Decreased Bone Volume and Bone Mineral Density in the Tibial Trabecular Bone Is Associated with Per2 Gene by 405 nm Laser Stimulation
Abstract
:1. Introduction
2. Results
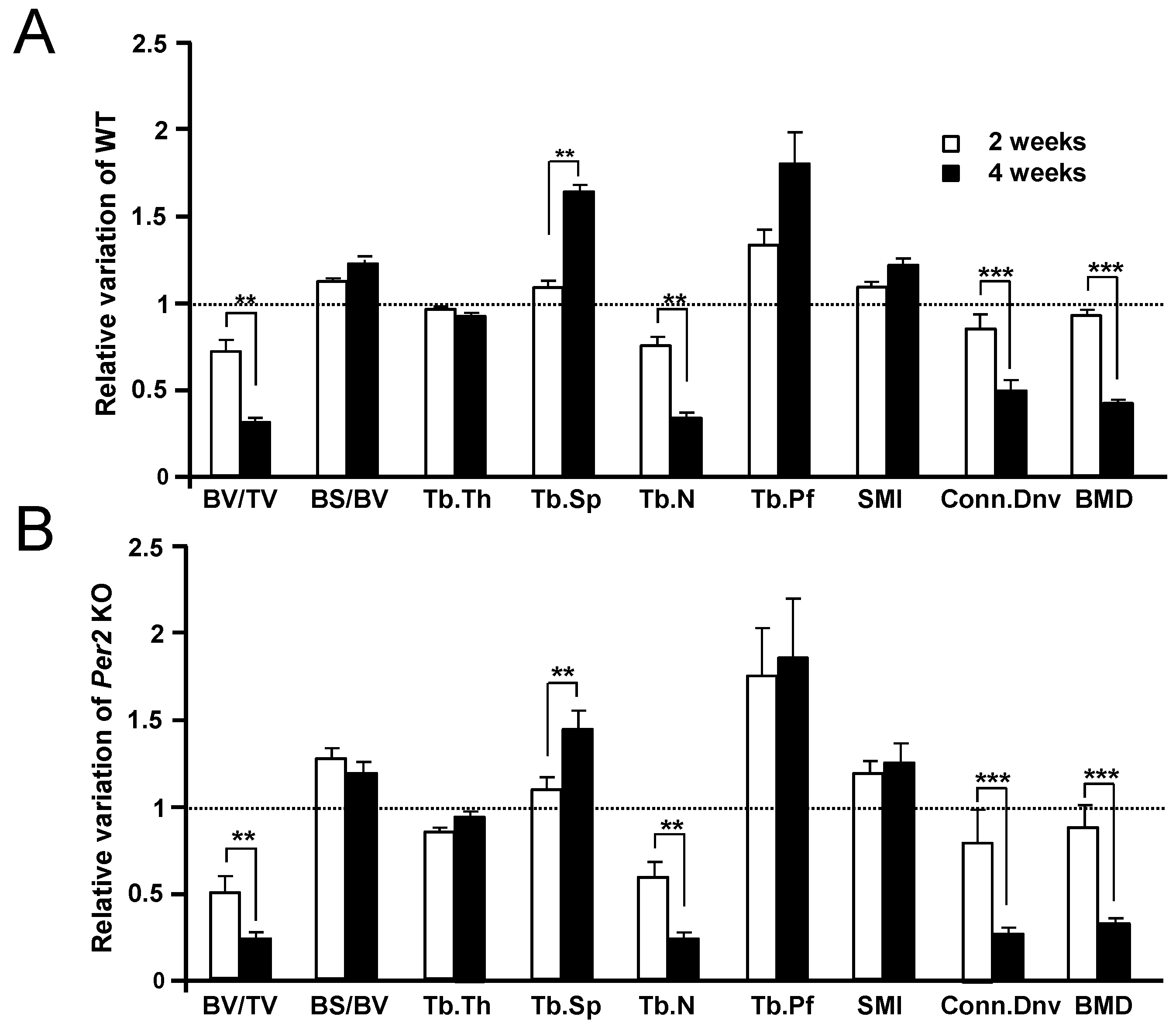
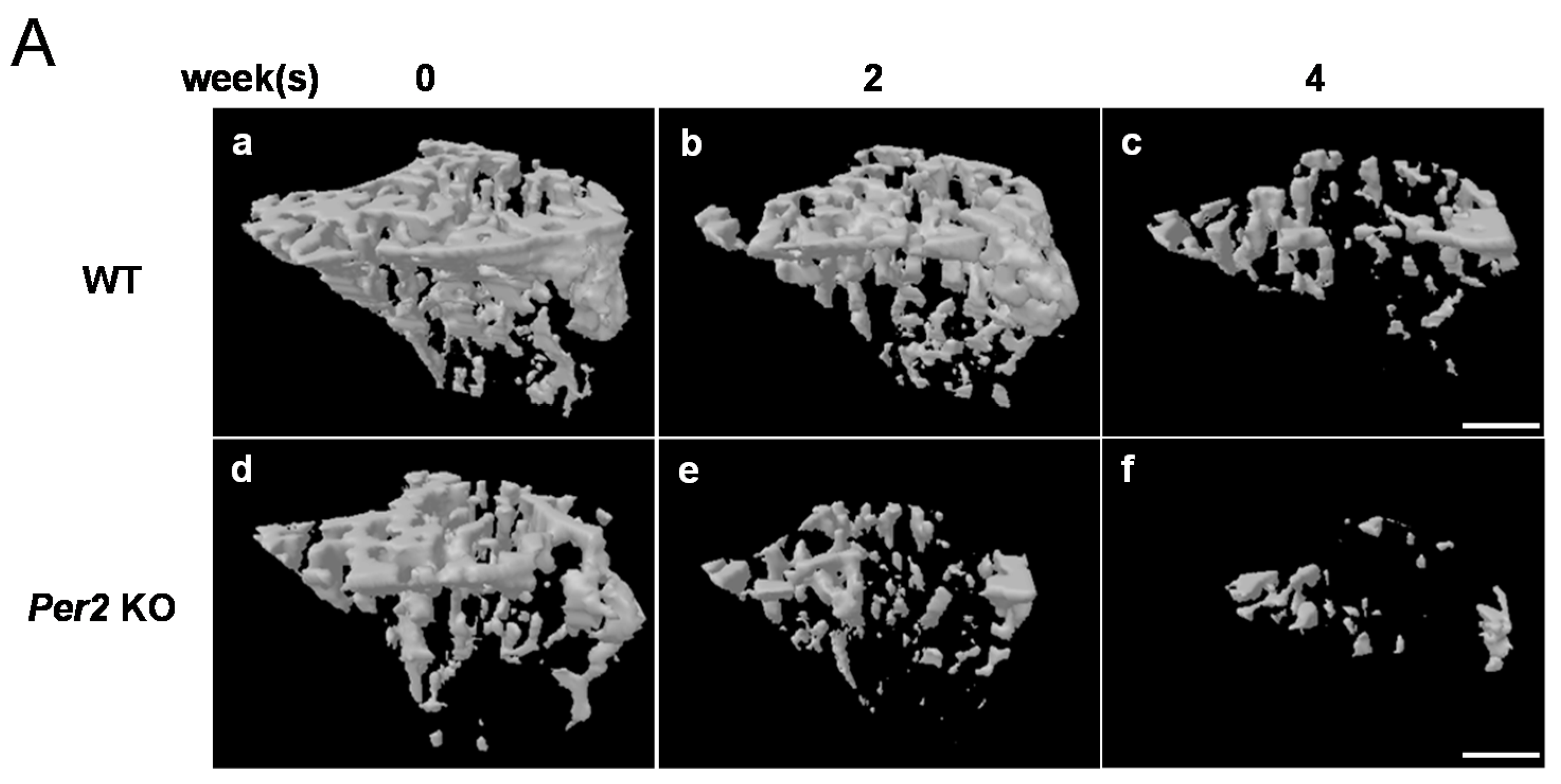
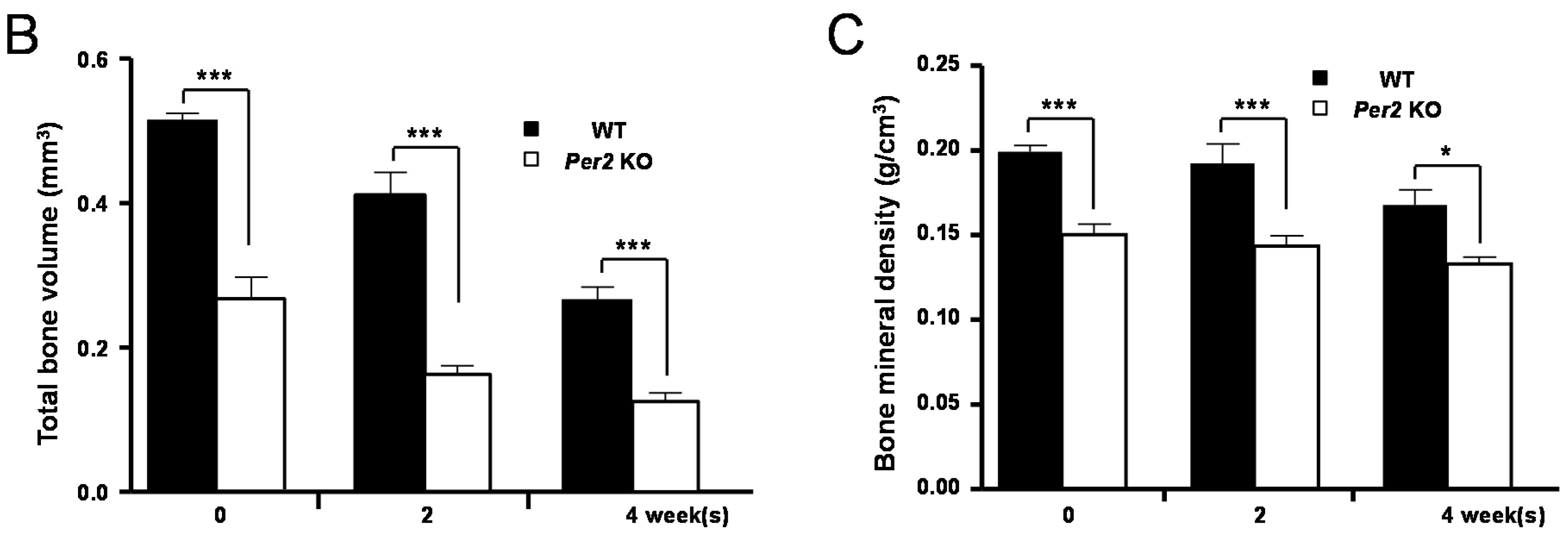
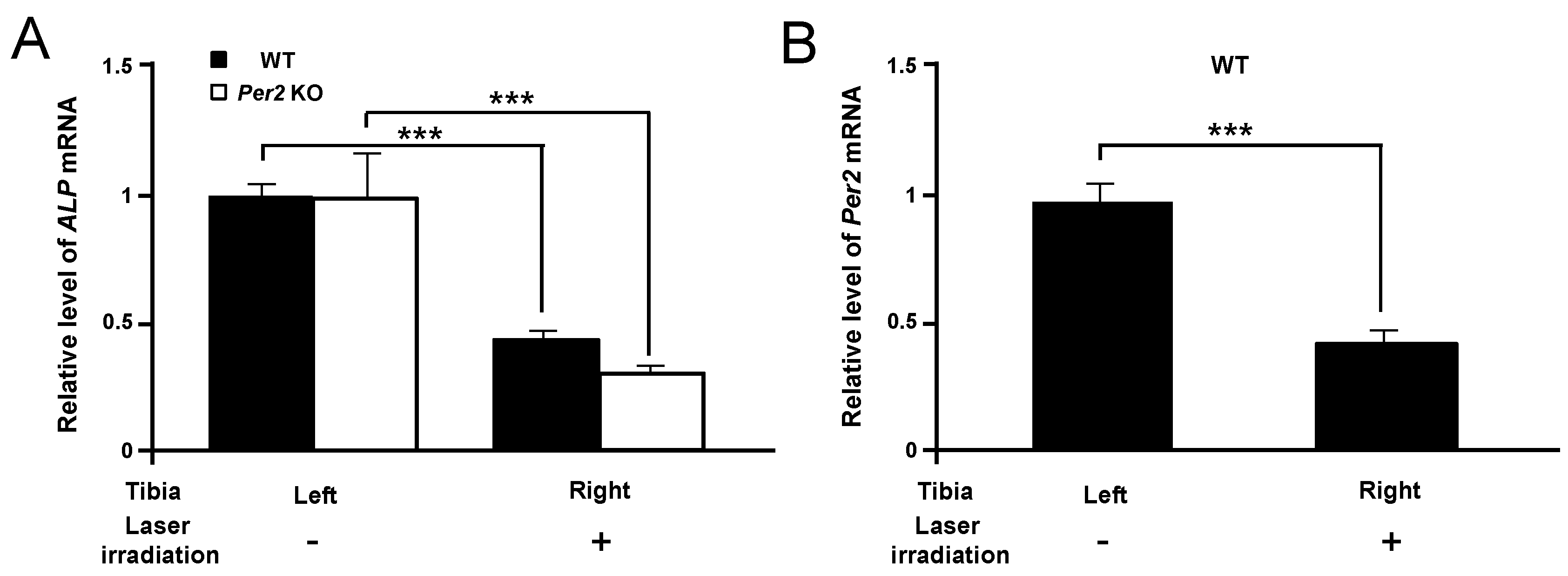
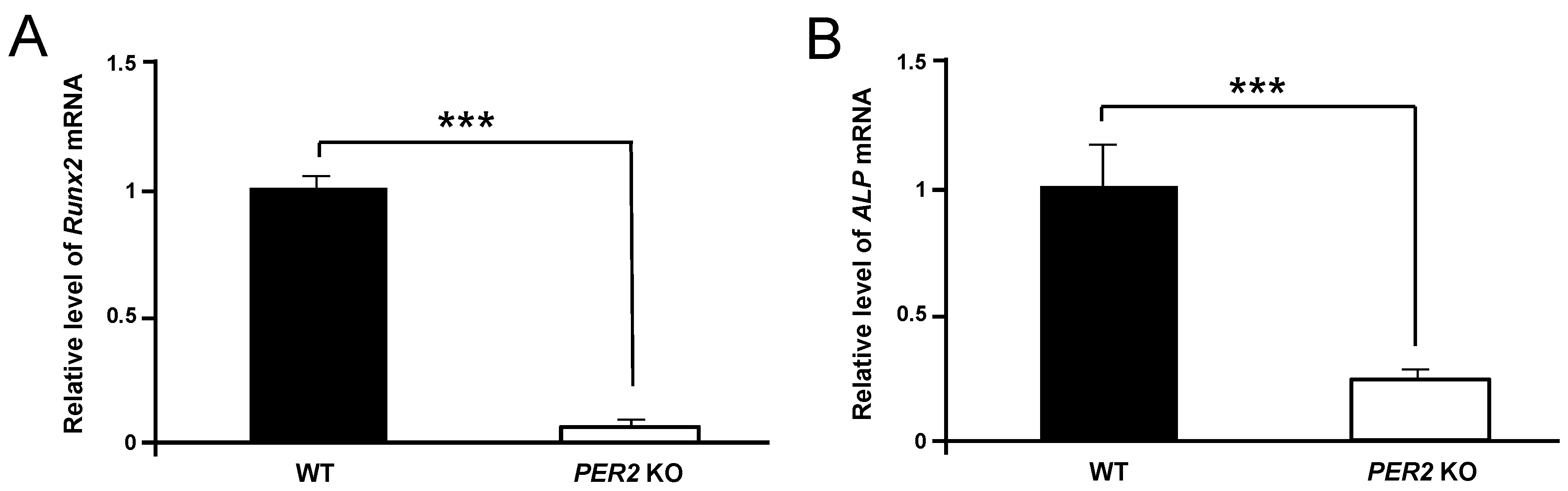
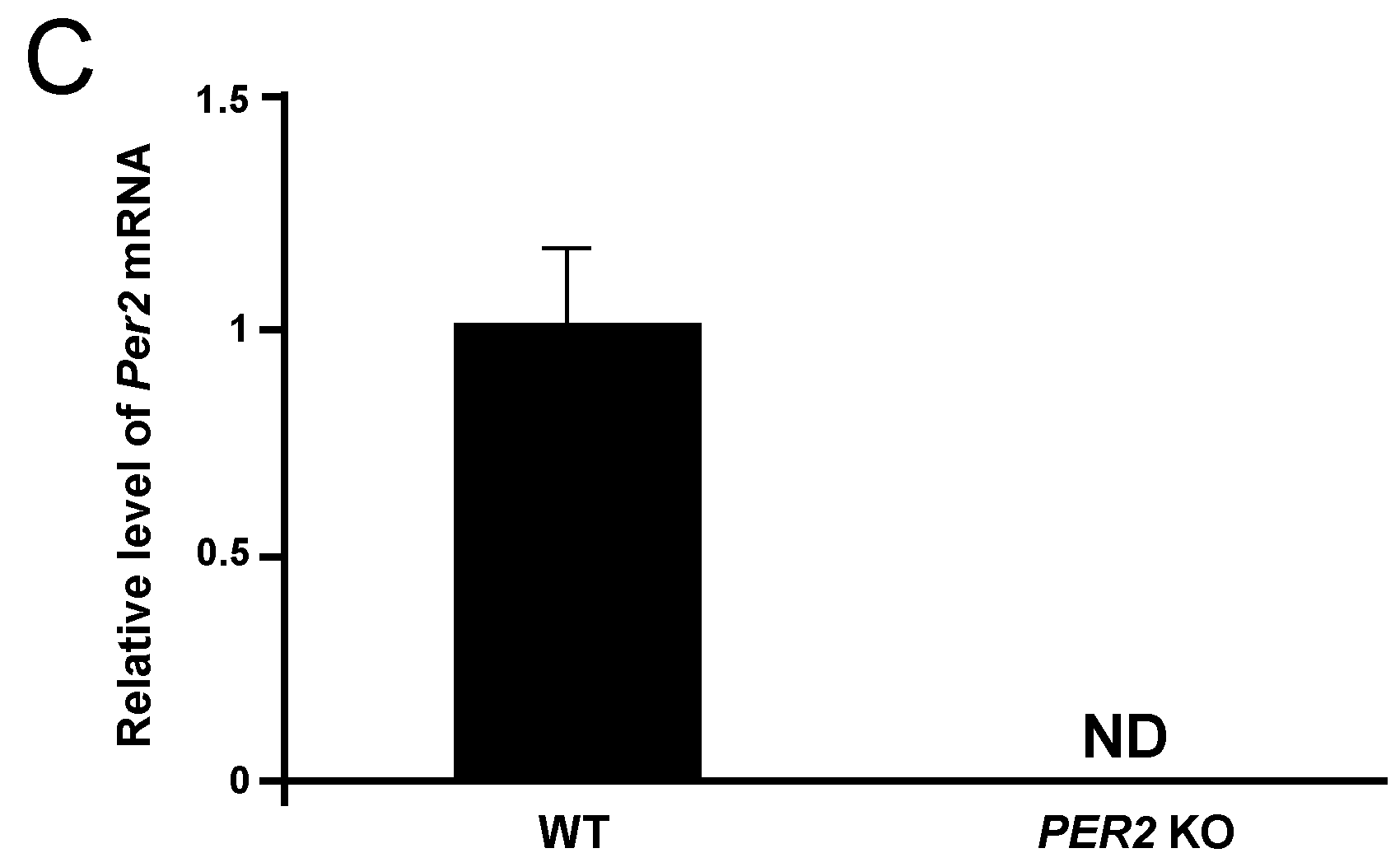
3. Discussion
4. Experimental Section
4.1. Experimental Animals
4.2. LLLT Using MILNS
4.3. Measurements of Structural Parameters
4.4. Preparation of cDNA and Real-Time Quantitative Polymerase Chain Reaction (qPCR)
| Gene | Strand | Sequence | Accession No. |
|---|---|---|---|
| Runx2 | Forward | 5′-TAG CCA GGT TCA ACG ATC TG-3′ | NM001145920.2 |
| Reverse | 5′-TTC TGT CTG TGC CTT CTT GG-3′ | ||
| ALP | Forward | 5′-ATA TAA CAC CAA CGC TCA GG-3′ | NM007431.3 |
| Reverse | 5′-AGG ATG GAT GTG ACC TCA TT-3′ | ||
| Per2 | Forward | 5′-TAT CGT GAA GAA CGC GGA TA-3′ | NM011066.3 |
| Reverse | 5′-AGC TGT GGA ACA CAC TGA CG-3′ | ||
| Gapdh | Forward | 5′-GAC ATC AAG AAG GTG GTG AAG C-3′ | NM008084.3 |
| Reverse | 5′-GAA GGT GGA AGA GTG GGA GTT-3′ |
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ebrahimi, T.; Moslemi, N.; Rokn, A.; Heidari, M.; Nokhbatolfoghahaie, H.; Fekrazad, R. The influence of low-intensity laser therapy on bone healing. J. Dent. 2012, 9, 238–248. [Google Scholar]
- Al Ghamdi, K.M.; Kumar, A.; Moussa, N.A. Low-level laser therapy: A useful technique for enhancing the proliferation of various cultured cells. Lasers Med. Sci. 2012, 27, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Tajali, B.S.; Macdermid, J.C.; Houghton, P.; Grewal, R. Effects of low power laser irradiation on bone healing in animals: A meta-analysis. J. Orthop. Surg. Res. 2010, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.R.; Ribeiro, D.A.; Rodrigues, N.C.; Tim, C.; Santos, A.A.; Parizotto, N.A.; de Araujo, H.S.; Driusso, P.; Rennó, A.C. Effects of low-level laser therapy on the expression of osteogenic genes related in the initial stages of bone defects in rats. J. Biomed. Opt. 2013, 18, 038002. [Google Scholar] [CrossRef] [PubMed]
- Sella, V.R.; do Bomfim, F.R.; Machado, P.C.; da Silva Morsoleto, M.J.; Chohfi, M.; Plapler, H. Effect of low-level laser therapy on bone repair: A randomized controlled experimental study. Lasers Med. Sci. 2015, 30, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Grassi, F.R.; Ciccolella, F.; d’Apolito, G.; Papa, F.; Iuso, A.; Salzo, A.E.; Trentadue, R.; Nardi, G.M.; Scivetti, M.; de Matteo, M.; et al. Effect of low-level laser irradiation on osteoblast proliferation and bone formation. J. Biol. Regul. Homeost. Agents 2011, 25, 603–614. [Google Scholar] [PubMed]
- Jawad, M.M.; Husein, A.; Azlina, A.; Alam, M.K.; Hassan, R.; Shaari, R. Effect of 940 nm low-level laser therapy on osteogenesis in vitro. J. Biomed. Opt. 2013, 18. [Google Scholar] [CrossRef] [PubMed]
- Kiyosaki, T.; Mitsui, N.; Suzuki, N.; Shimizu, N. Low-level laser therapy stimulates mineralization via increased Runx2 expression and ERK phosphorylation in osteoblasts. Photomed. Laser Surg. 2010, 28 (Suppl. 1), S167–S172. [Google Scholar] [CrossRef] [PubMed]
- Ghahroudi, A.A.R.; Rokn, A.R.; Kalhori, K.A.; Khorsand, A.; Pournabi, A.; Pinheiro, A.L.; Fekrazad, R. Effect of low-level laser therapy irradiation and Bio-Oss graft material on the osteogenesis process in rabbit calvarium defects: A double blind experimental study. Lasers Med. Sci. 2014, 29, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Scalize, P.H.; de Sousa, L.G.; Regalo, S.C.; Semprini, M.; Pitol, D.L.; da Silva, G.A.; de Almeida Coelho, J.; Coppi, A.A.; Laad, A.A.; Prado, K.F.; et al. Low-level laser therapy improves bone formation: Stereology findings for osteoporosis in rat model. Lasers Med. Sci. 2015, 30, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Batista, J.D.; Sargenti-Neto, S.; Dechichi, P.; Rocha, F.S.; Pagnoncelli, R.M. Low-level laser therapy on bone repair: Is there any effect outside the irradiated field? Lasers Med. Sci. 2015, 30, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Aras, M.H.; Erkilic, S.; Demir, T.; Demirkol, M.; Kaplan, D.S.; Yolcu, U. Effects of low-level laser therapy on osteoblastic bone formation and relapse in an experimental rapid maxillary expansion model. Niger J. Clin. Pract. 2015, 18, 607–611. [Google Scholar] [PubMed]
- Kang, H.; Ko, C.Y.; Ryu, Y.; Seo, D.H.; Kim, H.S.; Jung, B. Development of a minimally invasive laser needle system: Effects on cortical bone of osteoporotic mice. Lasers Med. Sci. 2012, 27, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, T.; Hosoya, A.; Nakamura, H.; Sano, K.; Nishisaka, T.; Ozawa, H. Increase of bone volume by a nanosecond pulsed laser irradiation is caused by a decreased osteoclast number and an activated osteoblasts. Bone 2007, 40, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.Y.; Kang, H.; Seo, D.H.; Jung, B.; Schreiber, J.; Kim, H.S. Low-level laser therapy using the minimally invasive laser needle system on osteoporotic bone in ovariectomized mice. Med. Eng. Phys. 2013, 35, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.Y.; Kang, H.; Ryu, Y.; Jung, B.; Kim, H.; Jeong, D.; Shin, H.I.; Lim, D.; Kim, H.S. The effects of minimally invasive laser needle system on suppression of trabecular bone loss induced by skeletal unloading. Lasers Med. Sci. 2013, 28, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Patel, M.S.; Bradley, A.; Wagner, E.F.; Karsenty, G. The molecular clock mediates leptin-regulated bone formation. Cell 2005, 122, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Maronde, E.; Schilling, A.F.; Seitz, S.; Schinke, T.; Schmutz, I.; van der Horst, G.; Amling, M.; Albrecht, U. The clock genes Period 2 and Cryptochrome 2 differentially balance bone formation. PLoS ONE 2010, 5, e11527. [Google Scholar] [CrossRef] [PubMed]
- Kushibiki, T.; Awazu, K. Controlling osteogenesis and adipogenesis of mesenchymal stromal cells by regulating a circadian clock protein with laser irradiation. Int. J. Med. Sci. 2008, 5, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Kushibiki, T.; Hirasawa, T.; Okawa, S.; Ishihara, M. Low reactive level laser therapy for mesenchymal stromal cells therapies. Stem Cells Int. 2015, 2015, 974864. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, G. Quantitative CT in the measurement of bone quantity and bone quality for assessing osteoporosis. Med. Eng. Phys. 1996, 18, 557–568. [Google Scholar] [CrossRef]
- Hohlweg-Majert, B.; Pautke, C.; Deppe, H.; Metzger, M.C.; Wagner, K.; Schulze, D. Qualitative and quantitative evaluation of bony structures based on DICOM dataset. J. Oral Maxillofac. Surg. 2011, 69, 2763–2770. [Google Scholar] [CrossRef] [PubMed]
- Munakata, M.; Tachikawa, N.; Honda, E.; Shiota, M.; Kasugai, S. Influence of menopause on mandibular bone quantity and quality in Japanese women receiving dental implants. Arch. Osteoporos. 2011, 6, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Tolstunov, L.; Thai, D.; Arellano, L. Implant-guided volumetric analysis of edentulous maxillary bone with cone beam CT scan. Maxillary sinus pneumatization classification. J. Oral Implantol. 2012, 38, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Langton, C.M.; Pisharody, S.; Keyak, J.H. Comparison of 3D finite element analysis derived stiffness and BMD to determine the failure load of the excised proximal femur. Med. Eng. Phys. 2009, 31, 668–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazarian, A.; von Stechow, D.; Zurakowski, D.; Müller, R.; Snyder, B.D. Bone volume fraction explains the variation in strength and stiffness of cancellous bone affected by metastatic cancer and osteoporosis. Calcif. Tissue Int. 2008, 83, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Zbranca, E.; Galusca, B.; Mogos, V. Bone mineral densitometry. The importance of BMD in endocrinologist practice. Timisoara Med. J. 2004, 3, 260–267. [Google Scholar]
- Gross, A.J.; Jelkmann, W. Helium-neon laser irradiation inhibits the growth of kidney epithelial cells in culture. Lasers Surg. Med. 1990, 10, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Sattayut, S.; Hughes, F.; Bradly, P. 820 nm gallium aluminium arsenide laser modulation of prostaglandin E2 production in interleukin-1 stimulated myoblasts. Laser Ther. 1999, 11, 88–95. [Google Scholar] [CrossRef]
- Shu, B.; Ni, G.X.; Zhang, L.Y.; Li, X.P.; Jiang, W.L.; Zhang, L.Q. High-power helium-neon laser irradiation inhibits the growth of traumatic scars in vitro and in vivo. Lasers Med. Sci. 2013, 28, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Nováková, M.; Nevsímalová, S.; Príhodová, I.; Sládek, M.; Sumová, A. Alteration of the circadian clock in children with Smith-Magenis syndrome. J. Clin. Endocrinol. Metab. 2012, 97, E312–E318. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Xing, L.; Shi, G.; Liu, Z.; Wang, X.; Qu, Z.; Wu, X.; Dong, Z.; Gao, X.; Liu, G.; et al. The circadian mutation PER2 (S662G) is linked to cell cycle progression and tumorigenesis. Cell Death Differ. 2012, 19, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Gery, S.; Virk, R.K.; Chumakov, K.; Yu, A.; Koeffler, H.P. The clock gene Per2 links the circadian system to the estrogen receptor. Oncogene 2007, 26, 7916–7920. [Google Scholar] [CrossRef] [PubMed]
- Okubo, N.; Fujiwara, H.; Minami, Y.; Kunimoto, T.; Hosokawa, T.; Umemura, Y.; Inokawa, H.; Asada, M.; Oda, R.; Kubo, T.; et al. Parathyroid hormone resets the cartilage circadian clock of the organ-cultured murine femur. Acta Orthop. 2015, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Regulation of osteoblast differentiation by Runx2. Adv. Exp. Med. Biol. 2010, 658, 43–49. [Google Scholar] [PubMed]
- Vimalraj, S.; Arumugam, B.; Miranda, P.J.; Selvamurugan, N. Runx2: Structure, function, and phosphorylation in osteoblast differentiation. Int. J. Biol. Macromol. 2015, 78, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Golub, E.E.; Boesze-Battaglia, K. The role of alkaline phosphatase in mineralization. Curr. Opin. Orthop. 2007, 18, 444–448. [Google Scholar] [CrossRef]
- Christenson, R.H. Biochemical markers of bone metabolism: An overview. Clin. Biochem. 1997, 30, 573–593. [Google Scholar] [CrossRef]
- Stein, E.; Koehn, J.; Sutter, W.; Wendtlandt, G.; Wanschitz, F.; Thurnher, D.; Baghestanian, M.; Turhani, D. Initial effects of low-level laser therapy on growth and differentiation of human osteoblast-like cells. Wien. Klin. Wochenschr. 2008, 120, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, Y.; Shimizu, N.; Kariya, G.; Abiko, Y. Lowenergy laser irradiation stimulates bone nodule formation at early stages of cell culture in rat calvarial cells. Bone 1998, 22, 347–354. [Google Scholar] [CrossRef]
- Ueda, Y.; Shimizu, N. Effects of pulse frequency of low-level laser therapy (LLLT) on bone nodule formation in rat calvarial cells. J. Clin. Laser Med. Surg. 2003, 21, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Chen, C.H.; Yeh, L.Y.; Yeh, M.L.; Ting, C.C.; Wang, Y.H. Low-power laser irradiation promotes the proliferation and osteogenic differentiation of human periodontal ligament cells via cyclic adenosine monophosphate. Int. J. Oral Sci. 2013, 5, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Hirata, S.; Kitamura, C.; Fukushima, H.; Nakamichi, I.; Abiko, Y.; Terashita, M.; Jimi, E. Low-level laser irradiation enhances BMP-induced osteoblast differentiation by stimulating the BMP/Smad signaling pathway. J. Cell. Biochem. 2010, 111, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, Y.-M.; Lee, M.-H.; Park, J.H.; Seo, D.-H.; Lee, S.; Jung, B.; Kim, H.S.; Bae, K. Decreased Bone Volume and Bone Mineral Density in the Tibial Trabecular Bone Is Associated with Per2 Gene by 405 nm Laser Stimulation. Int. J. Mol. Sci. 2015, 16, 27401-27410. https://doi.org/10.3390/ijms161126028
Yoo Y-M, Lee M-H, Park JH, Seo D-H, Lee S, Jung B, Kim HS, Bae K. Decreased Bone Volume and Bone Mineral Density in the Tibial Trabecular Bone Is Associated with Per2 Gene by 405 nm Laser Stimulation. International Journal of Molecular Sciences. 2015; 16(11):27401-27410. https://doi.org/10.3390/ijms161126028
Chicago/Turabian StyleYoo, Yeong-Min, Myung-Han Lee, Ji Hyung Park, Dong-Hyun Seo, Sangyeob Lee, Byungjo Jung, Han Sung Kim, and Kiho Bae. 2015. "Decreased Bone Volume and Bone Mineral Density in the Tibial Trabecular Bone Is Associated with Per2 Gene by 405 nm Laser Stimulation" International Journal of Molecular Sciences 16, no. 11: 27401-27410. https://doi.org/10.3390/ijms161126028






