Establishment and Comparison of Two Different Diagnostic Platforms for Detection of DENV1 NS1 Protein
Abstract
:1. Introduction
2. Results
2.1. Purification of DENV1 NS1 Protein
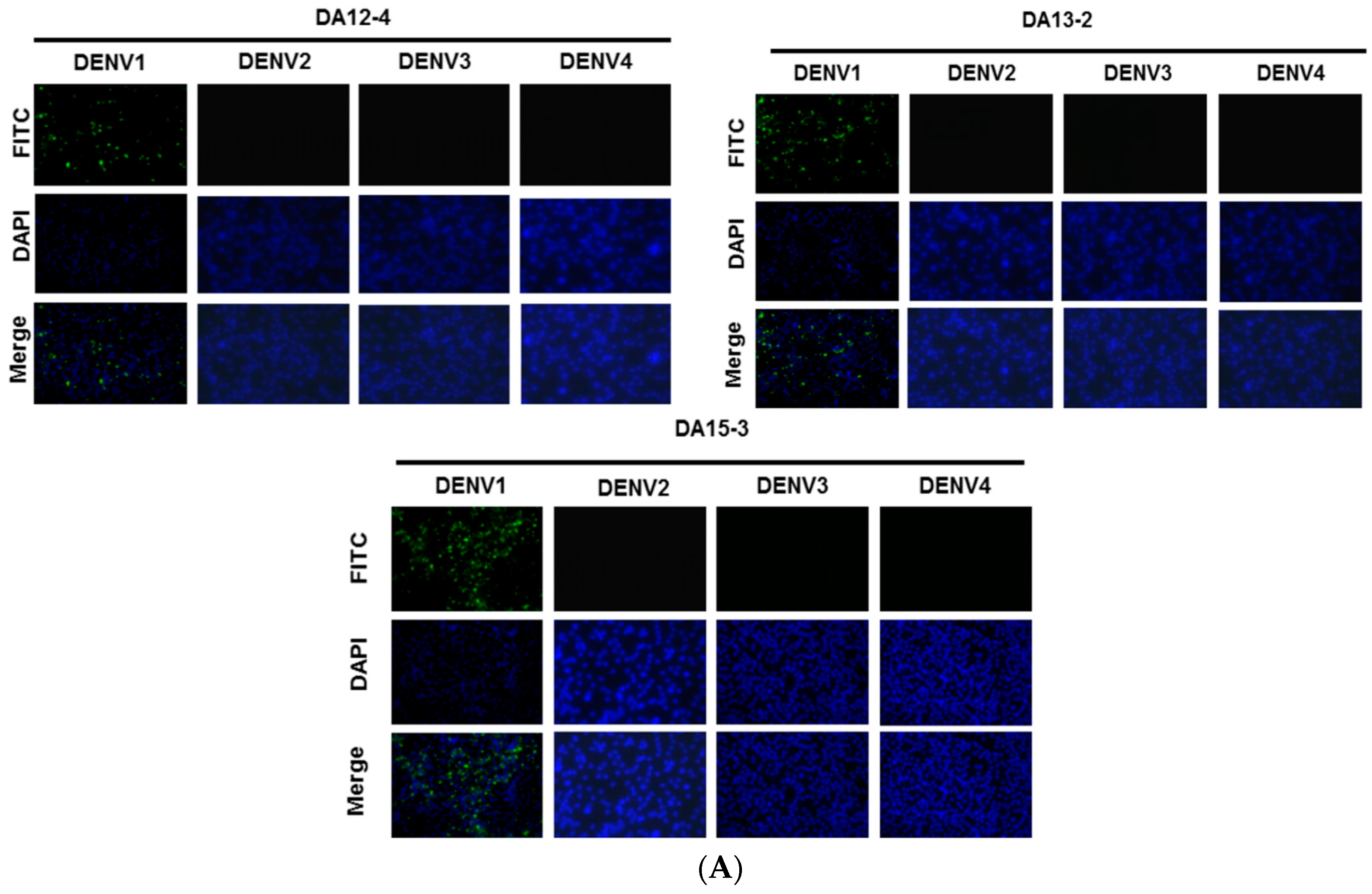
2.2. Generation and Identification of mAbs against DENV1 NS1 Protein

| mAbs | Isotype, Light chain | Specificity | IFA | ELISA | WB | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D1 | D1 | D2 | D3 | D4 | D1 | D2 | D3 | D4 | |||
| DA12-4 | IgG2b, λ | NS1 | + | + | − | − | − | + | − | − | − |
| DA13-2 | IgG1, κ | NS1 | + | + | − | − | − | + | − | − | − |
| DA15-3 | IgG1, κ | NS1 | + | + | − | − | − | + | − | − | − |
2.3. Establishment of an ELISA-Based Diagnostic Platform

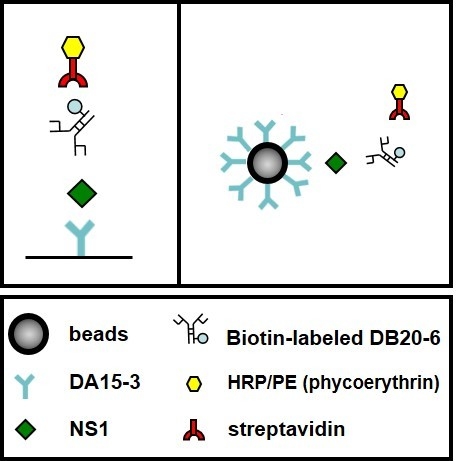
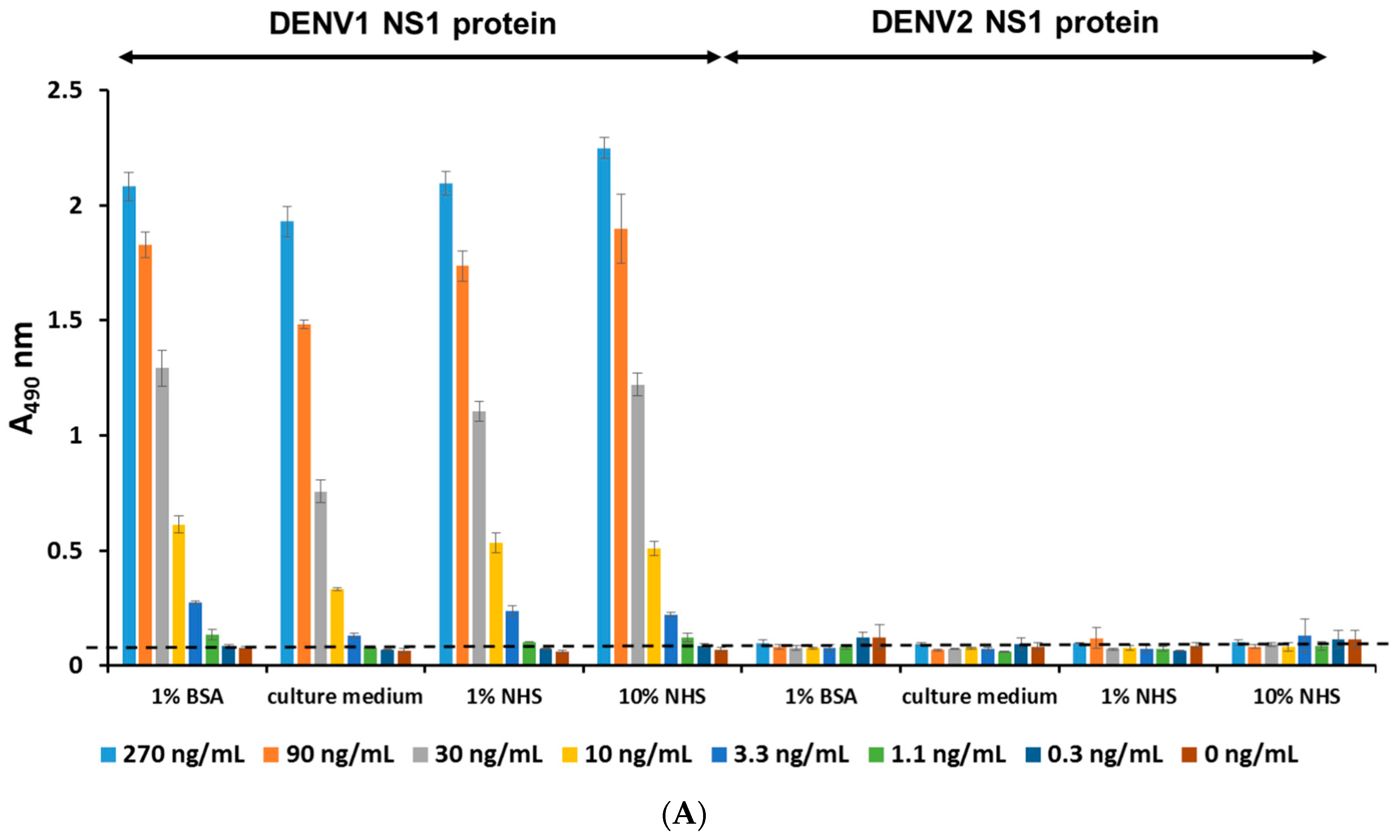
2.4. Establishment of a Flow Cytometry-Based Diagnostic Platform

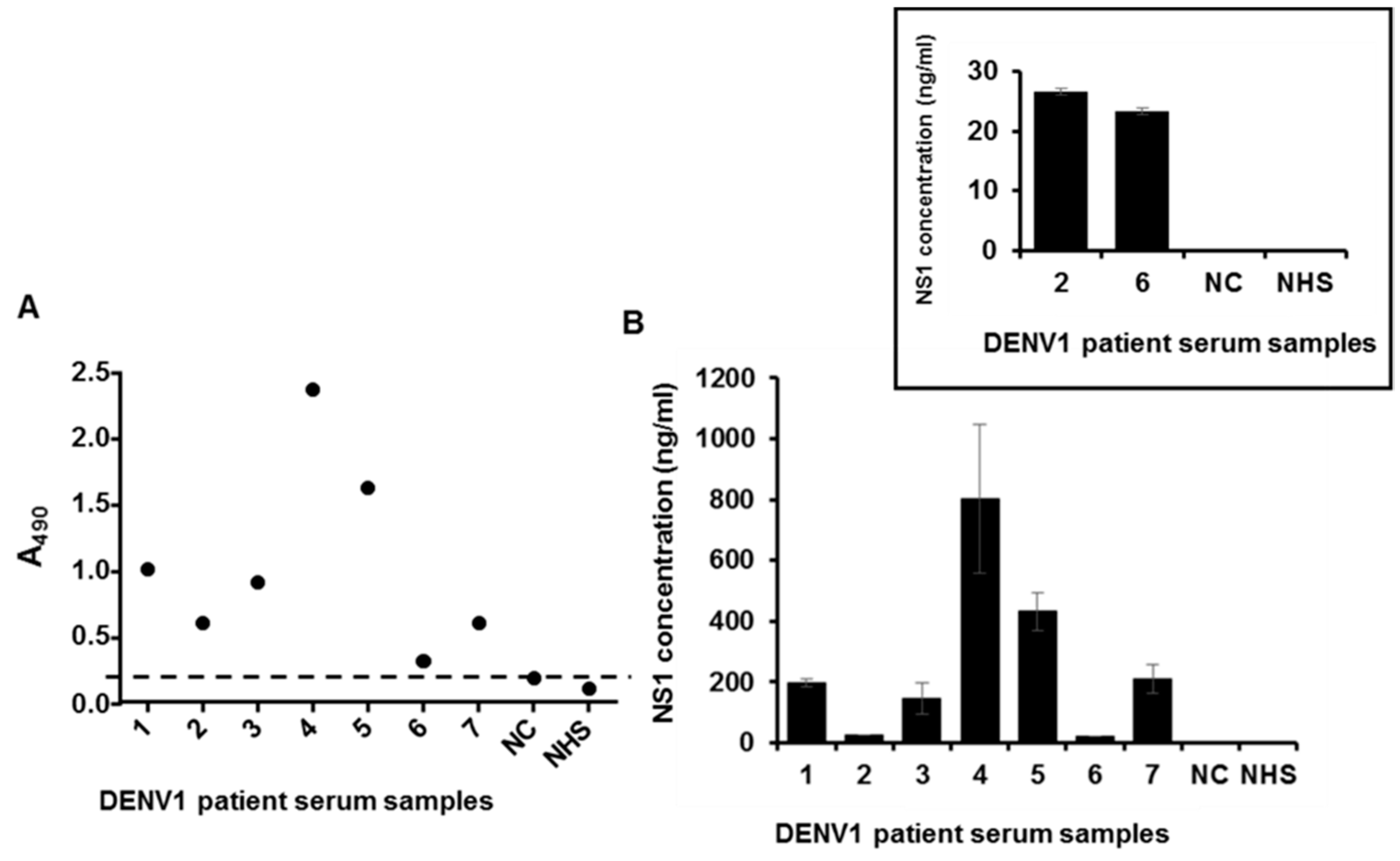
2.5. Detection of NS1 Protein in Sera from Patients Infected with DENV1

| DENV1 NS1 ELISA kit | |||
|---|---|---|---|
| Prototype platform | Mybiosource (Dengue early ELISA) | Euroimmun (Dengue virus NS1 ELISA) | |
| Sensitivity | 90% (45/50) | 4% (2/50) | 54% (27/50) |
| Specificity | 96% (48/50) | N/A | N/A |
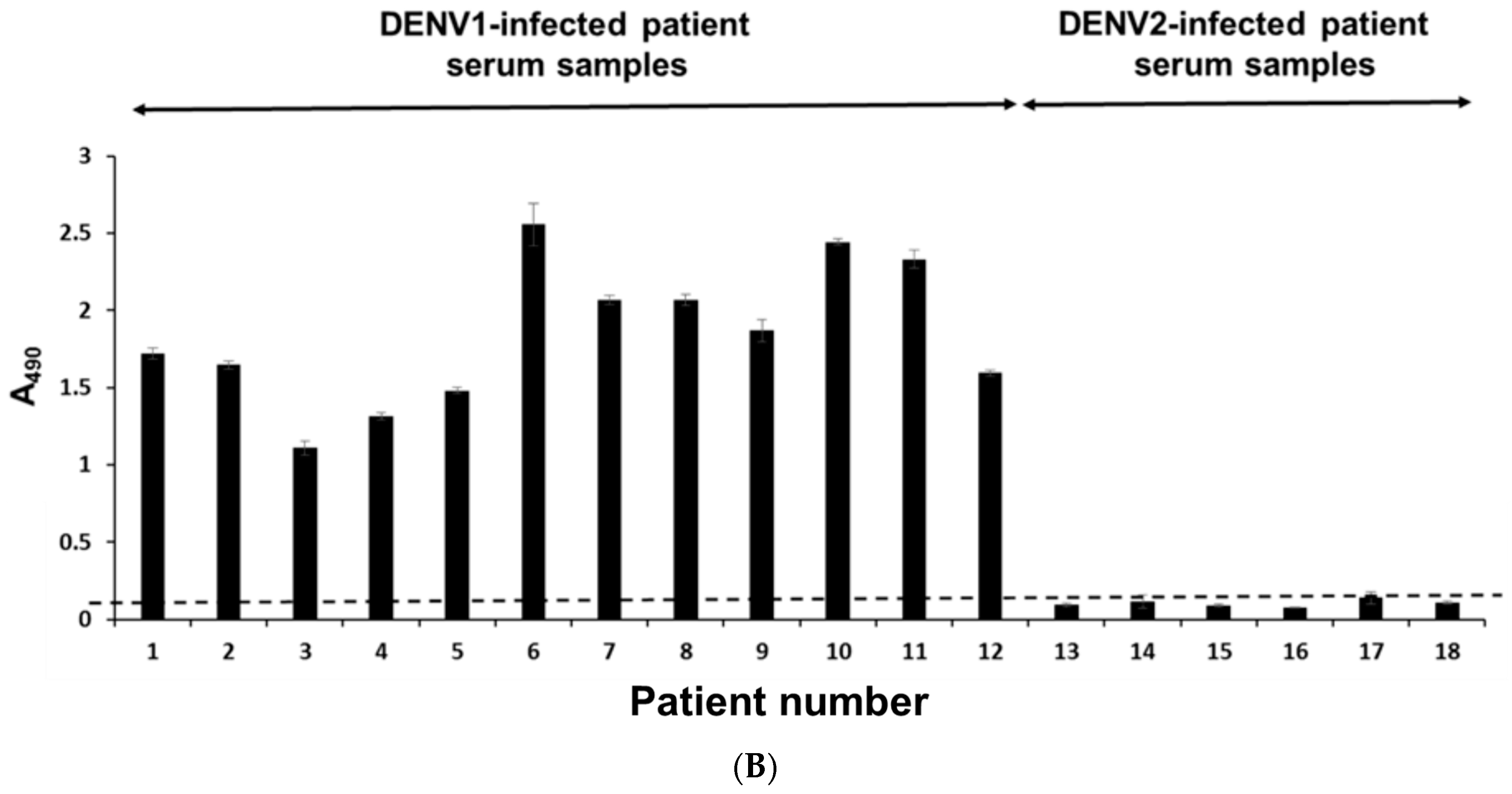
2.6. Serotyping Specificity of the NS1 Diagnostic Platform
3. Discussion
4. Experimental Section
4.1. Cells and Viruses
4.2. Human Serum Samples
4.3. Virus Infection
4.4. DENV Antigen Preparation
4.5. Purification of DENV1 NS1 Protein
4.6. Generation of mAbs against DENV1 NS1 Protein
4.7. Immunofluorescence Assay (IFA)
4.8. Cellular ELISA (Enzyme-Linked Immunosorbent Assay)
4.9. Western Blot Analysis
4.10. Direct ELISA
4.11. Biotinylation of mAb
4.12. Establishment of NS1 Standard Curves
4.13. Detection of NS1 Protein in Clinical Samples
4.14. Flow Cytometry Analysis
4.15. Detection of NS1 Protein in Clinical Samples by Commercial Kits
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, Y.C.; Huang, H.N.; Lin, C.T.; Chen, Y.F.; King, C.C.; Wu, H.C. Generation and characterization of monoclonal antibodies against dengue virus type 1 for epitope mapping and serological detection by epitope-based peptide antigens. Clin. Vaccine Immunol. 2007, 14, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Li, P.C.; Liao, M.Y.; Cheng, P.C.; Liang, J.J.; Liu, I.J.; Chiu, C.Y.; Lin, Y.L.; Chang, G.J.; Wu, H.C. Development of a humanized antibody with high therapeutic potential against dengue virus type 2. PLoS Negl. Trop. Dis. 2012. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Back, A.T.; Lundkvist, A. Dengue viruses—An overview. Infect. Ecol. Epidemiol. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Zidane, N.; Dussart, P.; Bremand, L.; Villani, M.E.; Bedouelle, H. Thermodynamic stability of domain III from the envelope protein of flaviviruses and its improvement by molecular design. Protein Eng. Des. Sel. 2013, 26, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Setthapramote, C.; Kurosu, T.; Nishimura, M.; Asai, A.; Omokoko, M.D.; Pipattanaboon, C.; Pitaksajjakul, P.; Limkittikul, K.; Subchareon, A.; et al. Dengue virus neutralization and antibody-dependent enhancement activities of human monoclonal antibodies derived from dengue patients at acute phase of secondary infection. Antivir. Res. 2013, 98, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Murrell, S.; Wu, S.C.; Butler, M. Review of dengue virus and the development of a vaccine. Biotechnol. Adv. 2011, 29, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Gutsche, I.; Coulibaly, F.; Voss, J.E.; Salmon, J.; D‘Alayer, J.; Ermonval, M.; Larquet, E.; Charneau, P.; Krey, T.; Megret, F.; et al. Secreted dengue virus nonstructural protein NS1 is an atypical barrel-shaped high-density lipoprotein. Proc. Natl. Acad. Sci. USA 2011, 108, 8003–8008. [Google Scholar] [CrossRef] [PubMed]
- Libraty, D.H.; Young, P.R.; Pickering, D.; Endy, T.P.; Kalayanarooj, S.; Green, S.; Vaughn, D.W.; Nisalak, A.; Ennis, F.A.; Rothman, A.L. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J. Infect. Dis. 2002, 186, 1165–1168. [Google Scholar] [CrossRef] [PubMed]
- Alcon, S.; Talarmin, A.; Debruyne, M.; Falconar, A.; Deubel, V.; Flamand, M. Enzyme-linked immunosorbent assay specific to Dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J. Clin. Microbiol. 2002, 40, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Young, P.R.; Hilditch, P.A.; Bletchly, C.; Halloran, W. An antigen capture enzyme-linked immunosorbent assay reveals high levels of the dengue virus protein NS1 in the sera of infected patients. J. Clin. Microbiol. 2000, 38, 1053–1057. [Google Scholar] [PubMed]
- Hu, D.; Di, B.; Ding, X.; Wang, Y.; Chen, Y.; Pan, Y.; Wen, K.; Wang, M.; Che, X. Kinetics of non-structural protein 1, IgM and IgG antibodies in dengue type 1 primary infection. Virol. J. 2011, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Puttikhunt, C.; Prommool, T.; U-thainual, N.; Ong-ajchaowlerd, P.; Yoosook, K.; Tawilert, C.; Duangchinda, T.; Jairangsri, A.; Tangthawornchaikul, N.; Malasit, P.; et al. The development of a novel serotyping-NS1-ELISA to identify serotypes of dengue virus. J. Clin. Virol. 2011, 50, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Bessoff, K.; Delorey, M.; Sun, W.; Hunsperger, E. Comparison of two commercially available dengue virus (DENV) NS1 capture enzyme-linked immunosorbent assays using a single clinical sample for diagnosis of acute DENV infection. Clin. Vaccine Immunol. 2008, 15, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Hang, V.T.; Nguyet, N.M.; Trung, D.T.; Tricou, V.; Yoksan, S.; Dung, N.M.; van Ngoc, T.; Hien, T.T.; Farrar, J.; Wills, B.; et al. Diagnostic accuracy of NS1 ELISA and lateral flow rapid tests for dengue sensitivity, specificity and relationship to viraemia and antibody responses. PLoS Negl. Trop. Dis. 2009. [Google Scholar] [CrossRef] [PubMed]
- Lapphra, K.; Sangcharaswichai, A.; Chokephaibulkit, K.; Tiengrim, S.; Piriyakarnsakul, W.; Chakorn, T.; Yoksan, S.; Wattanamongkolsil, L.; Thamlikitkul, V. Evaluation of an NS1 antigen detection for diagnosis of acute dengue infection in patients with acute febrile illness. Diagn. Microbiol. Infect. Dis. 2008, 60, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Sylvestre, G.; Gandini, M.; de Araujo, J.M.; Kubelka, C.F.; Lourenco-de-Oliveira, R.; Maciel-de-Freitas, R. Preliminary evaluation on the efficiency of the kit Platelia Dengue NS1 Ag-ELISA to detect dengue virus in dried Aedes aegypti: A potential tool to improve dengue surveillance. Parasit. Vectors 2014, 7, 155. [Google Scholar] [CrossRef] [PubMed]
- Koraka, P.; Burghoorn-Maas, C.P.; Falconar, A.; Setiati, T.E.; Djamiatun, K.; Groen, J.; Osterhaus, A.D. Detection of immune-complex-dissociated nonstructural-1 antigen in patients with acute dengue virus infections. J. Clin. Microbiol. 2003, 41, 4154–4159. [Google Scholar] [CrossRef] [PubMed]
- Hermann, L.L.; Thaisomboonsuk, B.; Poolpanichupatam, Y.; Jarman, R.G.; Kalayanarooj, S.; Nisalak, A.; Yoon, I.K.; Fernandez, S. Evaluation of a dengue NS1 antigen detection assay sensitivity and specificity for the diagnosis of acute dengue virus infection. PLoS Negl. Trop. Dis. 2014. [Google Scholar] [CrossRef] [PubMed]
- Barniol, J.; Gaczkowski, R.; Barbato, E.V.; da Cunha, R.V.; Salgado, D.; Martinez, E.; Segarra, C.S.; Pleites Sandoval, E.B.; Mishra, A.; Laksono, I.S.; et al. Usefulness and applicability of the revised dengue case classification by disease: Multi-centre study in 18 countries. BMC Infect. Dis. 2011, 11, 106. [Google Scholar] [CrossRef] [PubMed]
- Castleberry, J.S.; Mahon, C.R. Dengue fever in the Western Hemisphere. Clin. Lab. Sci. 2003, 16, 34–38. [Google Scholar] [PubMed]
- Dussart, P.; Petit, L.; Labeau, B.; Bremand, L.; Leduc, A.; Moua, D.; Matheus, S.; Baril, L. Evaluation of two new commercial tests for the diagnosis of acute dengue virus infection using NS1 antigen detection in human serum. PLoS Negl. Trop. Dis. 2008. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.K.; Tsai, C.H.; Chen, K.H.; Tang, C.T.; Leou, J.S.; Li, P.C.; Tang, Y.L.; Hsieh, H.J.; Wu, H.C.; Cheng, C.M. Cellulose-based diagnostic devices for diagnosing serotype-2 dengue fever in human serum. Adv. Healthc. Mater. 2014, 3, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Hu, D.; Chen, Y.; Di, B.; Jin, J.; Pan, Y.; Qiu, L.; Wang, Y.; Wen, K.; Wang, M.; et al. Full serotype- and group-specific NS1 capture enzyme-linked immunosorbent assay for rapid differential diagnosis of dengue virus infection. Clin. Vaccine Immunol. 2011, 18, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Liu, I.J.; Chiu, C.Y.; Chen, Y.C.; Wu, H.C. Molecular mimicry of human endothelial cell antigen by autoantibodies to nonstructural protein 1 of dengue virus. J. Biol. Chem. 2011, 286, 9726–9736. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.C.; Lin, Y.S.; Liu, C.C.; Liu, H.S.; Liao, S.H.; Shi, M.D.; Lei, H.Y.; Yeh, T.M. Factors contributing to the disturbance of coagulation and fibrinolysis in dengue virus infection. J. Formos. Med. Assoc. 2013, 112, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lee, S.T.; Kim, H.J.; Kim, S.H. Bone marrow flow cytometry in staging of patients with B-cell non-hodgkin lymphoma. Ann. Lab. Med. 2015, 35, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Jennings, C.D.; Foon, K.A. Recent advances in flow cytometry: Application to the diagnosis of hematologic malignancy. Blood 1997, 90, 2863–2892. [Google Scholar] [PubMed]
- Schupbach, J.; Flepp, M.; Pontelli, D.; Tomasik, Z.; Luthy, R.; Boni, J. Heat-mediated immune complex dissociation and enzyme-linked immunosorbent assay signal amplification render p24 antigen detection in plasma as sensitive as HIV-1 RNA detection by polymerase chain reaction. AIDS 1996, 10, 1085–1090. [Google Scholar] [PubMed]
- Nadal, D.; Boni, J.; Kind, C.; Varnier, O.E.; Steiner, F.; Tomasik, Z.; Schupbach, J. Prospective evaluation of amplification-boosted ELISA for heat-denatured p24 antigen for diagnosis and monitoring of pediatric human immunodeficiency virus type 1 infection. J. Infect. Dis. 1999, 180, 1089–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledergerber, B.; Flepp, M.; Boni, J.; Tomasik, Z.; Cone, R.W.; Luthy, R.; Schupbach, J. Human immunodeficiency virus type 1 p24 concentration measured by boosted ELISA of heat-denatured plasma correlates with decline in CD4 cells, progression to AIDS, and survival: Comparison with viral RNA measurement. J. Infect. Dis. 2000, 181, 1280–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.-L.; Chiu, C.-Y.; Lin, C.-Y.; Huang, C.-H.; Chen, Y.-H.; Destura, R.V.; Chao, D.-Y.; Wu, H.-C. Establishment and Comparison of Two Different Diagnostic Platforms for Detection of DENV1 NS1 Protein. Int. J. Mol. Sci. 2015, 16, 27850-27864. https://doi.org/10.3390/ijms161126069
Tang Y-L, Chiu C-Y, Lin C-Y, Huang C-H, Chen Y-H, Destura RV, Chao D-Y, Wu H-C. Establishment and Comparison of Two Different Diagnostic Platforms for Detection of DENV1 NS1 Protein. International Journal of Molecular Sciences. 2015; 16(11):27850-27864. https://doi.org/10.3390/ijms161126069
Chicago/Turabian StyleTang, Yin-Liang, Chien-Yu Chiu, Chun-Yu Lin, Chung-Hao Huang, Yen-Hsu Chen, Raul V. Destura, Day-Yu Chao, and Han-Chung Wu. 2015. "Establishment and Comparison of Two Different Diagnostic Platforms for Detection of DENV1 NS1 Protein" International Journal of Molecular Sciences 16, no. 11: 27850-27864. https://doi.org/10.3390/ijms161126069