The Correlation between Chitin and Acidic Mammalian Chitinase in Animal Models of Allergic Asthma
Abstract
:1. Introduction
2. Results and Discussion
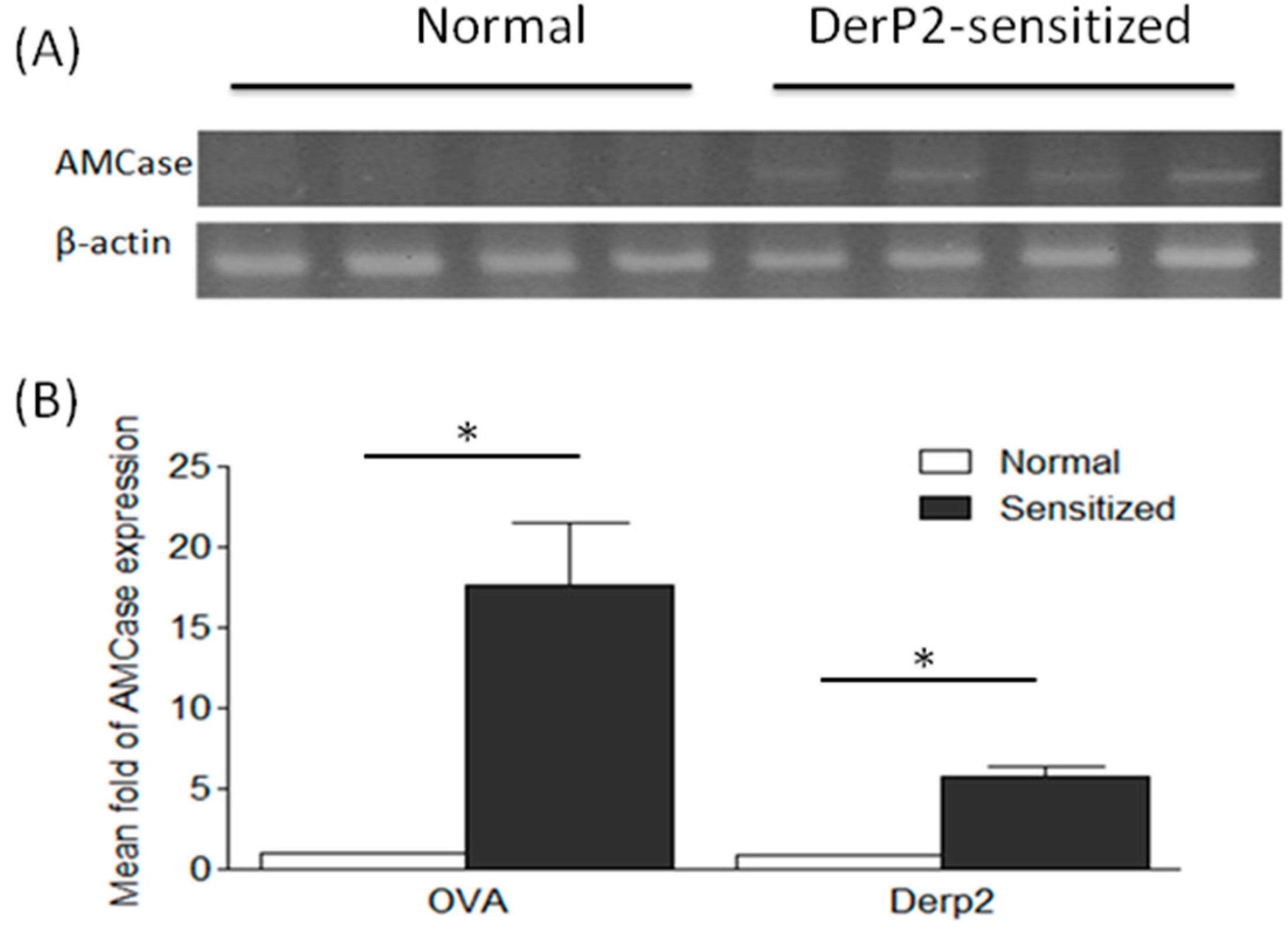
2.1. Levels of AMCase in the Lungs of Ovalbumin (OVA)- or Dermatophagoides Pteronyssinus Group II Allergen (Der P2)-Sensitized Mice
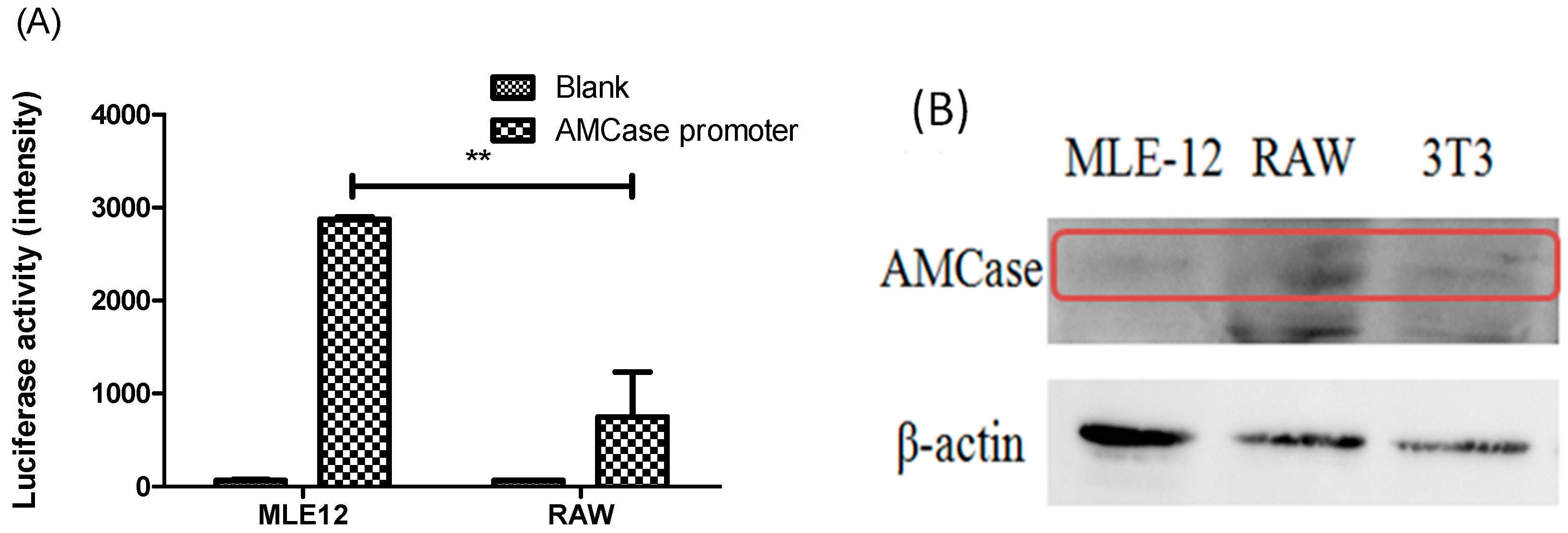
2.2. AMCase Reporter Vector Construction and AMCase Expression
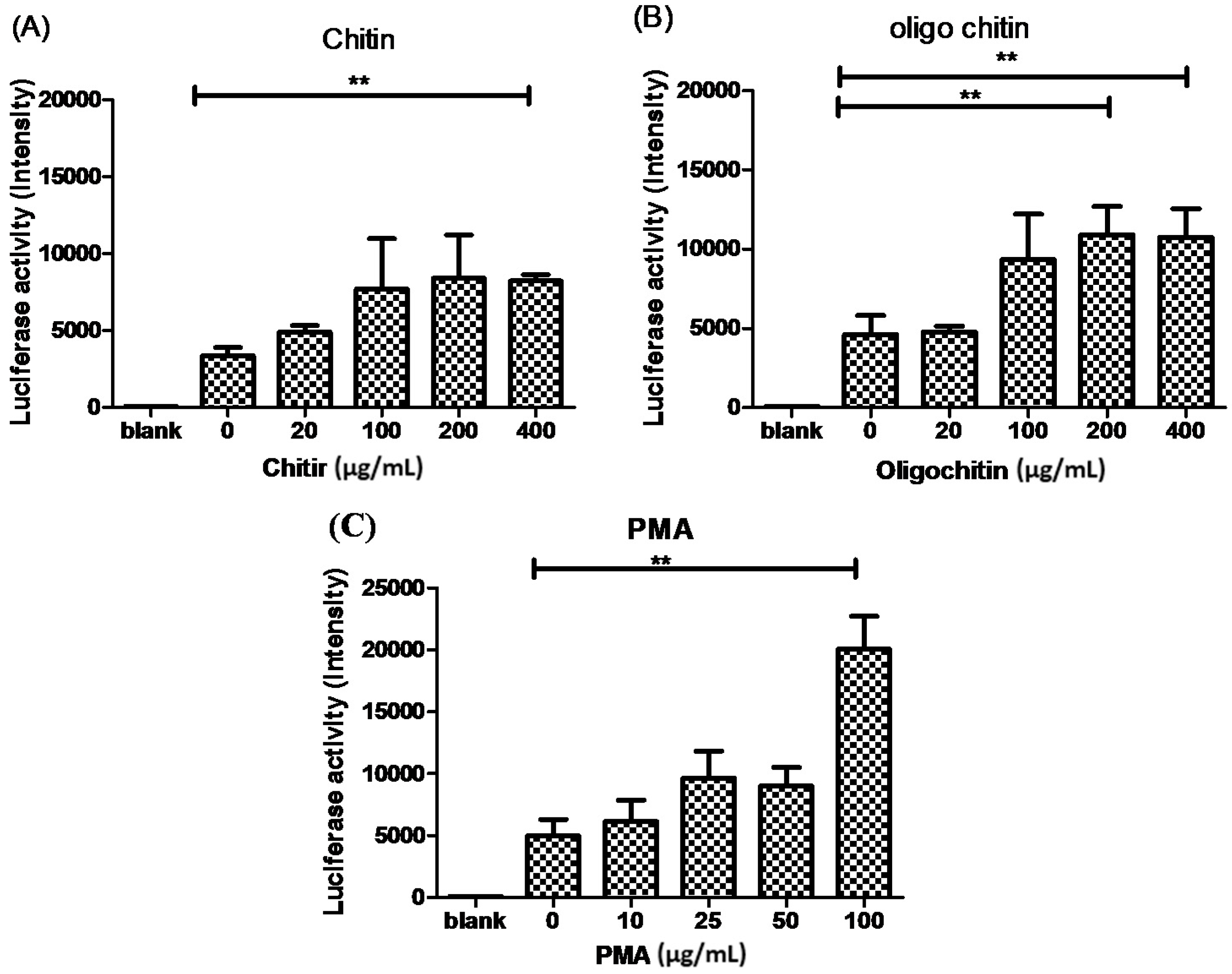
2.3. The Effects of Chitin on AMCase Expression
3. Materials and Methods
3.1. Cell Culture
3.2. Asthmatic Animal Induction Protocol
3.3. Real-Time Polymerase Chain Reaction (PCR)
3.4. Western Blot Analysis
3.5. Mouse AMCase Reporter Vector Construction and Transient Gene Expression Assay
3.6. Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Passalacqua, G.; Ciprandi, G. Allergy and the lung. Clin. Exp. Immunol. 2008, 1, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.R.; Smith, W.A. House dust mite allergens in asthma and allergy. Trends Mol. Med. 2010, 16, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.A.; Pochard, P.; Lee, C.G.; Elias, J.A. Chitin particles are multifaceted immune adjuvants. Am. J. Respir. Crit. Care Med. 2010, 182, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Blanc, P.; van Dyken, S.; Locksley, R.; Quinlan, P.J.; Balmes, J.R.; Iribarren, C.; Katz, P.P.; Yelin, E.H.; Trupin, L.; Eisner, M.D. Chitin detection in home dust sampling. Am. J. Respir. Crit. Care Med. 2010, 181, A4668. [Google Scholar]
- Boot, R.; Blommaart, E.; Swart, E.; Ghauharali-van der Vlugt, K.; Bijl, N.; Moe, C.; Place, A.; Aerts, J.M. Identification of a novel acidic mammalian chitinase distinct from chitotriosidase. J. Biol. Chem. 2001, 276, 6770–6778. [Google Scholar] [CrossRef] [PubMed]
- Hamid, R.; Khan, M.A.; Ahmad, M.; Ahmad, M.M.; Abdin, M.Z.; Musarrat, J.; Javed, S. Chitinases: An update. J. Pharm. Bioallied Sci. 2013, 5, 21–29. [Google Scholar] [PubMed]
- Chen, J.K.; Shen, C.R.; Liu, C.L. N-acetylglucosamine: Production and applications. Mar. Drugs 2010, 8, 2493–2516. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-J.; Liu, Y.-K.; Liu, C.-L.; Shen, C.-N.; Kuo, M.-L.; Su, C.-C.; Tseng, C.-P.; Yen, T.-C.; Shen, C.-R. Inhibition of acidic mammalian chitinase by RNA interference suppresses ovalbumin-sensitized allergic asthma. Hum. Gene Ther. 2009, 20, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Homer, R.J.; Kim, Y.-K.; Chen, N.Y.; Cohn, L.; Hamid, Q.; Elias, J.A. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science 2004, 304, 1678–1682. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Rosenthal, P.; Miller, M.; Pham, A.; Aceves, S.; Sakuda, S.; Broide, D.H. Targeting AMCase reduces esophageal eosinophilic inflammation and remodeling in a mouse model of egg induced eosinophilic esophagitis. Int. Immunopharmacol. 2014, 18, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; da Silva, C.A.; Dela Cruz, C.S.; Ahangari, F.; Ma, B.; Kang, M.J.; He, C.H.; Takyar, S.; Elias, J.A. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu. Rev. Physiol. 2011, 73, 479–501. [Google Scholar] [CrossRef] [PubMed]
- Erzurum, S.C.; Gaston, B.M. Biomarkers in asthma: A real hope to better manage asthma. Clin. Chest Med. 2012, 33, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Bierbaum, S.; Nickel, R.; Koch, A.; Lau, S.; Deichmann, K.A.; Wahn, U.; Superti-Furga, A.; Heinzmann, A. Polymorphisms and haplotypes of acid mammalian chitinase are associated with bronchial asthma. Am. J. Respir. Crit. Care Med. 2005, 172, 1505–1509. [Google Scholar] [CrossRef] [PubMed]
- Chupp, G.; Lee, C.G.; Jarjour, N.; Shim, Y.M.; Holm, C.T.; He, S.; Dziura, J.D.; Reed, J.; Coyle, A.J.; Kiener, P.; et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. N. Engl. J. Med. 2007, 357, 2016–2027. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, M.J.; Lee, W.; Lane, A.P. Increased expression of acidic mammalian chitinase in chronic rhinosinusitis with nasal polyps. Am. J. Rhinol. 2006, 20, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Kawada, M.; Hachiya, Y.; Arihiro, A.; Mizoguchi, E. Role of mammalian chitinases in inflammatory conditions. Keio J. Med. 2007, 56, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, C.; Musumeci, M.; Maltese, A.; Drago, F.; Musumeci, S. Effect of chitinase inhibitors on endotoxin-induced uveitis (EIU) in rabbits. Pharmacol. Res. 2008, 57, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, M.; Bellin, M.; Maltese, A.; Aragona, P.; Bucolo, C.; Musumeci, S. Chitinase levels in the tears of subjects with ocular allergies. Cornea 2008, 27, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Roby, D.; Broglie, K.; Gaynor, J.; Broglie, R. Regulation of a chitinase gene promoter by ethylene and elicitors in bean protoplasts. Plant Physiol. 1991, 97, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Hadwiger, L.A.; Beckman, J.M. Chitosan as a Component of Pea-Fusarium solani Interactions. Plant Physiol. 1980, 66, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Roby, D.; Gadelle, A.; Toppan, A. Chitin oligosaccharides as elicitors of chitinase activity in melon plants. Biochem. Biophys. Res. Commun. 1987, 143, 885–892. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, C.-R.; Juang, H.-H.; Chen, H.-S.; Yang, C.-J.; Wu, C.-J.; Lee, M.-H.; Hwang, Y.-S.; Kuo, M.-L.; Chen, Y.-S.; Chen, J.-K.; et al. The Correlation between Chitin and Acidic Mammalian Chitinase in Animal Models of Allergic Asthma. Int. J. Mol. Sci. 2015, 16, 27371-27377. https://doi.org/10.3390/ijms161126033
Shen C-R, Juang H-H, Chen H-S, Yang C-J, Wu C-J, Lee M-H, Hwang Y-S, Kuo M-L, Chen Y-S, Chen J-K, et al. The Correlation between Chitin and Acidic Mammalian Chitinase in Animal Models of Allergic Asthma. International Journal of Molecular Sciences. 2015; 16(11):27371-27377. https://doi.org/10.3390/ijms161126033
Chicago/Turabian StyleShen, Chia-Rui, Horng-Heng Juang, Hui-Shan Chen, Ching-Jen Yang, Chia-Jen Wu, Meng-Hua Lee, Yih-Shiou Hwang, Ming-Ling Kuo, Ya-Shan Chen, Jeen-Kuan Chen, and et al. 2015. "The Correlation between Chitin and Acidic Mammalian Chitinase in Animal Models of Allergic Asthma" International Journal of Molecular Sciences 16, no. 11: 27371-27377. https://doi.org/10.3390/ijms161126033





