A Quantitative Real-Time PCR-Based Strategy for Molecular Evaluation of Nicotine Conversion in Burley Tobacco
Abstract
:1. Introduction
2. Results
2.1. Development of Quantitative Real-Time PCR Assay
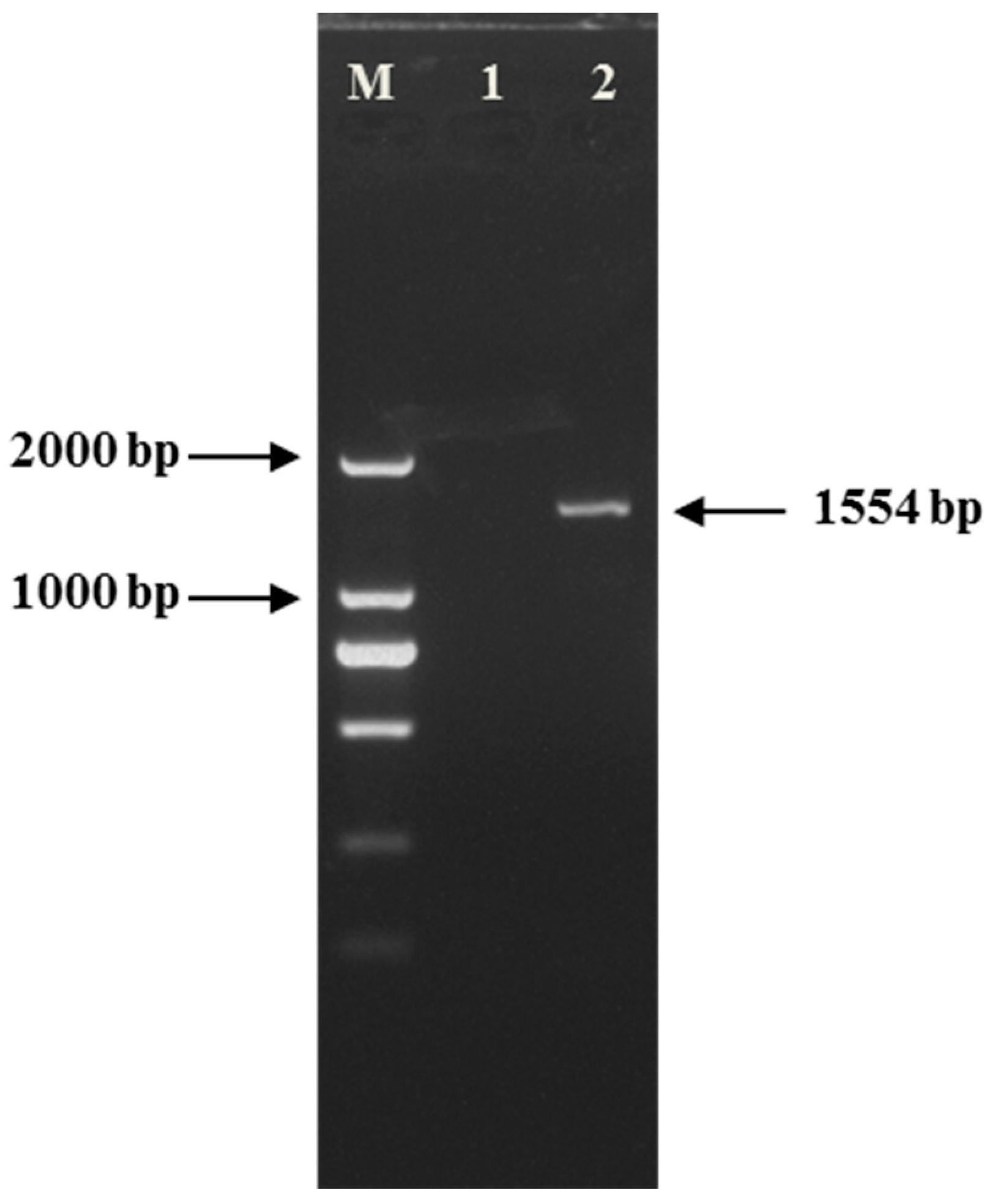
2.1.1. Gel Electrophoresis
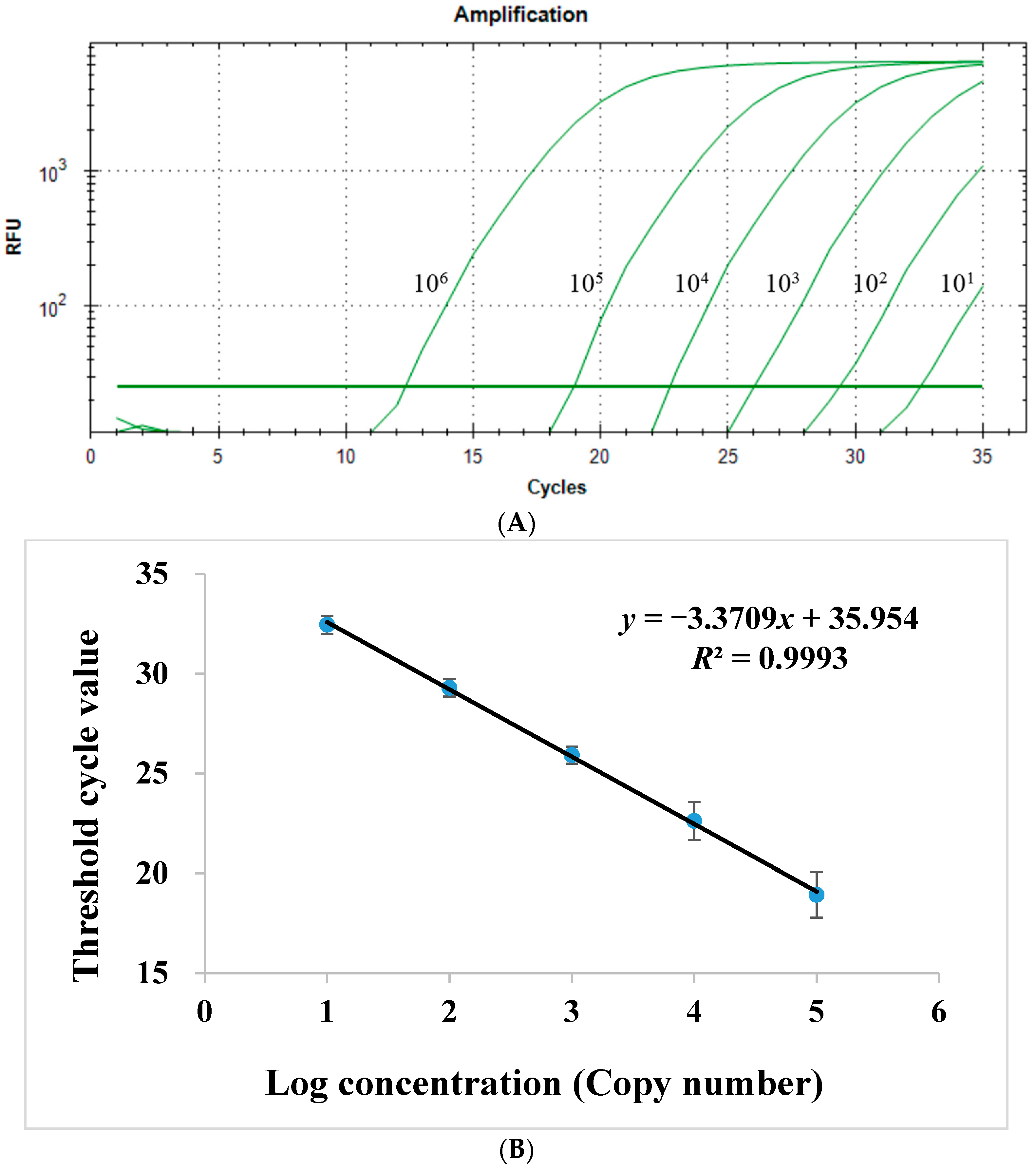
2.1.2. Sensitivity of Quantitative Real-Time PCR Assay

2.1.3. Specificity and Reproducibility of Quantitative Real-Time PCR Assay
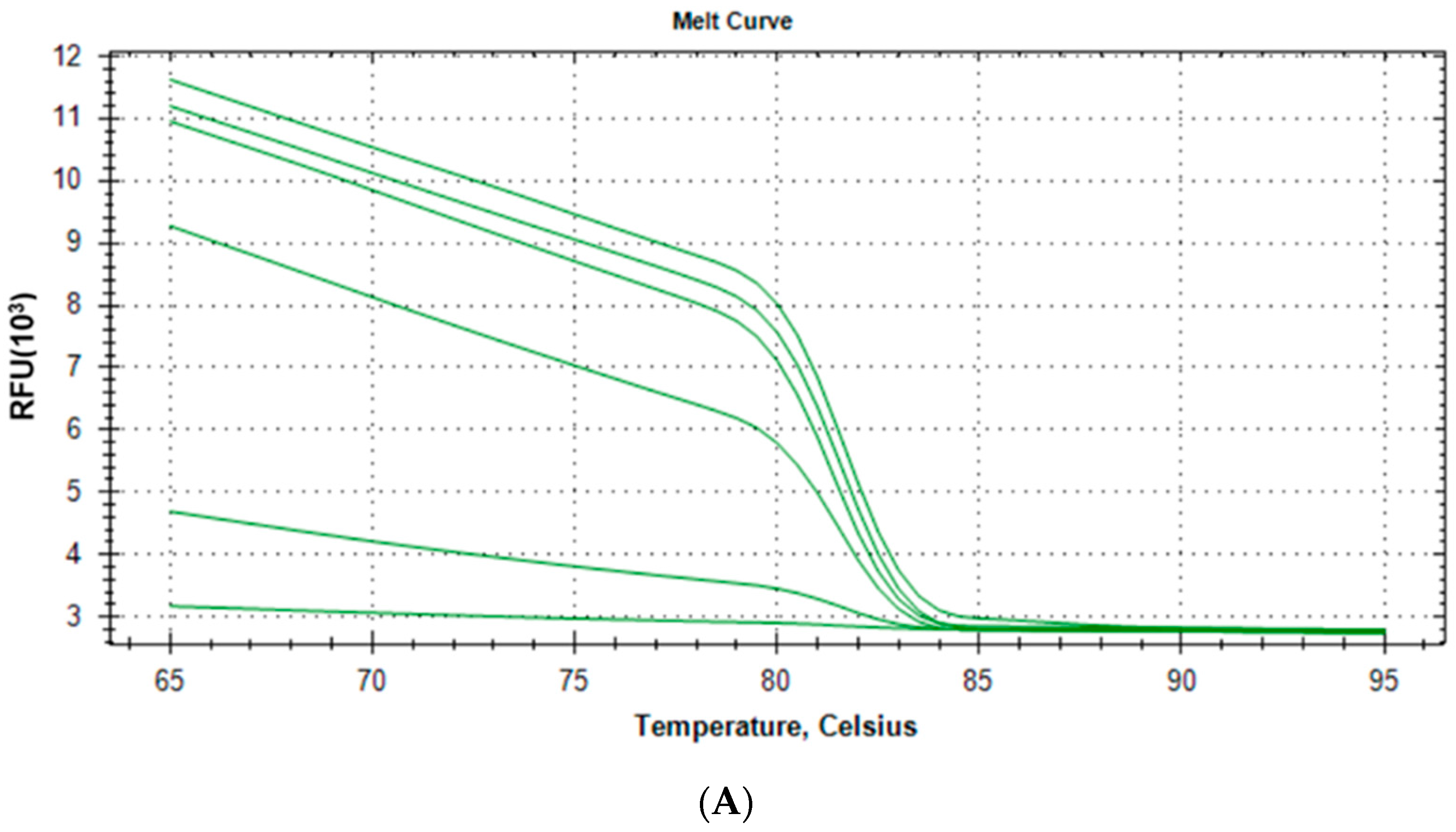
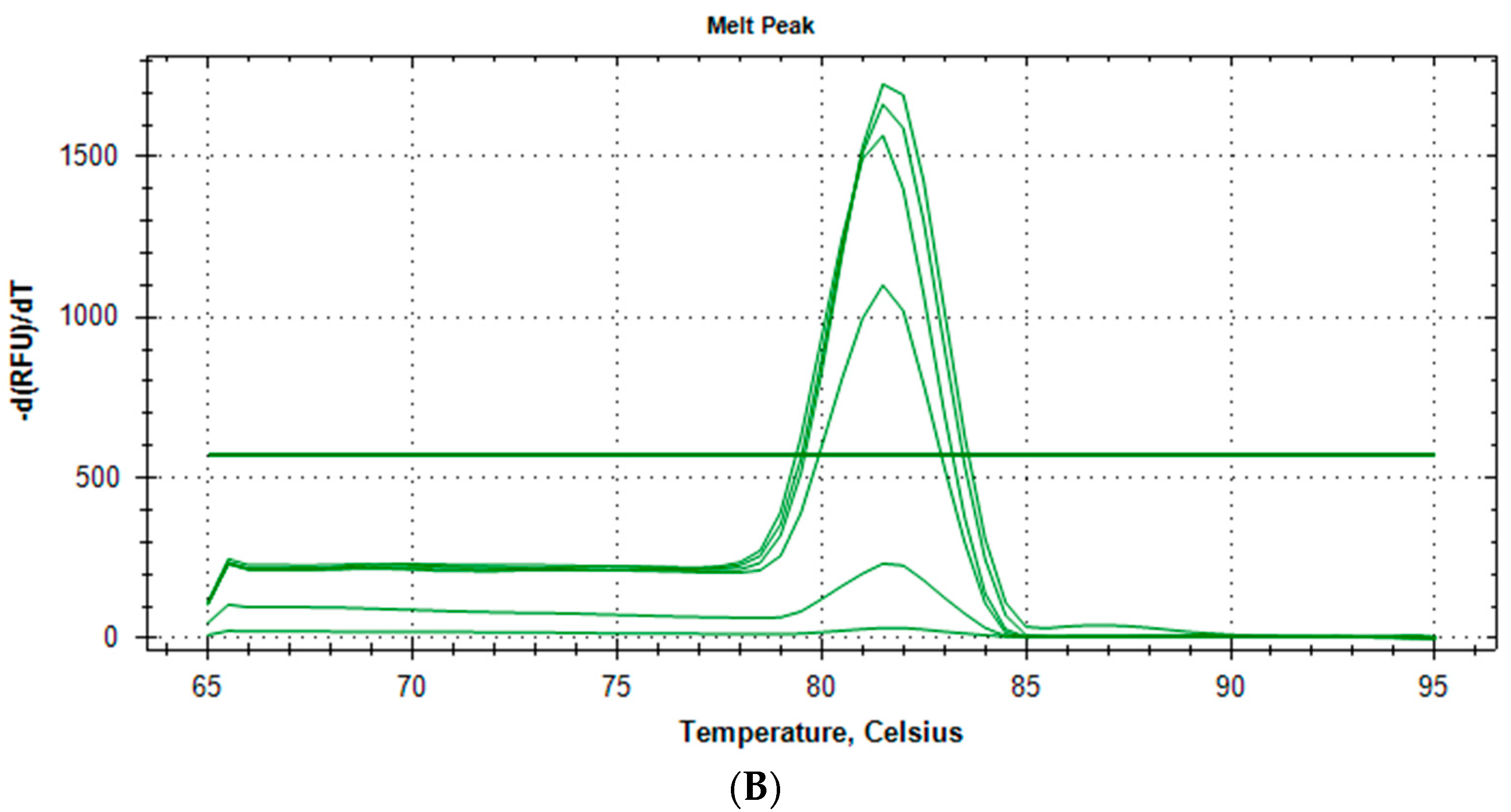
| The Standard Concentration (copies/mL) | Variation Coefficient within Batches (%) | Variation Coefficient between Batches (%) |
|---|---|---|
| 1 × 101 | 1.39 | 2.71 |
| 1 × 102 | 1.47 | 2.46 |
| 1 × 103 | 1.65 | 2.83 |
| 1 × 104 | 4.21 | 4.72 |
| 1 × 105 | 6.49 | 7.12 |
2.1.4. Linear Range of Quantitative Real-Time PCR Assay
2.2. Application of Quantitative Real-Time PCR Assay with Test Samples
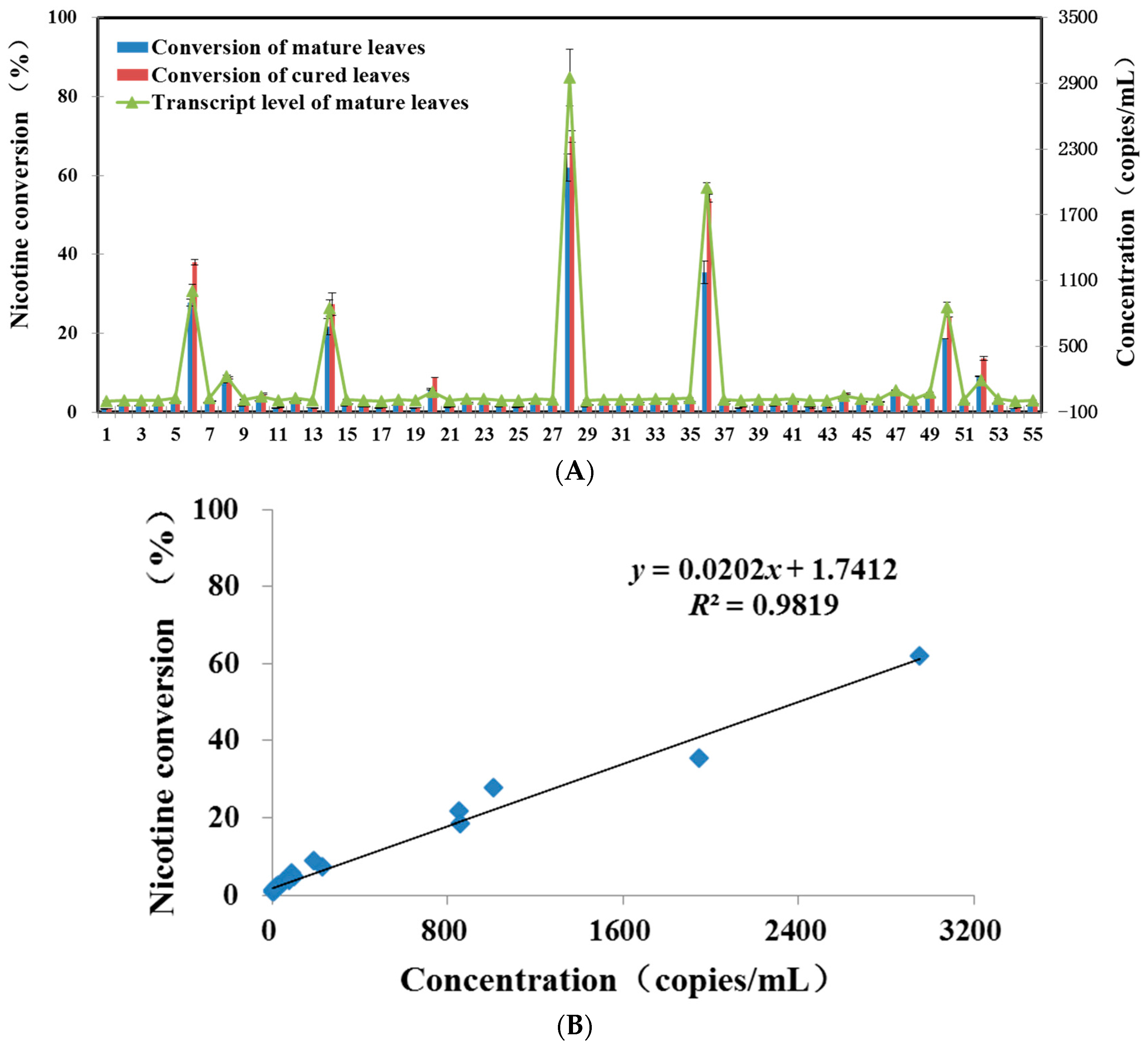
2.3. Compared with GC-MS Method
3. Discussion
4. Experimental Section
4.1. Development of Quantitative Real-Time PCR Method
4.1.1. Preparation of the Standard of Quantitative Real-Time PCR
4.1.2. Quantification of the Standard
4.1.3. Quantitative Real-Time PCR Analysis
4.1.4. Gel Electrophoresis
4.2. Evaluation of Nicotine Conversion by Quantitative Real-Time PCR Method
4.3. Analysis of Nicotine Conversion by GC-MS Method
4.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Djordjevic, M.V.; Gay, S.L.; Bush, L.P.; Chaplin, J.F. Tobacco-specific nitrosamine accumulation and distribution in flue-cured tobacco alkaloid isolines. J. Agric. Food Chem. 1989, 37, 752–756. [Google Scholar] [CrossRef]
- Burton, H.R.; Dye, N.K.; Bush, L.P. Distribution of tobacco constituents in tobacco leaf tissue. 1. Tobacco-specific nitrosamines, nitrate, nitrite, and alkaloids. J. Agric. Food Chem. 1992, 40, 1050–1055. [Google Scholar] [CrossRef]
- Siminszky, B.; Gavilano, L.; Bowen, S.W.; Dewey, R.E. Conversion of nicotine to nornicotine in Nicotiana tabacum is mediated by CYP82E4, a cytochrome P450 monooxygenase. Proc. Natl. Acad. Sci. USA 2005, 102, 14919–14924. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Jack, A.M.; Lewis, R.S.; Dewey, R.E.; Bush, L.P. (R)-nicotine biosynthesis, metabolism and translocation in tobacco as determined by nicotine demethylase mutants. Phytochemistry 2013, 95, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.S.; Hoffmann, D. The relevance of tobacco-specific nitrosamines to human cancer. Cancer Surv. 1989, 8, 273–294. [Google Scholar] [PubMed]
- Hoffmann, D.; Brunnemann, K.D.; Prokopczyk, B.; Djordjevic, M.V. Tobacco-specific N-nitrosamines and ARECA-derived N-nitrosamines: Chemistry, biochemistry, carcinogenicity, and relevance to humans. J. Toxicol. Environ. Health 1994, 41, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.S. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem. Res. Toxicol. 1998, 11, 559–603. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.L. Natural tobacco flavor. Recent Adv. Tob. Sci. 1988, 14, 49–81. [Google Scholar]
- Gavilano, L.B.; Coleman, N.P.; Burnley, L.E.; Bowman, M.L.; Kalengamaliro, N.E.; Hayes, A.; Bush, L.; Siminszky, B. Genetic engineering of Nicotiana tabacum for reduced nornicotine content. J. Agric. Food Chem. 2006, 54, 9071–9078. [Google Scholar] [CrossRef] [PubMed]
- Jack, A.; Bush, L.P. The “LC” Protocol. Available online: http://www.uky.edu/Ag/Tobacco/Pdf/LC-Protocol.pdf (accessed on 3 March 2007).
- Lewis, R.S.; Jack, A.M.; Morris, J.W.; Robert, V.J.M.; Gavilano, L.B.; Siminszky, B.; Bush, L.P.; Hayes, A.; Dewey, R.E. RNA interference (RNAi)-induced suppression of nicotine demethylase activity reduces levels of a key carcinogen in cured tobacco leaves. Plant Biotechnol. J. 2008, 6, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.S.; Bowen, S.W.; Keogh, M.R.; Dewey, R.E. Three nicotine demethylase genes mediate nornicotine biosynthesis in Nicotiana tabacum L.: Functional characterization of the CYP82E10 gene. Phytochemistry 2010, 71, 1988–1998. [Google Scholar] [CrossRef] [PubMed]
- CORESTA Guide N° 7. A Scale for Coding Growth Stages in Tobacco Crops. Available online: http://www.coresta.org/Guides/Guide-No07-Growth-Stages_Feb09.pdf (accessed on 11 April 2012).
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Method 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yu, H.W.; Tang, H.R.; Wang, X.R. Identification and expression analysis of genes involved in anthocyanin and proanthocyanidin biosynthesis in the fruit of blackberry. Sci. Hortic. 2012, 141, 61–68. [Google Scholar] [CrossRef]
- Bogani, P.; Lio, P.; Intrieri, M.C.; Buiatti, M. A physiological and molecular analysis of the genus Nicotiana. Mol. Phylogenet. Evol. 1997, 7, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, M.; Meekins, K.M.; Gavilano, L.B.; Siminszky, B. Inactivation of the cytochrome P450 gene CYP82E2 by degenerative mutations was a key event in the evolution of the alkaloid profile of modern tobacco. New Phytol. 2007, 175, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Shen, Y.; Chappell, J.; Cui, M.; Nielsen, M. Biochemical and molecular characterizations of nicotine demethylase in tobacco. Physiol. Plant. 2007, 129, 307–319. [Google Scholar] [CrossRef]
- Gavilano, L.B.; Siminszky, B. Isolation and characterization of the cytochrome P450 gene CYP82E5v2 that mediates nicotine to nornicotine conversion in the green leaves of tobacco. Plant Cell Physiol. 2007, 48, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Gavilano, L.B.; Coleman, N.P.; Bowen, S.W.; Siminszky, B. Functional analysis of nicotine demethylase genes reveals insights into the evolution of modern tobacco. J. Biol. Chem. 2007, 282, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhang, F.; Zhou, G.J.; Chu, G.H.; Huang, F.F.; Wang, Q.M.; Jin, L.F.; Lin, F.C.; Yang, J. Genetic variation in alkaloid accumulation in leaves of Nicotiana. J. Zhejiang Univ. Sci. B 2013, 14, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhang, F.; Chu, G.H.; Li, F.; Wang, R.; Luo, Z.P.; Wei, C.Y.; Huang, F.F.; Lin, F.C.; Zhou, G.J.; et al. Effects of different environmental locations on alkaloid accumulation in tobacco leaves in China. J. Food Agric. Environ. 2013, 11, 1337–1342. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, B.; Xue, S.-L.; Zhang, F.; Luo, Z.-P.; Wu, M.-Z.; Chen, Q.; Tang, H.-R.; Lin, F.-C.; Yang, J. A Quantitative Real-Time PCR-Based Strategy for Molecular Evaluation of Nicotine Conversion in Burley Tobacco. Int. J. Mol. Sci. 2015, 16, 27422-27432. https://doi.org/10.3390/ijms161126038
Sun B, Xue S-L, Zhang F, Luo Z-P, Wu M-Z, Chen Q, Tang H-R, Lin F-C, Yang J. A Quantitative Real-Time PCR-Based Strategy for Molecular Evaluation of Nicotine Conversion in Burley Tobacco. International Journal of Molecular Sciences. 2015; 16(11):27422-27432. https://doi.org/10.3390/ijms161126038
Chicago/Turabian StyleSun, Bo, Sheng-Ling Xue, Fen Zhang, Zhao-Peng Luo, Ming-Zhu Wu, Qing Chen, Hao-Ru Tang, Fu-Cheng Lin, and Jun Yang. 2015. "A Quantitative Real-Time PCR-Based Strategy for Molecular Evaluation of Nicotine Conversion in Burley Tobacco" International Journal of Molecular Sciences 16, no. 11: 27422-27432. https://doi.org/10.3390/ijms161126038






