Berberine Sulfate Attenuates Osteoclast Differentiation through RANKL Induced NF-κB and NFAT Pathways
Abstract
:1. Introduction
2. Results
2.1. Berberine Sulfate Inhibits RANKL-Induced Osteoclastogenesis
2.2. Berberine Sulfate Suppresses RANKL-Induced Osteoclast Function
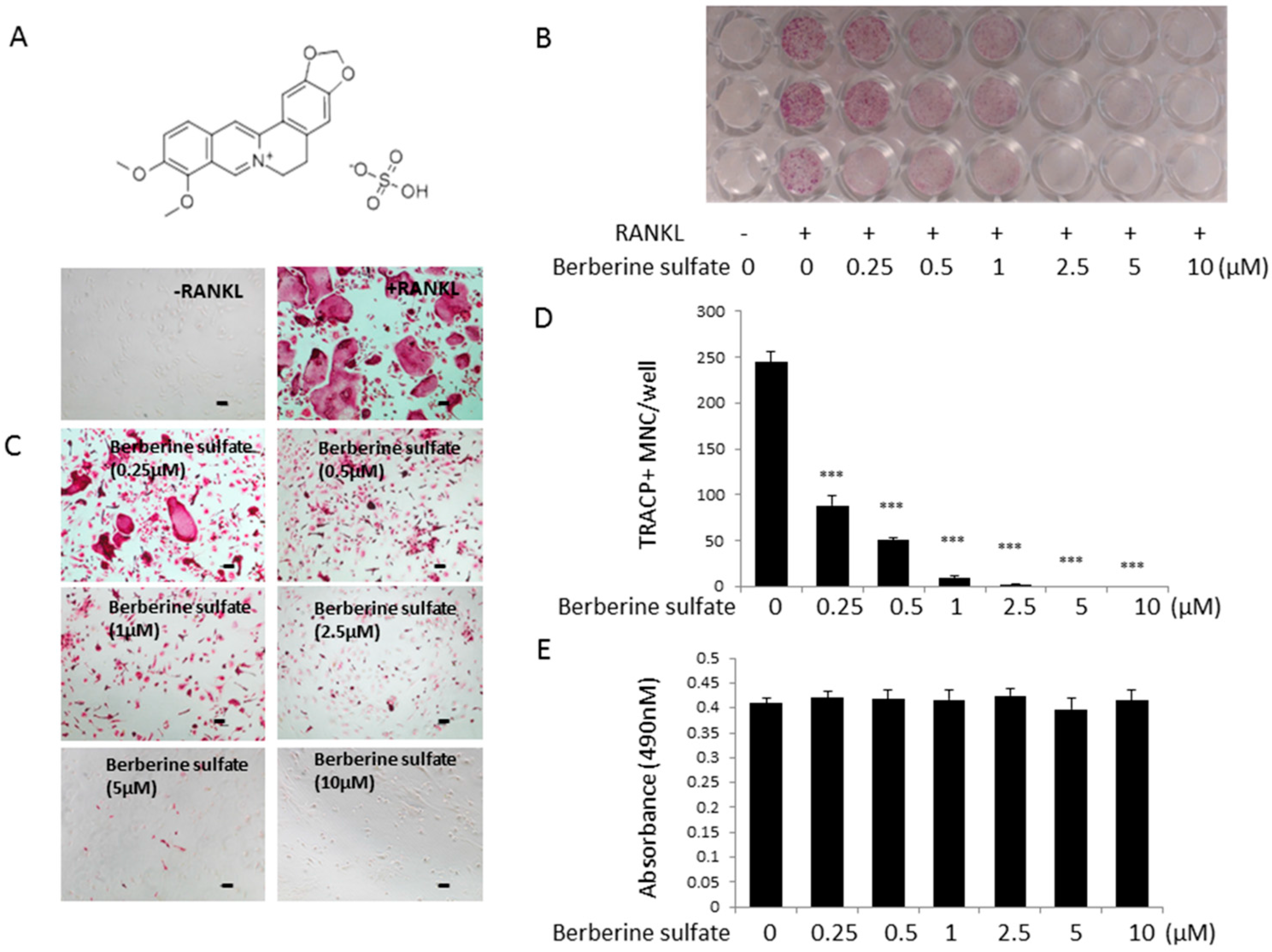

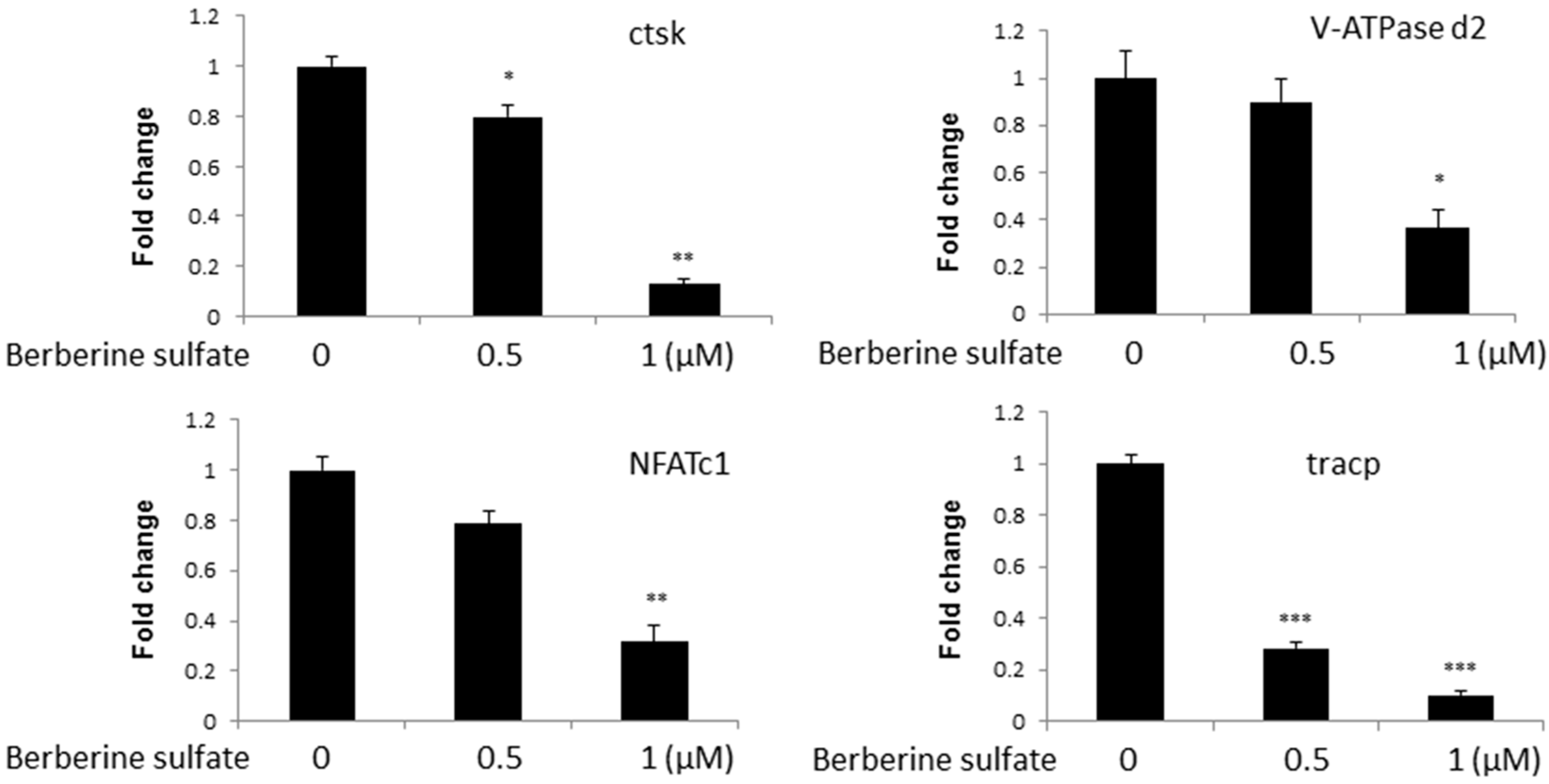
2.3. Berberine Sulfate Suppresses RANKL-Induced Osteoclast-Associated Gene Expression
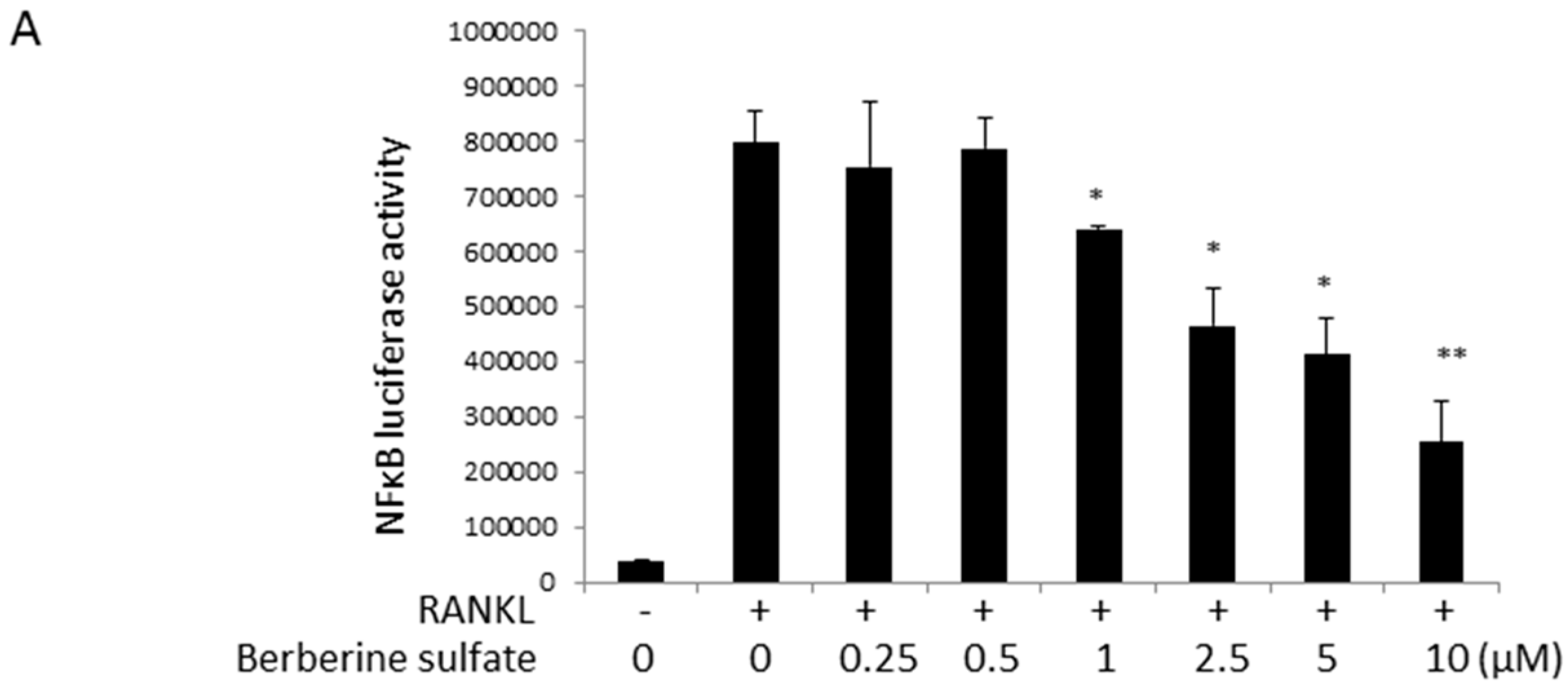
2.4. Berberine Sulfate Inhibits RANKL-Induced NF-κB and NFAT Activity

2.5. Berberine Sulfate Inhibits RANKL-Induced IKBα Protein Degradation and NFATc1 Protein Expression

3. Discussion
4. Materials and Methods
4.1. Media and Reagents
4.2. Cell Culture
4.3. In Vitro Osteoclastogenesis Assay
4.4. Cytotoxicity Assays
4.5. Hydroxyapatite Resorption Assay
4.6. RNA Isolation and Analysis
4.7. NF-κB and NFAT Luciferase Reporter Gene Assays
4.8. Western Blot Assays
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Udagawa, N.; Takahashi, N.; Akatsu, T.; Tanaka, H.; Sasaki, T.; Nishihara, T.; Koga, T.; Martin, T.J.; Suda, T. Origin of osteoclasts: Mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc. Natl. Acad. Sci. USA 1990, 87, 7260–7264. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C.; Jilka, R.L. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N. Engl. J. Med. 1995, 332, 305–311. [Google Scholar] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef]
- Hu, J.P.; Takahashi, N.; Yamada, T. Coptidis rhizoma inhibits growth and proteases of oral bacteria. Oral Dis. 2000, 6, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Anis, K.V.; Rajeshkumar, N.V.; Kuttan, R. Inhibition of chemical carcinogenesis by berberine in rats and mice. J. Pharm. Pharmacol. 2001, 53, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.M.; Kuo, H.C.; Tseng, T.H.; Liu, J.Y.; Chu, C.Y. Berberine induces apoptosis through a mitochondria/caspases pathway in human hepatoma cells. Arch. Toxicol. 2006, 80, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.L.; Chou, C.C.; Yung, B.Y. Berberine complexes with DNA in the berberine-induced apoptosis in human leukemic HL-60 cells. Cancer Lett. 1995, 93, 193–200. [Google Scholar] [CrossRef]
- Mantena, S.K.; Sharma, S.D.; Katiyar, S.K. Berberine, a natural product, induces G1-phase cell cycle arrest and caspase-3-dependent apoptosis in human prostate carcinoma cells. Mol. Cancer Ther. 2006, 5, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Jiao, L.; Qin, L.P.; Yan, F.; Han, T.; Zhang, Q.Y. Effects of berberine on differentiation and bone resorption of osteoclasts derived from rat bone marrow cells. J. Chi. Integr. Med. 2009, 7, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Miyahara, T.; Tezuka, Y.; Namba, T.; Suzuki, T.; Dowaki, R.; Watanabe, M.; Nemoto, N.; Tonami, S.; Seto, H.; et al. The effect of kampo formulae on bone resorption in vitro and in vivo. II. Detailed study of berberine. Biol. Pharm. Bull. 1999, 22, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.P.; Nishishita, K.; Sakai, E.; Yoshida, H.; Kato, Y.; Tsukuba, T.; Okamoto, K. Berberine inhibits RANKL-induced osteoclast formation and survival through suppressing the NF-κB and Akt pathways. Eur. J. Pharmacol. 2008, 580, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wu, H.; Chim, S.M.; Zhou, L.; Zhao, J.; Feng, H.; Wei, Q.; Wang, Q.; Zheng, M.H.; Tan, R.X.; et al. SC-514, a selective inhibitor of IKKβ attenuates RANKL-induced osteoclastogenesis and NF-κB activation. Biochem. Pharmacol. 2013, 86, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhou, L.; Wu, H.; Pavlos, N.; Chim, S.M.; Liu, Q.; Zhao, J.; Xue, W.; Tan, R.X.; Ye, J.; et al. Triptolide inhibits osteoclast formation, bone resorption, RANKL-mediated NF-κB activation and titanium particle-induced osteolysis in a mouse model. Mol. Cell. Endocrinol. 2015, 399, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.P.; Mahajan, S. Berberine and its derivatives: A patent review (2009–2012). Expert Opin. Ther. Pat. 2013, 23, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Baird, A.W.; Taylor, C.T.; Brayden, D.J. Non-antibiotic anti-diarrhoeal drugs: Factors affecting oral bioavailability of berberine and loperamide in intestinal tissue. Adv. Drug Deliver. Rev. 1997, 23, 111–120. [Google Scholar] [CrossRef]
- Yu, C.P.; Shia, C.S.; Tsai, S.Y.; Hou, Y.C. Pharmacokinetics and relative bioavailability of flavonoids between two dosage forms of gegen-qinlian-tang in rats. Evid. Based Complement. Alternat. Med. 2012, 2012, 308018. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Zhu, Z.; Kang, N.; Piao, S.; Qin, G.; Yao, X. Isolation and identification of urinary metabolites of berberine in rats and humans. Drug Metab. Dispos. 2008, 36, 2159–2165. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Maraskovsky, E.; Billingsley, W.L.; Dougall, W.C.; Tometsko, M.E.; Roux, E.R.; Teepe, M.C.; DuBose, R.F.; Cosman, D.; Galibert, L. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 1997, 390, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Nakagomi, D.; Suzuki, K.; Nakajima, H. Critical roles of IκB kinase subunits in mast cell degranulation. Int. Arch. Allergy Immunol. 2012, 158, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Regnier, C.H.; Song, H.Y.; Gao, X.; Goeddel, D.V.; Cao, Z.; Rothe, M. Identification and characterization of an IκB kinase. Cell 1997, 90, 373–383. [Google Scholar] [CrossRef]
- Brown, K.D.; Claudio, E.; Siebenlist, U. The roles of the classical and alternative nuclear factor-κB pathways: potential implications for autoimmunity and rheumatoid arthritis. Arthritis Res. Ther. 2008, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, F.; Manning, A.M. Multiple signals converging on NF-κB. Curr. Opin. Cell Biol. 1999, 11, 226–232. [Google Scholar] [CrossRef]
- Dixit, V.; Mak, T.W. NF-κB signaling: Many roads lead to madrid. Cell 2002, 111, 615–619. [Google Scholar] [CrossRef]
- Xu, J.; Tan, J.W.; Huang, L.; Gao, X.H.; Laird, R.; Liu, D.; Wysocki, S.; Zheng, M.H. Cloning, sequencing, and functional characterization of the rat homologue of receptor activator of NF-κB ligand. J. Bone Miner. Res. 2000, 15, 2178–2186. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Steer, J.H.; Joyce, D.A.; Yip, K.H.; Zheng, M.H.; Xu, J. 12-O-tetradecanoylphorbol-13-acetate (TPA) inhibits osteoclastogenesis by suppressing RANKL-induced NF-κB activation. J. Bone Miner. Res. 2003, 18, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Van der Kraan, A.G.; Chai, R.C.; Singh, P.P.; Lang, B.J.; Xu, J.; Gillespie, M.T.; Julian, M.W. HSP90 inhibitors enhance differentiation and MITF (microphthalmia transcription factor) activity in osteoclast progenitors. Biochem. J. 2013, 451, 235–244. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Song, F.; Liu, Q.; Yang, M.; Zhao, J.; Tan, R.; Xu, J.; Zhang, G.; Quinn, J.M.W.; Tickner, J.; et al. Berberine Sulfate Attenuates Osteoclast Differentiation through RANKL Induced NF-κB and NFAT Pathways. Int. J. Mol. Sci. 2015, 16, 27087-27096. https://doi.org/10.3390/ijms161125998
Zhou L, Song F, Liu Q, Yang M, Zhao J, Tan R, Xu J, Zhang G, Quinn JMW, Tickner J, et al. Berberine Sulfate Attenuates Osteoclast Differentiation through RANKL Induced NF-κB and NFAT Pathways. International Journal of Molecular Sciences. 2015; 16(11):27087-27096. https://doi.org/10.3390/ijms161125998
Chicago/Turabian StyleZhou, Lin, Fangming Song, Qian Liu, Mingli Yang, Jinmin Zhao, Renxiang Tan, Jun Xu, Ge Zhang, Julian M. W. Quinn, Jennifer Tickner, and et al. 2015. "Berberine Sulfate Attenuates Osteoclast Differentiation through RANKL Induced NF-κB and NFAT Pathways" International Journal of Molecular Sciences 16, no. 11: 27087-27096. https://doi.org/10.3390/ijms161125998






