Allyl Isothiocyanate Inhibits Actin-Dependent Intracellular Transport in Arabidopsis thaliana
Abstract
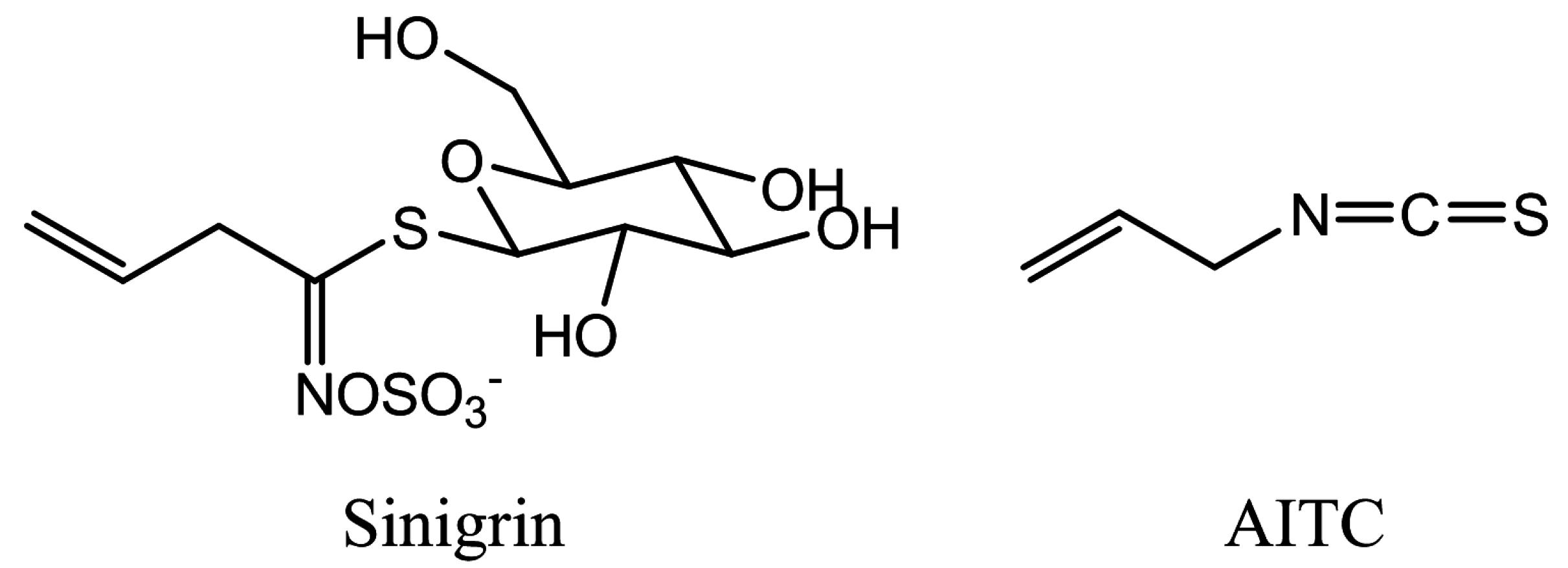
:1. Introduction
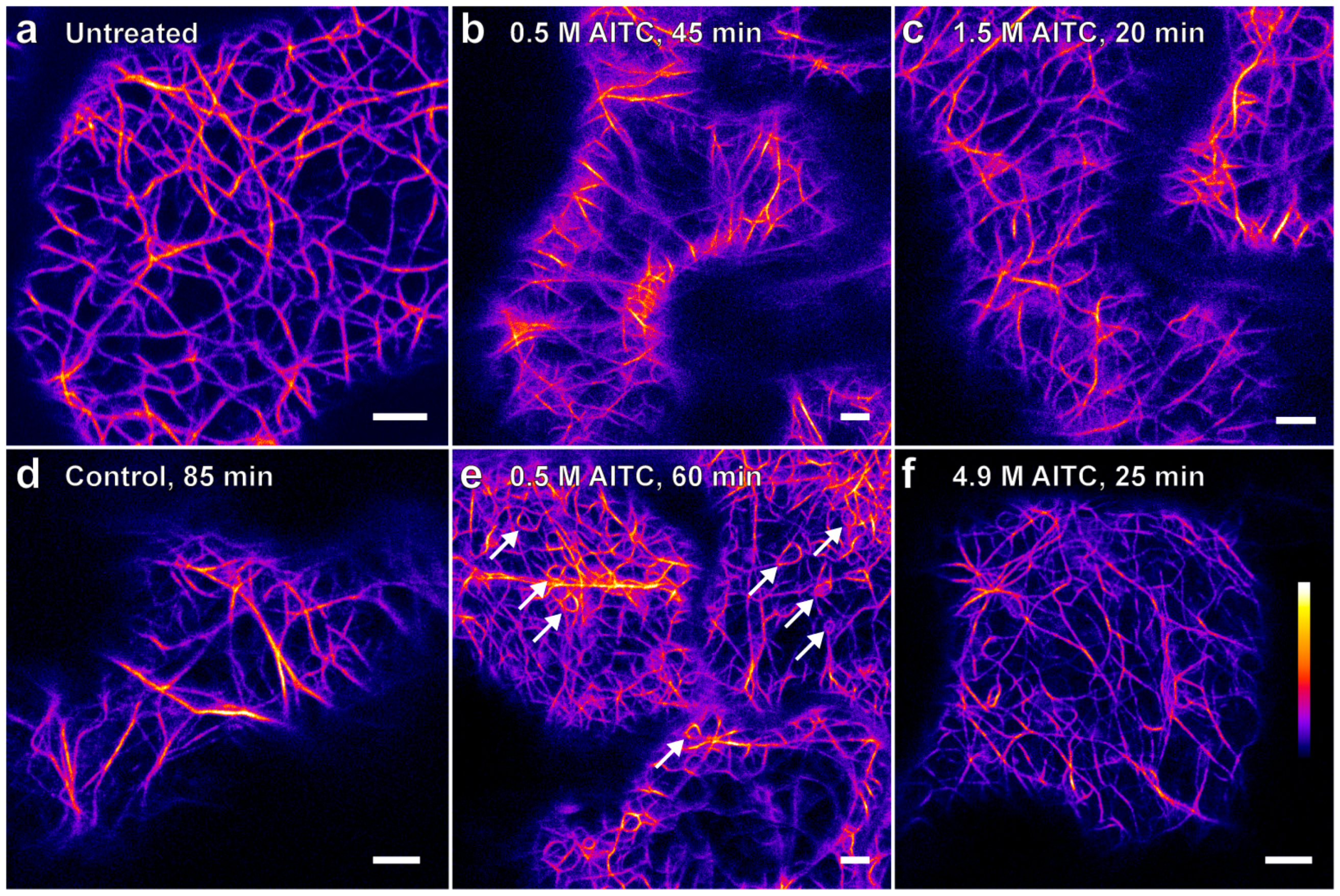
2. Results
2.1. AITC Reduces the Movement of Actin Filaments
| Transgenic Line | YFP-Tagged Protein | Fluorescent Marker | References |
|---|---|---|---|
| YFP-mTalin | Talin | Actin filaments | [26] |
| GOLGI-YFP | Man1 | Golgi apparatus | [27,28] |
| ER-YFP | HDEL and AtWAK2 | Endoplasmic reticulum | [27,29] |
| VAC-YFP | γ-TIP | Tonoplast | [27,30] |
| YFP-PER | PTS1 | Peroxisomes | [27,31] |

2.2. Reduced Actin-Dependent Intracellular Transport by AITC
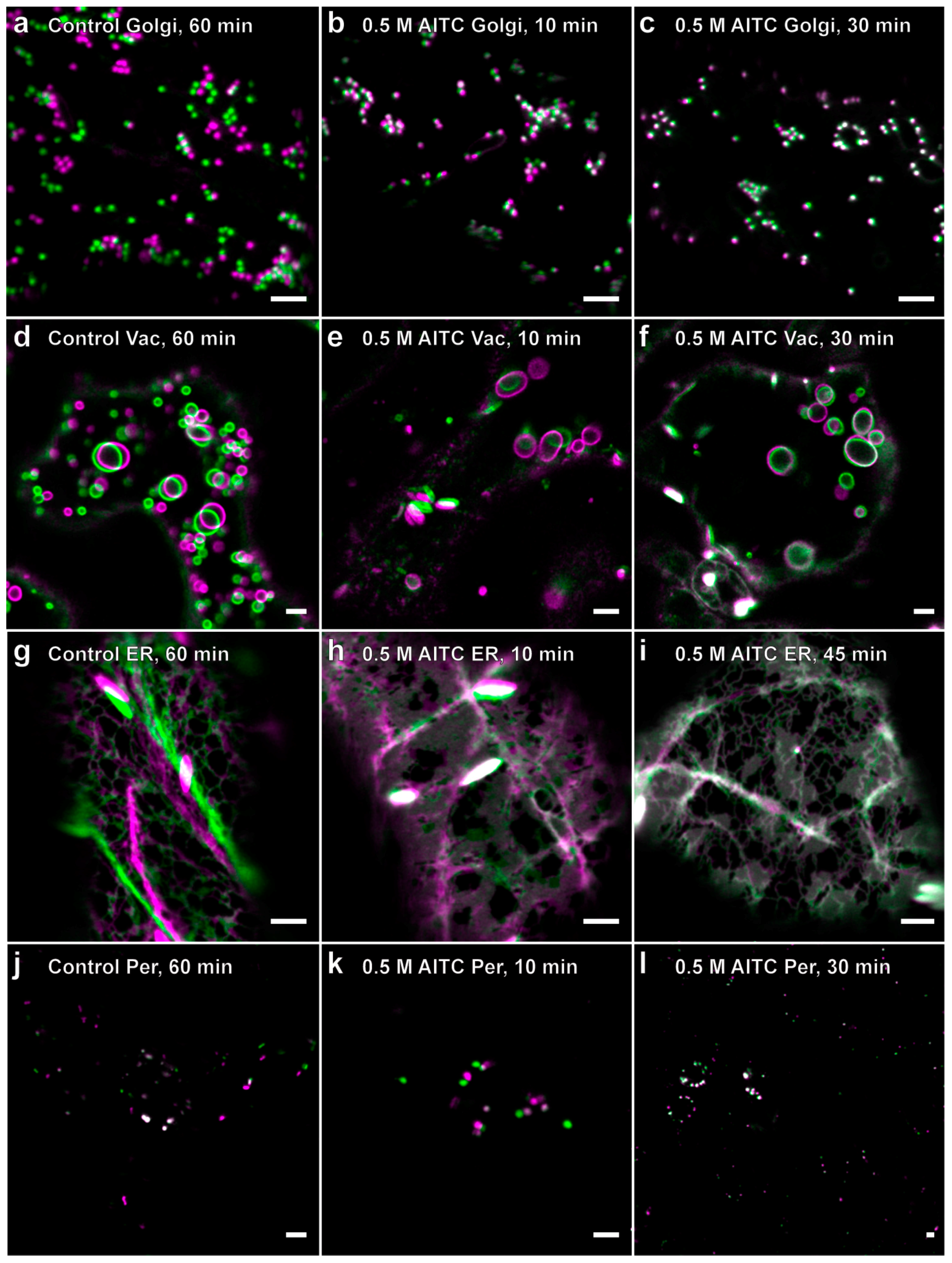

2.3. Recovery of Actin Filament Movement and Actin-Dependent Transport after AITC Exposure

3. Discussion
4. Experimental Section
4.1. Chemicals
4.2. Plant Growth and AITC Treatment in the Vapor Phase
4.3. Molecular Imaging
4.4. Confocal Laser Scanning Microscopy
4.5. Image Processing
4.6. Golgi Detection and Tracking
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kissen, R.; Bones, A.M. Nitrile-specifier proteins involved in glucosinolate hydrolysis in Arabidopsis thaliana. J. Biol. Chem. 2009, 284, 12057–12070. [Google Scholar] [CrossRef] [PubMed]
- Halkier, B.A.; Gershenzon, J. Biology and biochemistry of glucosinolates. In Annual Review of Plant Biology; Annual Reviews: Palo Alto, CA, USA, 2006; Volume 57, pp. 303–333. [Google Scholar]
- Kissen, R.; Rossiter, J.T.; Bones, A.M. The “mustard oil bomb”: Not so easy to assemble?! Localization, expression and distribution of the components of the myrosinase enzyme system. Phytochem. Rev. 2009, 8, 69–86. [Google Scholar] [CrossRef]
- Bones, A.M.; Rossiter, J.T. The myrosinase-glucosinolate system, its organisation and biochemistry. Physiol. Plant. 1996, 97, 194–208. [Google Scholar] [CrossRef]
- Mi, L.; Hood, B.L.; Stewart, N.A.; Xiao, Z.; Govind, S.; Wang, X.; Conrads, T.P.; Veenstra, T.D.; Chung, F.-L. Identification of potential protein targets of isothiocyanates by proteomics. Chem. Res. Toxicol. 2011, 24, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Bialy, Z.; Oleszek, W.; Lewis, J.; Fenwick, G.R. Allelopathic potential of glucosinolates (mustard oil glycosides) and their degradation products against wheat. Plant Soil 1990, 129, 277–281. [Google Scholar]
- Vaughn, S.F.; Boydston, R.A. Volatile allelochemicals released by crucifer green manures. J. Chem. Ecol. 1997, 23, 2107–2116. [Google Scholar] [CrossRef]
- Norsworthy, J.K.; Meehan, J.T. Use of isothiocyanates for suppression of palmer amaranth (amaranthus palmeri), pitted morningglory (ipomoea lacunosa), and yellow nutsedge (cyperus esculentus). Weed Sci. 2005, 53, 884–890. [Google Scholar] [CrossRef]
- Hara, M.; Yatsuzuka, Y.; Tabata, K.; Kuboi, T. Exogenously applied isothiocyanates enhance glutathione s-transferase expression in Arabidopsis but act as herbicides at higher concentrations. J. Plant Physiol. 2010, 167, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Haramoto, E.R.; Gallandt, E.R. Brassica cover cropping: I. Effects on weed and crop establishment. Weed Sci. 2005, 53, 695–701. [Google Scholar] [CrossRef]
- Boydston, R.A.; Hang, A. Rapeseed (Brassica napus) green manure crop suppresses weeds in potato (solanum tuberosum). Weed Technol. 1995, 9, 669–675. [Google Scholar]
- AlKhatib, K.; Libbey, C.; Boydston, R. Weed suppression with brassica green manure crops in green pea. Weed Sci. 1997, 45, 439–445. [Google Scholar]
- Norsworthy, J.K.; Malik, M.S.; Jha, P.; Oliveira, M.J. Effects of isothiocyanates on purple (Cyperus rotundus L.) and yellow nutsedge (Cyperus esculentus L.). Weed Biol. Manag. 2006, 6, 131–138. [Google Scholar] [CrossRef]
- Gimsing, A.L.; Kirkegaard, J.A. Glucosinolates and biofumigation: Fate of glucosinolates and their hydrolysis products in soil. Phytochem. Rev. 2009, 8, 299–310. [Google Scholar] [CrossRef]
- Asberg, S.E.; Bones, A.M.; Overby, A. Allyl isothiocyanate affects the cell cycle of Arabidopsis thaliana. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Overby, A.; Baevre, M.S.; Thangstad, O.P.; Bones, A.M. Disintegration of microtubules in Arabidopsis thaliana and bladder cancer cells by isothiocyanates. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Hara, M.; Harazaki, A.; Tabata, K. Administration of isothiocyanates enhances heat tolerance in Arabidopsis thaliana. Plant Growth Regul. 2013, 69, 71–77. [Google Scholar] [CrossRef]
- Overby, A.; Stokland, R.A.; Asberg, S.E.; Sporsheim, B.; Bones, A.M. Allyl isothiocyanate depletes glutathione and upregulates expression of glutathione S-transferases in Arabidopsis thaliana. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khokon, M.A.R.; Jahan, M.S.; Rahman, T.; Hossain, M.A.; Muroyama, D.; Minami, I.; Munemasa, S.; Mori, I.C.; Nakamura, Y.; Murata, Y. Allyl isothiocyanate (AITC) induces stomatal closure in Arabidopsis. Plant Cell Environ. 2011, 34, 1900–1906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. The molecular basis that unifies the metabolism, cellular uptake and chemopreventive activities of dietary isothiocyanates. Carcinogenesis 2012, 33, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.L.; Kong, A.N. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. Aaps J. 2010, 12, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.X.; Chung, F.L. Binding to protein by isothiocyanates: A potential mechanism for apoptosis induction in human nonsmall lung cancer cells. Nutr. Cancer 2008, 60, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.X.; Xiao, Z.; Hood, B.L.; Dakshanamurthy, S.; Wang, X.T.; Govind, S.; Conrads, T.P.; Veenstra, T.D.; Chung, F.L. Covalent binding to tubulin by isothiocyanates—A mechanism of cell growth arrest and apoptosis. J. Biol. Chem. 2008, 283, 22136–22146. [Google Scholar] [CrossRef] [PubMed]
- Overby, A.; Zhao, C.-M.; Bones, A.M.; Chen, D. Naturally occurring phenethyl isothiocyanate-induced inhibition of gastric cancer cell growth by disruption of microtubules. J. Gastroenterol. Hepatol. 2014, 29, 99–106. [Google Scholar] [PubMed]
- Sparkes, I. Recent advances in understanding plant myosin function: Life in the fast lane. Mol. Plant 2011, 4, 805–812. [Google Scholar] [PubMed]
- Brembu, T.; Winge, P.; Seem, M.; Bones, A.M. NAPP and PIRP encode subunit of a putative wave regulatory protein complex involved in plant cell morphogenesis. Plant Cell 2004, 16, 2335–2349. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.K.; Cai, X.; Nebenfuehr, A. A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J. 2007, 51, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Saint-Jore-Dupas, C.; Nebenfuhr, A.; Boulaflous, A.; Follet-Gueye, M.-L.; Plasson, C.; Hawes, C.; Driouich, A.; Faye, L.; Gomord, V. Plant n-glycan processing enzymes employ different targeting mechanisms for their spatial arrangement along the secretory pathway. Plant Cell 2006, 18, 3182–3200. [Google Scholar] [CrossRef] [PubMed]
- He, Z.H.; Cheeseman, I.; He, D.Z.; Kohorn, B.D. A cluster of five cell wall-associated receptor kinase genes, wak1–5, are expressed in specific organs of Arabidopsis. Plant Mol. Biol. 1999, 39, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Saito, C.; Ueda, T.; Abe, H.; Wada, Y.; Kuroiwa, T.; Hisada, A.; Furuya, M.; Nakano, A. A complex and mobile structure forms a distinct subregion within the continuous vacuolar membrane in young cotyledons of Arabidopsis. Plant J. 2002, 29, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Reumann, S. Specification of the peroxisome targeting signals type 1 and type 2 of plant peroxisomes by bioinformatics analyses. Plant Physiol. 2004, 135, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Norsworthy, J.K.; Meehan, J.T. Herbicidal activity of eight isothiocyanates on texas panicurn (Panicum texanum), large crabgrass (Digitaria sanguinalis), and sicklepod (Senna obtusifolia). Weed Sci. 2005, 53, 515–520. [Google Scholar] [CrossRef]
- Wolf, R.B.; Spencer, G.F.; Kwolek, W.F. Inhibition of velvetleaf (Abutilon-theophrasti) germination and growth by benzyl isothiocyanate, a natural toxicant. Weed Sci. 1984, 32, 612–615. [Google Scholar]
- Uremis, I.; Arslan, M.; Sangun, M.K.; Uygur, V.; Isler, N. Allelopathic potential of rapeseed cultivars on germination and seedling growth of weeds. Asian J. Chem. 2009, 21, 2170–2184. [Google Scholar]
- Olivier, C.; Vaughn, S.F.; Mizubuti, E.S.G.; Loria, R. Variation in allyl isothiocyanate production within brassica species and correlation with fungicidal activity. J. Chem. Ecol. 1999, 25, 2687–2701. [Google Scholar] [CrossRef]
- McDowell, J.M.; Huang, S.R.; McKinney, E.C.; An, Y.Q.; Meagher, R.B. Structure and evolution of the actin gene family in Arabidopsis thaliana. Genetics 1996, 142, 587–602. [Google Scholar] [PubMed]
- Elzinga, M.; Collins, J.H.; Kuehl, W.M.; Adelstei, R.S. Complete amino-acid sequence of actin of rabbit skeletal-muscle. Proc. Natl. Acad. Sci. USA 1973, 70, 2687–2691. [Google Scholar] [CrossRef] [PubMed]
- DalleDonne, I.; Milzani, A.; Colombo, R. The tert-butyl hydroperoxide-induced oxidation of actin Cys-374 is coupled with structural changes in distant regions of the protein. Biochemistry 1999, 38, 12471–12480. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Rossi, R.; Milzani, A.; di Simplicio, P.; Colombo, R. The actin cytoskeleton response to oxidants: From small heat shock protein phosphorylation to changes in the redox state of actin itself. Free Radic. Biol. Med. 2001, 31, 1624–1632. [Google Scholar] [CrossRef]
- Malm, B. Chemical modification of Cys-374 of actin interferes with the formation of the profilactin complex. FEBS Lett. 1984, 173, 399–402. [Google Scholar] [CrossRef]
- Day, B.; Henty, J.L.; Porter, K.J.; Staiger, C.J. The pathogen-actin connection: A platform for defense signaling in plants. Annu. Rev. Phytopathol. 2011, 49, 483–506. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.Q.; Wang, X.L.; Ren, F.; Chen, J.; Wang, X.C. Dynamics of vacuoles and actin filaments in guard cells and their roles in stomatal movement. Plant Cell Environ. 2009, 32, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Melotto, M.; Underwood, W.; He, S.Y. Role of stomata in plant innate immunity and foliar bacterial diseases. Annu. Rev. Phytopathol. 2008, 46, 101–122. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Melotto, M.; He, S.Y. Plant stomata: A checkpoint of host immunity and pathogen virulence. Curr. Opin. Biotechnol. 2010, 21, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Eun, S.O.; Lee, Y. Stomatal opening by fusicoccin is accompanied by depolymerization of actin filaments in guard cells. Planta 2000, 210, 1014–1017. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Hepler, P.K.; Fun, S.O.; Ha, K.S.; Lee, Y. Actin-filaments in mature guard-cells are radially distributed and involved in stomatal movement. Plant Physiol. 1995, 109, 1077–1084. [Google Scholar] [PubMed]
- Clement, M.; Ketelaar, T.; Rodiuc, N.; Banora, M.Y.; Smertenko, A.; Engler, G.; Abad, P.; Hussey, P.J.; Engler, J.D.A. Actin-depolymerizing factor2-mediated actin dynamics are essential for root-knot nematode infection of Arabidopsis. Plant Cell 2009, 21, 2963–2979. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Ye, W.X.; Hossain, M.A.; Okuma, E.; Uraji, M.; Nakamura, Y.; Mori, I.C.; Murata, Y. Glucosinolate degradation products, isothiocyanates, nitriles, and thiocyanates, induce stomatal closure accompanied by peroxidase-mediated reactive oxygen species production in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2013, 77, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Avisar, D.; Abu-Abied, M.; Belausov, E.; Sadot, E.; Hawes, C.; Sparkes, I.A. A comparative study of the involvement of 17 Arabidopsis myosin family members on the motility of golgi and other organelles. Plant Physiol. 2009, 150, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Higashifujime, S. Active movement invitro of bundles of microfilaments isolated from nitella cell. J. Cell Biol. 1980, 87, 569–578. [Google Scholar] [CrossRef]
- Chaidee, A.; Foissner, I.; Pfeiffer, W. Cell-specific association of heat shock-induced proton flux with actin ring formation in chenopodium cells: Comparison of auto- and heterotroph cultures. Protoplasma 2008, 234, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Smertenko, A.P.; Deeks, M.J.; Hussey, P.J. Strategies of actin reorganisation in plant cells. J. Cell Sci. 2010, 123, 3019–3029. [Google Scholar] [CrossRef] [PubMed]
- Foissner, I.; Grolig, F.; Obermeyer, G. Reversible protein phosphorylation regulates the dynamic organization of the pollen tube cytoskeleton: Effects of calyculin a and okadaic acid. Protoplasma 2002, 220, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Foissner, I.; Wasteneys, G.O. The characean internodal cell as a model system for studying wound healing. J. Microsc. 2012, 247, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Smertenko, A.; Franklin-Tong, V.E. Organisation and regulation of the cytoskeleton in plant programmed cell death. Cell Death Differ. 2011, 18, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, S.J.; Hosford, M.A.; Molitoris, B.A. Mechanism of actin polymerization in cellular atp depletion. J. Biol. Chem. 2004, 279, 5194–5199. [Google Scholar] [CrossRef] [PubMed]
- Shelden, E.A.; Weinberg, J.M.; Sorenson, D.R.; Edwards, C.A.; Pollock, F.M. Site-specific alteration of actin assembly visualized in living renal epithelial cells during atp depletion. J. Am. Soc. Nephrol. 2002, 13, 2667–2680. [Google Scholar] [CrossRef] [PubMed]
- Rasband, W.S.; Imagej, U.S. National Institutes of Health, Bethesda, Maryland, USA 1997–2014. Available online: http://imagej.nih.gov/ij/ (accessed on 25 November 2015).
- Li, Q.; Lau, A.; Morris, T.J.; Guo, L.; Fordyce, C.B.; Stanley, E.F. A syntaxin 1, Gαo, and N-type calcium channel complex at a presynaptic nerve terminal: Analysis by quantitative immunocolocalization. J. Neurosci. 2004, 24, 4070–4081. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sporsheim, B.; Øverby, A.; Bones, A.M. Allyl Isothiocyanate Inhibits Actin-Dependent Intracellular Transport in Arabidopsis thaliana. Int. J. Mol. Sci. 2015, 16, 29134-29147. https://doi.org/10.3390/ijms161226154
Sporsheim B, Øverby A, Bones AM. Allyl Isothiocyanate Inhibits Actin-Dependent Intracellular Transport in Arabidopsis thaliana. International Journal of Molecular Sciences. 2015; 16(12):29134-29147. https://doi.org/10.3390/ijms161226154
Chicago/Turabian StyleSporsheim, Bjørnar, Anders Øverby, and Atle Magnar Bones. 2015. "Allyl Isothiocyanate Inhibits Actin-Dependent Intracellular Transport in Arabidopsis thaliana" International Journal of Molecular Sciences 16, no. 12: 29134-29147. https://doi.org/10.3390/ijms161226154






