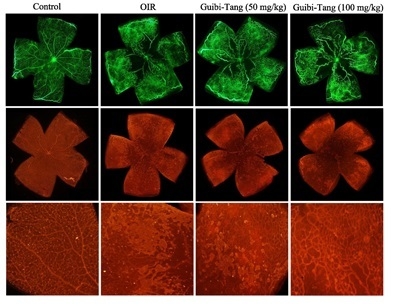
2.1. GBT Treatment Significantly Downregulated the Central Non-Perfusion Area and Retinal Tufts in Flat Mounts
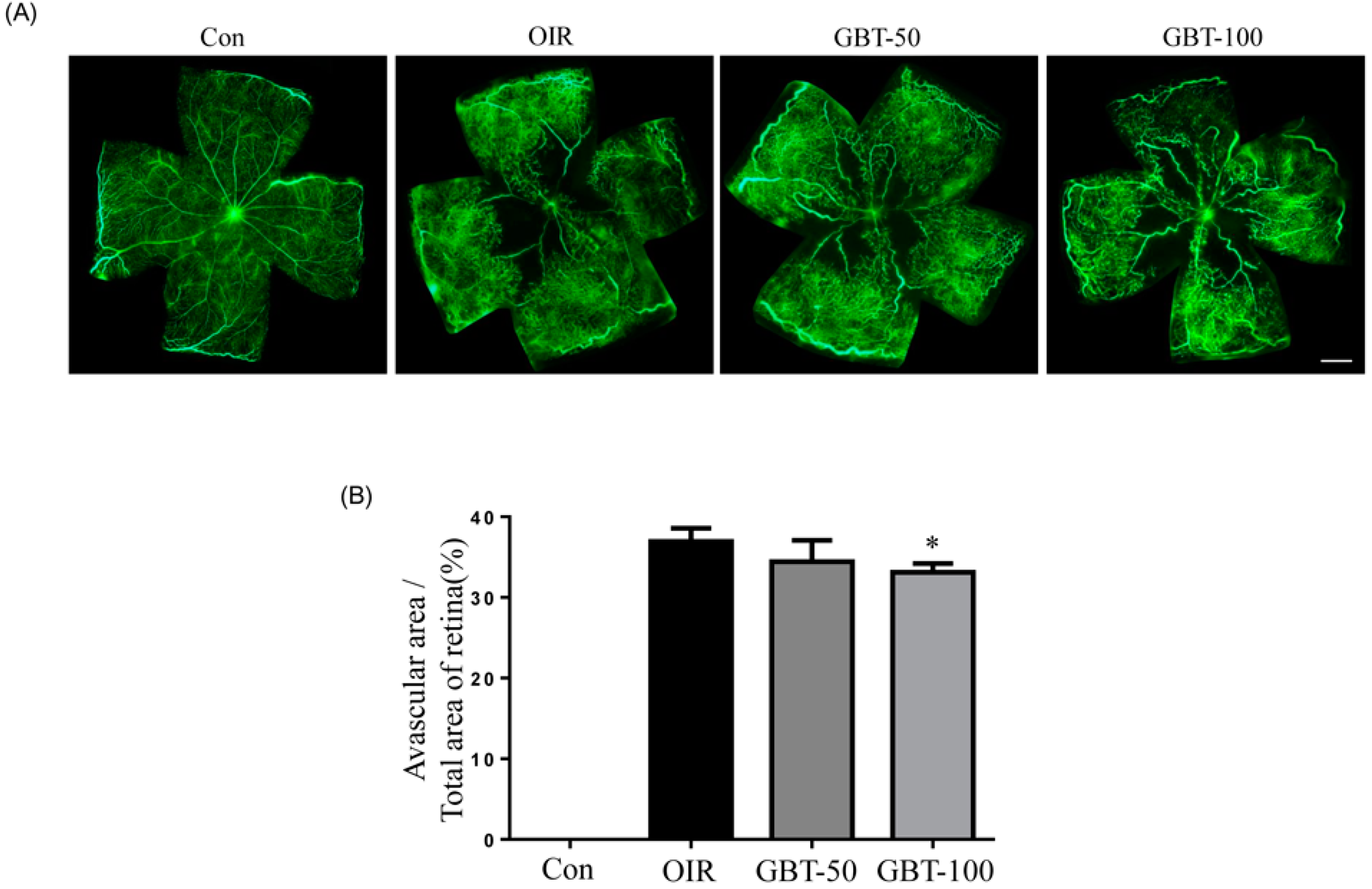
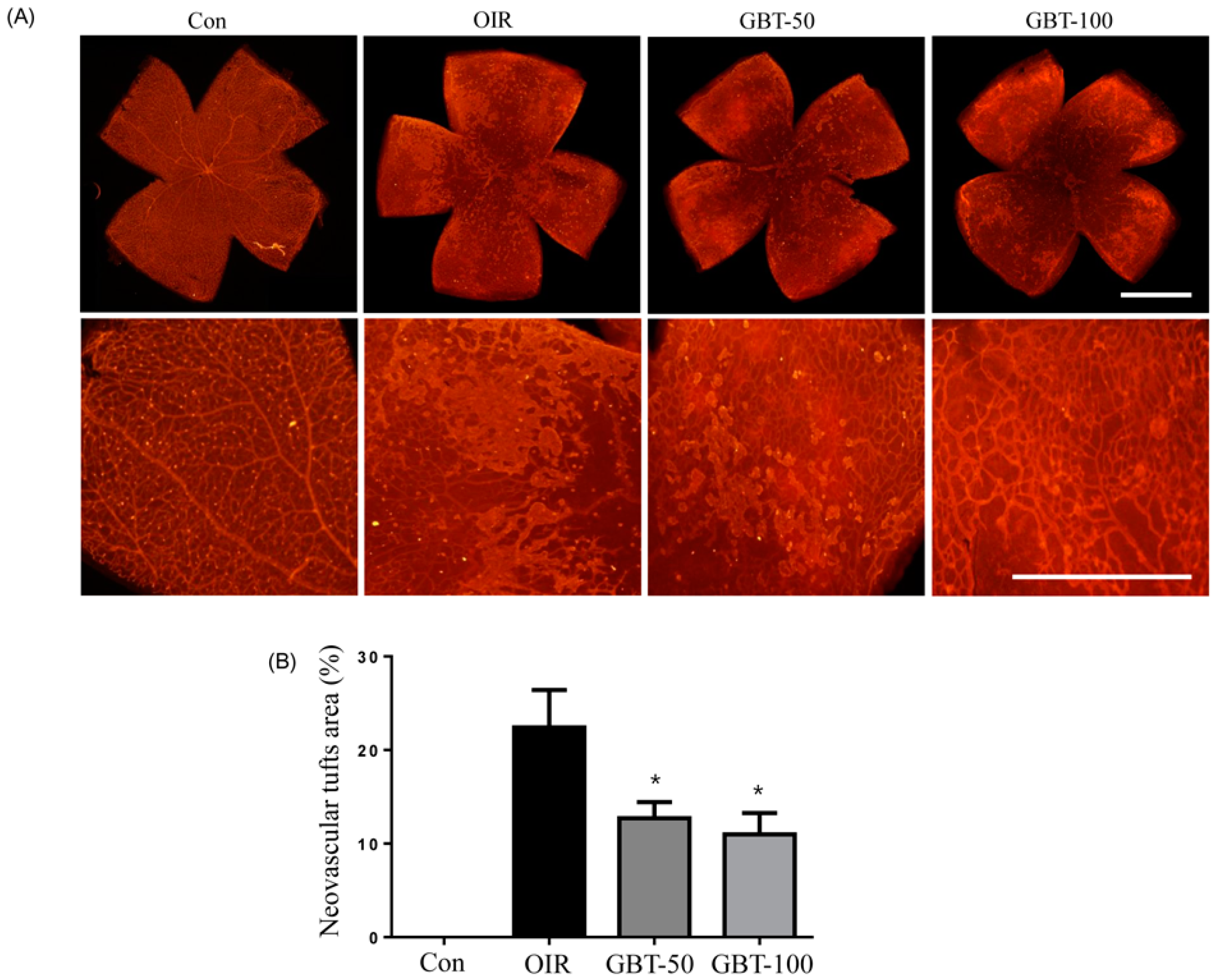
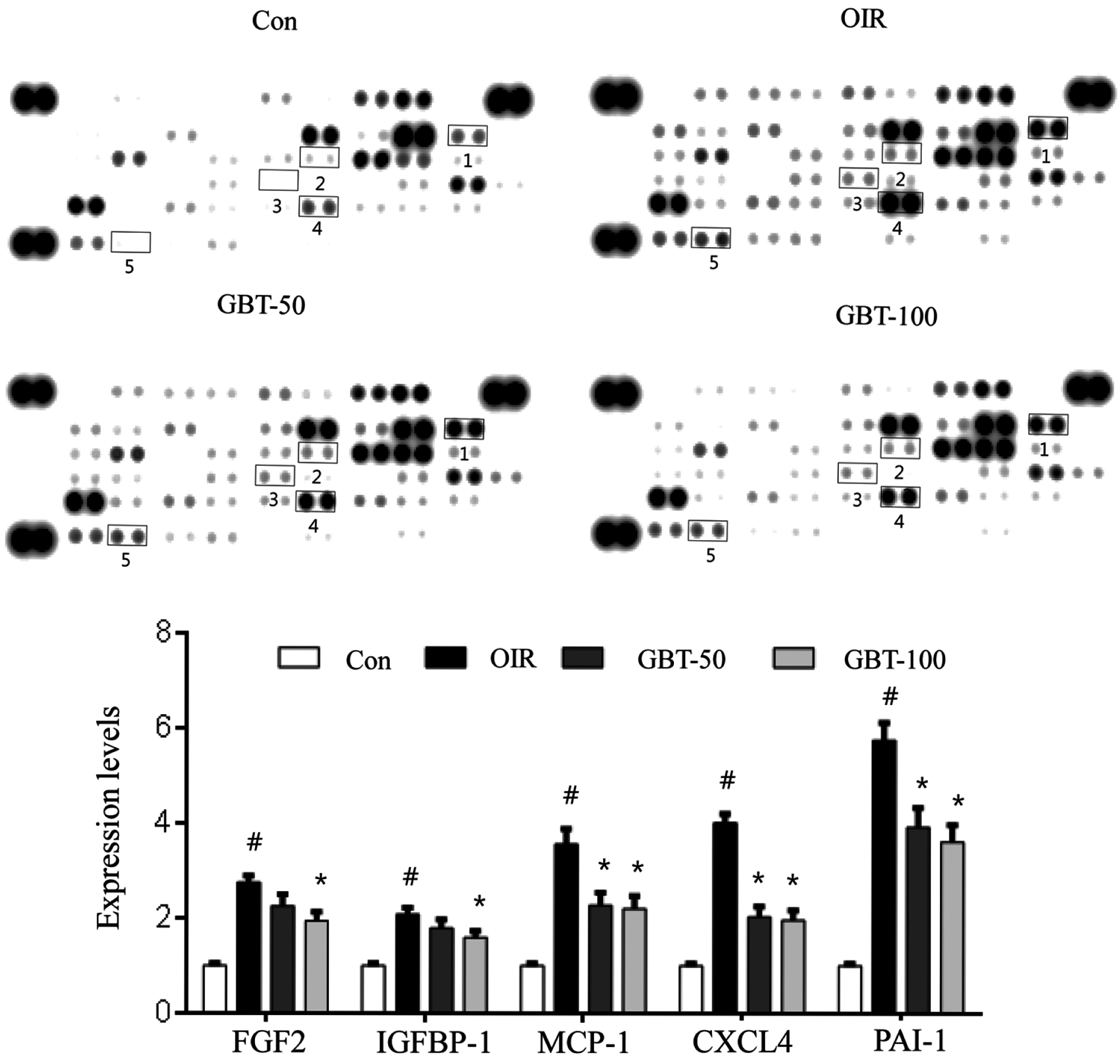
2.2. GBT Significantly Reduced Angiogenesis-Related Factor Protein Expressions
2.3. GBT Treatment Downregulates VEGF, FGF2 and PAI-1 mRNAs Expression

3. Discussion
4. Experimental Section
4.1. GBT Preparation
4.2. A Mouse Model of Oxygen-Induced Retinopathy
4.3. Fluorescein-Dextran Microscopy
4.4. Lectin Staining
4.5. Angiogenesis-Related Protein Array
4.6. Real-Time PCR Analysis
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Patz, A. Studies on retinal neovascularization. Friedenwald Lecture. Investig. Ophthalmol. Vis. Sci. 1980, 19, 1133–1138. [Google Scholar]
- Damico, F.M. Angiogenesis and retinal diseases. Arq. Bras. Oftalmol. 2007, 70, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Sennlaub, F.; Chemtob, S. VEGFR-1: A safe target for prophylaxis of retinopathy of prematurity? Pediatr. Res. 2004, 55, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.E.; Kopchick, J.J.; Chen, W.; Knapp, J.; Kinose, F.; Daley, D.; Foley, E.; Smith, R.G.; Schaeffer, J.M. Essential role of growth hormone in ischemia-induced retinal neovascularization. Science 1997, 276, 1706–1709. [Google Scholar] [CrossRef] [PubMed]
- Asahara, T.; Bauters, C.; Zheng, L.P.; Takeshita, S.; Bunting, S.; Ferrara, N.; Symes, J.F.; Isner, J.M. Synergistic effect of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in vivo. Circulation 1995, 92, 365–371. [Google Scholar] [CrossRef]
- Pepper, M.S.; Ferrara, N.; Orci, L.; Montesano, R. Potent synergism between vascular endothelial growth factor and basic fibroblast growth factor in the induction of angiogenesis in vitro. Biochem. Biophys. Res. Commun. 1992, 189, 824–831. [Google Scholar] [CrossRef]
- Goto, F.; Goto, K.; Weindel, K.; Folkman, J. Synergistic effects of vascular endothelial growth factor and basic fibroblast growth factor on the proliferation and cord formation of bovine capillary endothelial cells within collagen gels. Lab. Investig. 1993, 69, 508–517. [Google Scholar] [PubMed]
- Hu, D.E.; Fan, T.P. Suppression of VEGF-induced angiogenesis by the protein tyrosine kinase inhibitor, lavendustin A. Br. J. Pharmacol. 1995, 114, 262–268. [Google Scholar] [CrossRef] [PubMed]
- D’Amore, P.A. Mechanisms of retinal and choroidal neovascularization. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3974–3979. [Google Scholar]
- Pepper, M.S.; Ferrara, N.; Orci, L.; Montesano, R. Vascular endothelial growth factor (VEGF) induces plasminogen activators and plasminogen activator inhibitor-1 in microvascular endothelial cells. Biochem. Biophys. Res. Commun. 1991, 181, 902–906. [Google Scholar] [CrossRef]
- Fotsis, T.; Pepper, M.; Adlercreutz, H.; Fleischmann, G.; Hase, T.; Montesano, R.; Schweigerer, L. Genistein, a dietary-derived inhibitor of in vitro angiogenesis. Proc. Natl. Acad. Sci. USA 1993, 90, 2690–2694. [Google Scholar] [CrossRef] [PubMed]
- Gils, A.; Declerck, P.J. The structural basis for the pathophysiological relevance of PAI-I in cardiovascular diseases and the development of potential PAI-I inhibitors. Thromb. Haemost. 2004, 91, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, Y.; Nishida, C.; Sato-Kusubata, K.; Ohki-Koizumi, M.; Ishihara, M.; Sato, A.; Gritli, I.; Komiyama, H.; Sato, Y.; Dan, T.; et al. Inhibition of PAI-1 induces neutrophil-driven neoangiogenesis and promotes tissue regeneration via production of angiocrine factors in mice. Blood 2012, 119, 6382–6393. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Menicucci, G.; Maestas, J.; Das, A.; McGuire, P. Plasminogen activator inhibitor-1 (PAI-1) facilitates retinal angiogenesis in a model of oxygen-induced retinopathy. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4974–4981. [Google Scholar] [CrossRef] [PubMed]
- Hur, J. Donguibogam; Namsandang: Seoul, Korea, 2007; p. 98. [Google Scholar]
- Busta, I.; Xie, H.; Kim, M.S. The use of Gui-Pi-Tang in small animals with immune-mediated blood disorders. J. Vet. Clin. 2009, 26, 181–184. [Google Scholar]
- Eun, J.S.; Song, J.M. Effects of Kwibi-tang on serum levels of hormone and the non-specific immune response after immobilization stress in mice. Korean J. Orient. Med. Physiol. Pathol. 2004, 18, 172–178. [Google Scholar]
- Lim, J.W.; Kim, J.W.; Chung, S.Y.; Cho, S.H.; Oh, M.S.; Hwang, W.W. The Antioxidative and neuroprotective effect of guibi-tang(Guipitang) and guibi-tang gamibang(guipitang jiaweijang) on PC12 cells. J. Orient. Neuropsychiatry 2009, 20, 1–19. [Google Scholar]
- Kim, H.J.; Choi, J.H.; Lim, S.W. The Defensive Effect of Keuibi-tang on the Gastric Mucous Membrane of Mouse Injured by Stress and Ethanol. J. Korean Orient. Med. 2003, 24, 155–168. [Google Scholar]
- Rosenfarb, A. Healing Your Eyes with Chinese Medicine: Acupuncture, Acupressure, & Chinese Herbs; North Atlantic Books: Berkeley, CA, USA, 2007. [Google Scholar]
- Kim, J.H.; Lee, Y.M.; Ahn, E.M.; Kim, K.W.; Yu, Y.S. Decursin inhibits retinal neovascularization via suppression of VEGFR-2 activation. Mol. Vis. 2009, 15, 1868–1875. [Google Scholar] [PubMed]
- Lee, Y.M.; Kim, C.S.; Sohn, E.; Jo, K.; Lim, H.R.; Kim, S.K.; Kim, J.S.; Kim, J. Sipjeondaebo-tang, a traditional herbal formula, inhibits retinal neovascularization in a mouse model of oxygen-induced retinopathy. Tohoku. J. Exp. Med. 2014, 234, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Stahl, A.; Connor, K.M.; Sapieha, P.; Chen, J.; Dennison, R.J.; Krah, N.M.; Seaward, M.R.; Willett, K.L.; Aderman, C.M.; Guerin, K.I.; et al. The mouse retina as an angiogenesis model. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2813–2826. [Google Scholar] [CrossRef] [PubMed]
- Stahl, A.; Connor, K.M.; Sapieha, P.; Willett, K.L.; Krah, N.M.; Dennison, R.J.; Chen, J.; Guerin, K.I.; Smith, L.E. Computer-aided quantification of retinal neovascularization. Angiogenesis 2009, 12, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Brower, V. Antiangiogenesis research is booming, as questions and studies proliferate. J. Natl. Cancer Inst. 2009, 101, 780–781. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Armaiz Pena, G.N.; Pradeep, S.; Cho, M.S.; Coleman, R.L.; Sood, A.K. Anti-vascular therapies in ovarian cancer: Moving beyond anti-VEGF approaches. Cancer Metastasis Rev. 2014, 34, 19–40. [Google Scholar] [CrossRef] [PubMed]
- Casanovas, O.; Hicklin, D.J.; Bergers, G.; Hanahan, D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 2005, 8, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Brower, V. How well do angiogenesis inhibitors work? Biomarkers of response prove elusive. J. Natl. Cancer Inst. 2009, 101, 846–847. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, R.S.; Basavarajappa, H.D.; Corson, T.W. Natural product inhibitors of ocular angiogenesis. Exp. Eye Res. 2014, 129, 161–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.J.; Lee, E.O.; Rhee, Y.H.; Ahn, K.S.; Li, G.X.; Jiang, C.; Lu, J.; Kim, S.H. An oriental herbal cocktail, ka-mi-kae-kyuk-tang, exerts anti-cancer activities by targeting angiogenesis, apoptosis and metastasis. Carcinogenesis 2006, 27, 2455–2463. [Google Scholar] [CrossRef] [PubMed]
- Redington, A.E.; Roche, W.R.; Madden, J.; Frew, A.J.; Djukanovic, R.; Holgate, S.T.; Howarth, P.H. Basic fibroblast growth factor in asthma: measurement in bronchoalveolar lavage fluid basally and following allergen challenge. J. Allergy Clin. Immunol. 2001, 107, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Yonemitsu, Y.; Okano, S.; Nakagawa, K.; Nakashima, Y.; Irisa, T.; Iwamoto, Y.; Nagai, Y.; Hasegawa, M.; Sueishi, K. Fibroblast growth factor-2 determines severity of joint disease in adjuvant-induced arthritis in rats. J. Immunol. 2002, 168, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.W.; Bae, S.M.; Lee, M.; Al-Hilal, T.A.; Lee, C.K.; Kim, J.K.; Kim, I.S.; Kim, S.Y.; Byun, Y. LHT7, a chemically modified heparin, inhibits multiple stages of angiogenesis by blocking VEGF, FGF2 and PDGF-B signaling pathways. Biomaterials 2015, 37, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Cross, M.J.; Claesson-Welsh, L. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol. Sci. 2001, 22, 201–207. [Google Scholar] [CrossRef]
- Mandriota, S.J.; Pepper, M.S. Vascular endothelial growth factor-induced in vitro angiogenesis and plasminogen activator expression are dependent on endogenous basic fibroblast growth factor. J. Cell Sci. 1997, 110, 2293–2302. [Google Scholar] [PubMed]
- Brodsky, S.; Chen, J.; Lee, A.; Akassoglou, K.; Norman, J.; Goligorsky, M.S. Plasmin-dependent and -independent effects of plasminogen activators and inhibitor-1 on ex vivo angiogenesis. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H1784–H1792. [Google Scholar] [PubMed]
- Kwaan, H.C.; Wang, J.; Svoboda, K.; Declerck, P.J. Plasminogen activator inhibitor 1 may promote tumour growth through inhibition of apoptosis. Br. J. Cancer 2000, 82, 1702–1708. [Google Scholar] [PubMed]
- Gutierrez, L.S.; Schulman, A.; Brito-Robinson, T.; Noria, F.; Ploplis, V.A.; Castellino, F.J. Tumor development is retarded in mice lacking the gene for urokinase-type plasminogen activator or its inhibitor, plasminogen activator inhibitor-1. Cancer Res. 2000, 60, 5839–5847. [Google Scholar] [PubMed]
- Bajou, K.; Noel, A.; Gerard, R.D.; Masson, V.; Brunner, N.; Holst-Hansen, C.; Skobe, M.; Fusenig, N.E.; Carmeliet, P.; Collen, D.; et al. Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat. Med. 1998, 4, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A. Ocular neovascularization. J. Mol. Med. 2013, 91, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Ambati, J.; Anand, A.; Fernandez, S.; Sakurai, E.; Lynn, B.C.; Kuziel, W.A.; Rollins, B.J.; Ambati, B.K. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat. Med. 2003, 9, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Ehlken, C.; Grundel, B.; Michels, D.; Junker, B.; Stahl, A.; Schlunck, G.; Hansen, L.L.; Feltgen, N.; Martin, G.; Agostini, H.T.; et al. Increased expression of angiogenic and inflammatory proteins in the vitreous of patients with ischemic central retinal vein occlusion. PLoS ONE 2015, 10, e0126859. [Google Scholar] [CrossRef] [PubMed]
- Lofqvist, C.; Willett, K.L.; Aspegren, O.; Smith, A.C.; Aderman, C.M.; Connor, K.M.; Chen, J.; Hellstrom, A.; Smith, L.E. Quantification and localization of the IGF/insulin system expression in retinal blood vessels and neurons during oxygen-induced retinopathy in mice. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1831–1837. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Fu, Z.J.; Lo, A.C. Hypoxia-induced oxidative stress in ischemic retinopathy. Oxid. Med. Cell. Longev. 2012, 2012, 426769. [Google Scholar] [CrossRef] [PubMed]
- Natoli, R.; Valter, K.; Barbosa, M.; Dahlstrom, J.; Rutar, M.; Kent, A.; Provis, J. 670nm photobiomodulation as a novel protection against retinopathy of prematurity: evidence from oxygen induced retinopathy models. PLoS ONE 2013, 8, e72135. [Google Scholar] [CrossRef] [PubMed]
- Bodrova, M.E.; Brailovskaya, I.V.; Efron, G.I.; Starkov, A.A.; Mokhova, E.N. Cyclosporin A-sensitive decrease in the transmembrane potential across the inner membrane of liver mitochondria induced by low concentrations of fatty acids and Ca2+. Biochemistry 2003, 68, 391–398. [Google Scholar] [PubMed]
- Greco, T.; Shafer, J.; Fiskum, G. Sulforaphane inhibits mitochondrial permeability transition and oxidative stress. Free Radic. Biol. Med. 2011, 51, 2164–2171. [Google Scholar] [CrossRef] [PubMed]
- Panfoli, I.; Ravera, S.; Bruschi, M.; Candiano, G.; Morelli, A. Proteomics unravels the exportability of mitochondrial respiratory chains. Expert Rev. Proteom. 2011, 8, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Shimizu, N.; Obi, S.; Kumagaya, S.; Taketani, Y.; Kamiya, A.; Ando, J. Involvement of cell surface ATP synthase in flow-induced ATP release by vascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, 1646–1653. [Google Scholar] [CrossRef] [PubMed]
- Moser, T.L.; Stack, M.S.; Asplin, I.; Enghild, J.J.; Hojrup, P.; Everitt, L.; Hubchak, S.; Schnaper, H.W.; Pizzo, S.V. Angiostatin binds ATP synthase on the surface of human endothelial cells. Proc. Natl. Acad. Sci. USA 1999, 96, 2811–2816. [Google Scholar] [CrossRef] [PubMed]
- Calzia, D.; Oneto, M.; Caicci, F.; Bianchini, P.; Ravera, S.; Bartolucci, M.; Diaspro, A.; Degan, P.; Manni, L.; Traverso, C.E.; et al. Effect of polyphenolic phytochemicals on ectopic oxidative phosphorylation in rod outer segments of bovine retina. Br. J. Pharmacol. 2015, 172, 3890–3903. [Google Scholar] [CrossRef] [PubMed]
- Seo, C.S.; Kim, J.H.; Shin, H.K. Simultaneous determination of liquiritin, nodakenin and glycyrrhizinin Guibi-tang, a traditional herbal prescription by HPLC-PDA. Pak. J. Pharm. Sci. 2014, 27, 819–824. [Google Scholar] [PubMed]
- Liang, C.; Lee, K.J.; Jeong, S.W.; Ha, J.H.; Ma, J.Y. Analysis of Constituents from Guibi-Tang with Lactobacillus. Int. J. Biosci. Biochem. Bioinform. 2012, 2, 374–376. [Google Scholar] [CrossRef]
- Kim, K.J.; Choi, J.S.; Kim, K.W.; Jeong, J.W. The anti-angiogenic activities of glycyrrhizic acid in tumor progression. Phytother. Res. 2013, 27, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Kim, J.; Jo, K.; Shin, S.D.; Kim, C.S.; Sohn, E.J.; Kim, S.G.; Kim, J.S. Ethyl pyruvate inhibits retinal pathogenic neovascularization by downregulating HMGB1 expression. J. Diabetes Res. 2013, 2013, 245271. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.H.; Lee, S.H.; Ahn, E.M.; Lee, Y.M. Decursin and decursinol angelate inhibit VEGF-induced angiogenesis via suppression of the VEGFR-2-signaling pathway. Carcinogenesis 2009, 30, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Son, S.H.; Kim, M.J.; Chung, W.Y.; Son, J.A.; Kim, Y.S.; Kim, Y.C.; Kang, S.S.; Lee, S.K.; Park, K.K. Decursin and decursinol inhibit VEGF-induced angiogenesis by blocking the activation of extracellular signal-regulated kinase and c-Jun N-terminal kinase. Cancer Lett. 2009, 280, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, C.; Ward, K.W.; Mazzon, E.; Cuzzocrea, S.; Drago, F. Protective effects of a coumarin derivative in diabetic rats. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3846–3852. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Maher, P.; Hanneken, A. The flavonoid, eriodictyol, induces long-term protection in ARPE-19 cells through its effects on Nrf2 activation and phase 2 gene expression. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2398–2406. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, C.; Leggio, G.M.; Drago, F.; Salomone, S. Eriodictyol prevents early retinal and plasma abnormalities in streptozotocin-induced diabetic rats. Biochem. Pharmacol. 2012, 84, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H. Flavonoids inhibit VEGF/bFGF-induced angiogenesis in vitro by inhibiting the matrix-degrading proteases. J. Cell. Biochem. 2003, 89, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Cai, X.; Lu, W.; Zhou, F.; Huo, J. Growth inhibition and induction of apoptosis in SHG-44 glioma cells by Chinese medicine formula “Pingliu Keli”. Evid. Based Complement. Alternat. Med. 2011. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.M.; Lee, Y.-R.; Kim, C.-S.; Jo, K.; Sohn, E.; Kim, J.S.; Kim, J. Effect of Guibi-Tang, a Traditional Herbal Formula, on Retinal Neovascularization in a Mouse Model of Proliferative Retinopathy. Int. J. Mol. Sci. 2015, 16, 29900-29910. https://doi.org/10.3390/ijms161226211
Lee YM, Lee Y-R, Kim C-S, Jo K, Sohn E, Kim JS, Kim J. Effect of Guibi-Tang, a Traditional Herbal Formula, on Retinal Neovascularization in a Mouse Model of Proliferative Retinopathy. International Journal of Molecular Sciences. 2015; 16(12):29900-29910. https://doi.org/10.3390/ijms161226211
Chicago/Turabian StyleLee, Yun Mi, Yu-Ri Lee, Chan-Sik Kim, Kyuhyung Jo, Eunjin Sohn, Jin Sook Kim, and Junghyun Kim. 2015. "Effect of Guibi-Tang, a Traditional Herbal Formula, on Retinal Neovascularization in a Mouse Model of Proliferative Retinopathy" International Journal of Molecular Sciences 16, no. 12: 29900-29910. https://doi.org/10.3390/ijms161226211