The Effect of Alendronate Loaded Biphasic Calcium Phosphate Scaffolds on Bone Regeneration in a Rat Tibial Defect Model
Abstract
:1. Introduction
2. Results
2.1. Characterization of Biphasic Calcium Phosphate (BCP) and Modified BCP Scaffolds
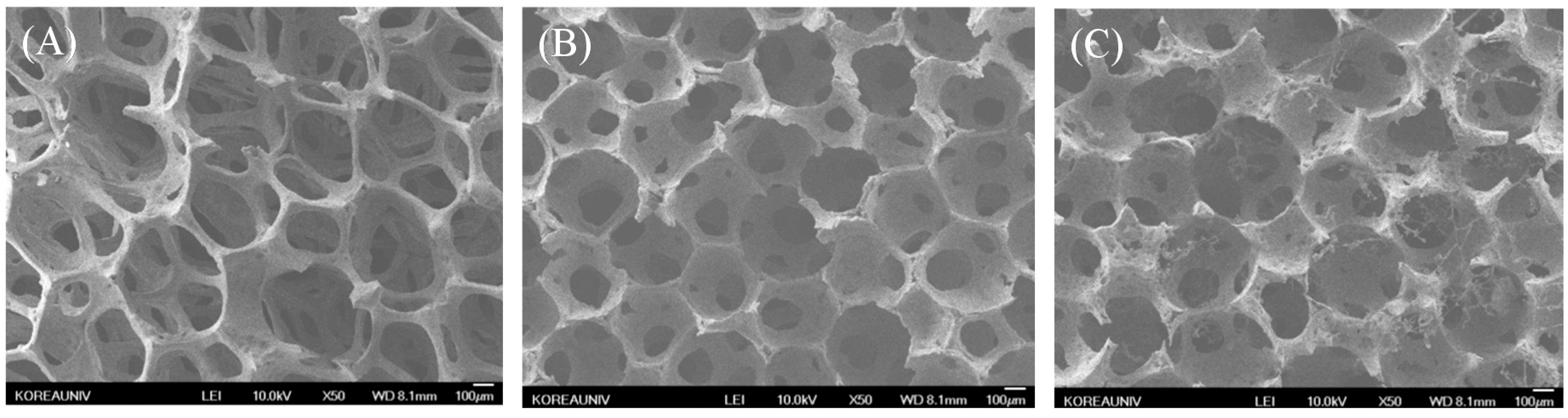

| Samples | Elements | |||||
|---|---|---|---|---|---|---|
| C (%) | O (%) | P (%) | Ca (%) | N (%) | Total (%) | |
| BCP | 22.20 | 53.19 | 12.32 | 12.29 | 0 | 100 |
| Aln (1 mg)/BCP | 9.87 | 45.66 | 11.76 | 21.68 | 11.03 | 100 |
| Aln (5 mg)/BCP | 16.55 | 38.75 | 10.36 | 22.87 | 11.47 | 100 |
| Samples | Loading Amount (µg) | Loading Efficiency (%) |
|---|---|---|
| Aln (1 mg)/BCP | 786.28 ± 6.68 | 78.63 ± 0.67 |
| Aln (5 mg)/BCP | 3638.49 ± 7.12 | 72.77 ± 0.14 |
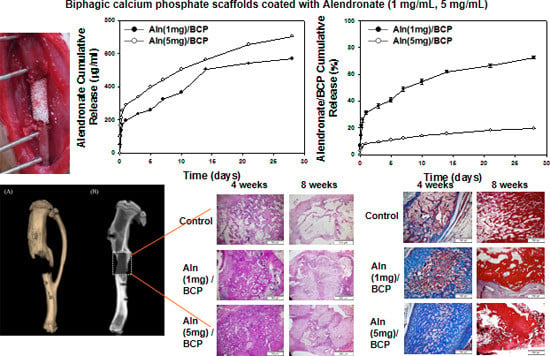
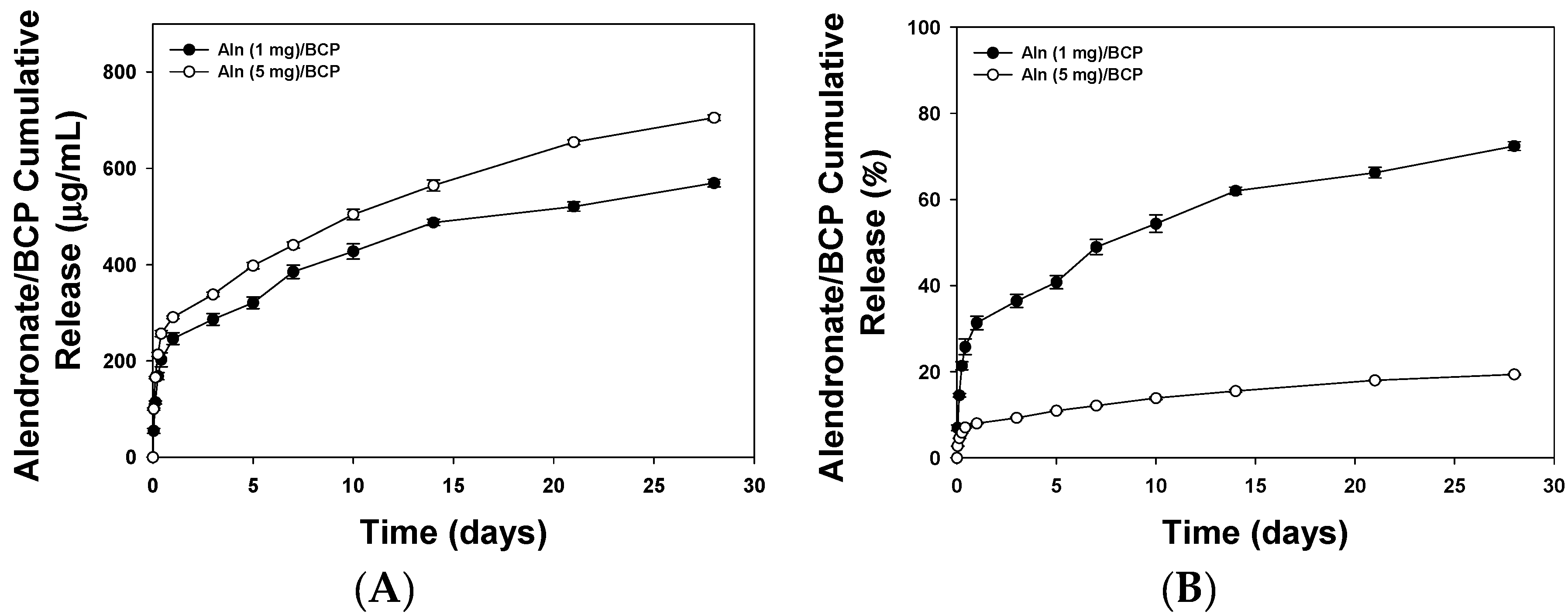
2.2. Release Kinetics of Alendronate (Aln) from BCP Scaffold
2.3. Alkaline Phosphatase (ALP) Activity and Calcium Contents


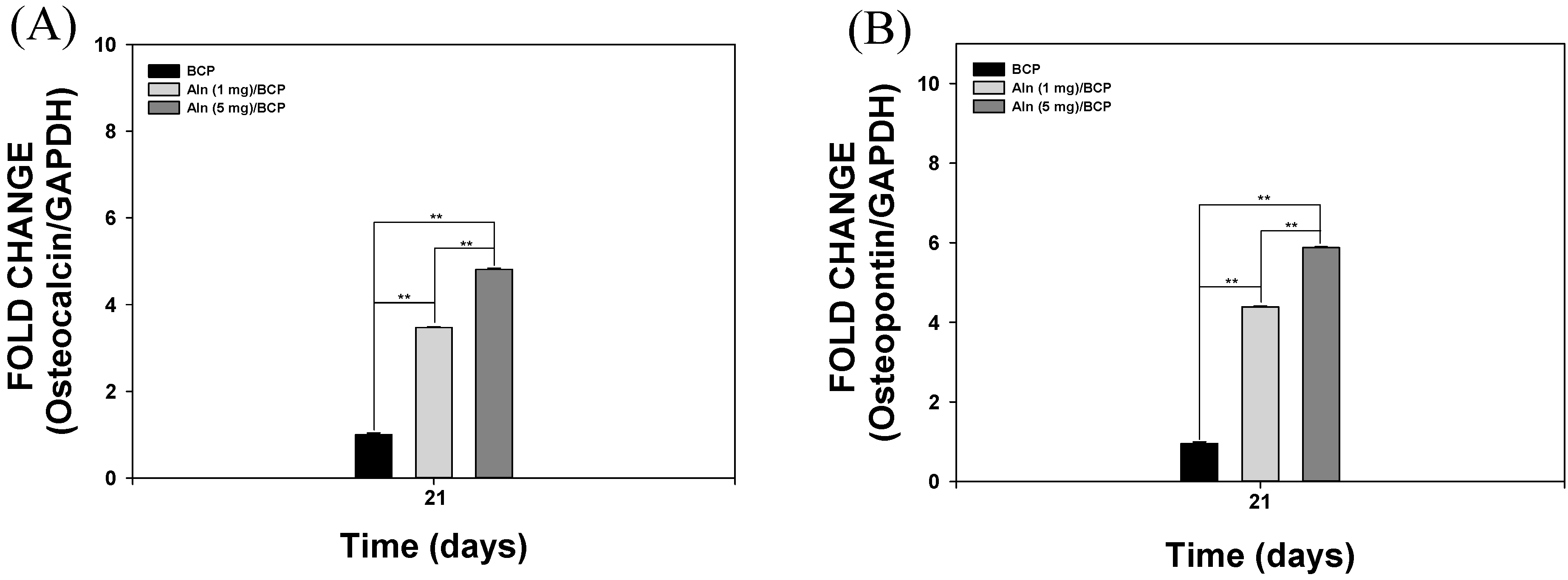
2.4. Gene Expression
2.5. Bone Formation Evaluation
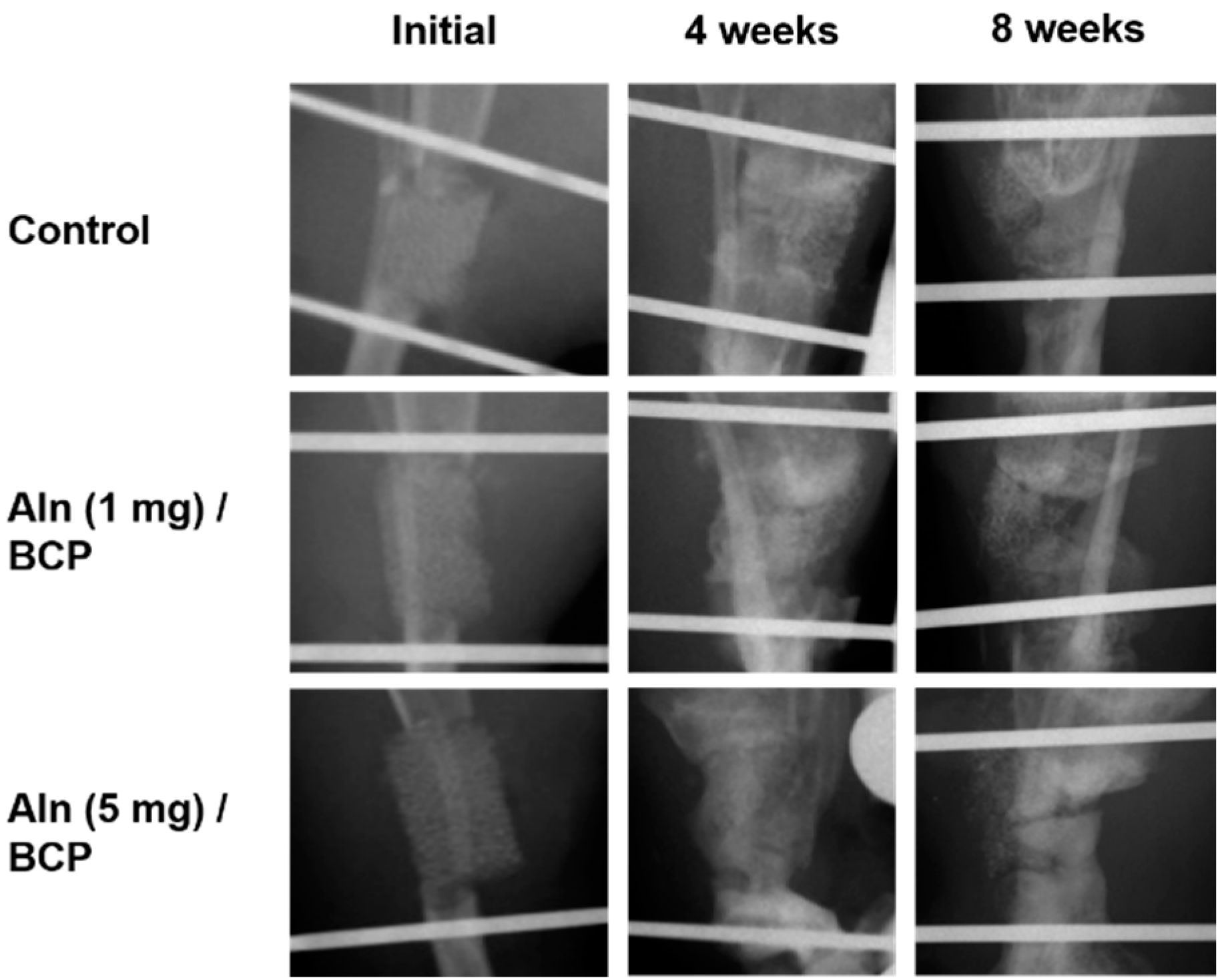
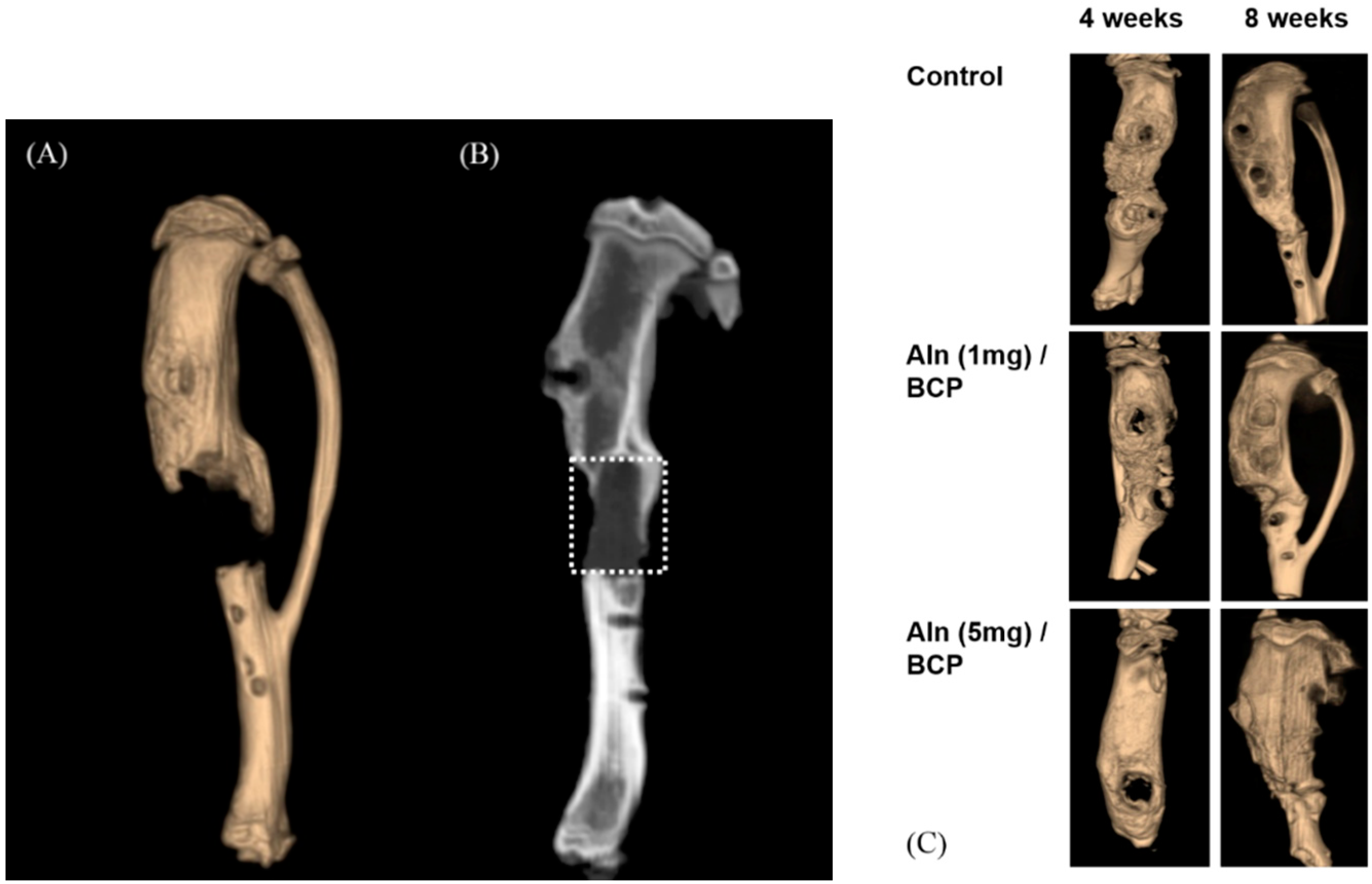
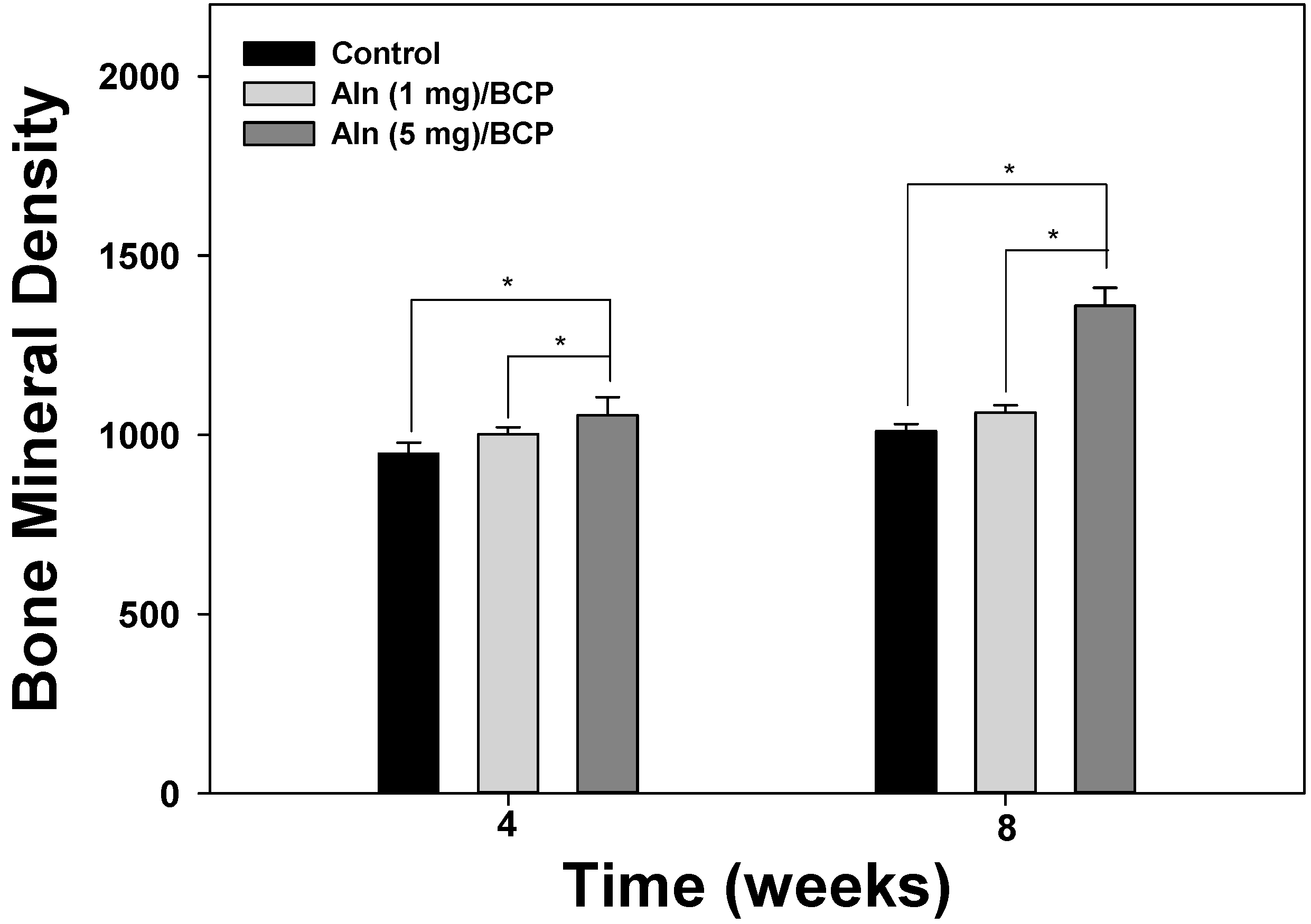
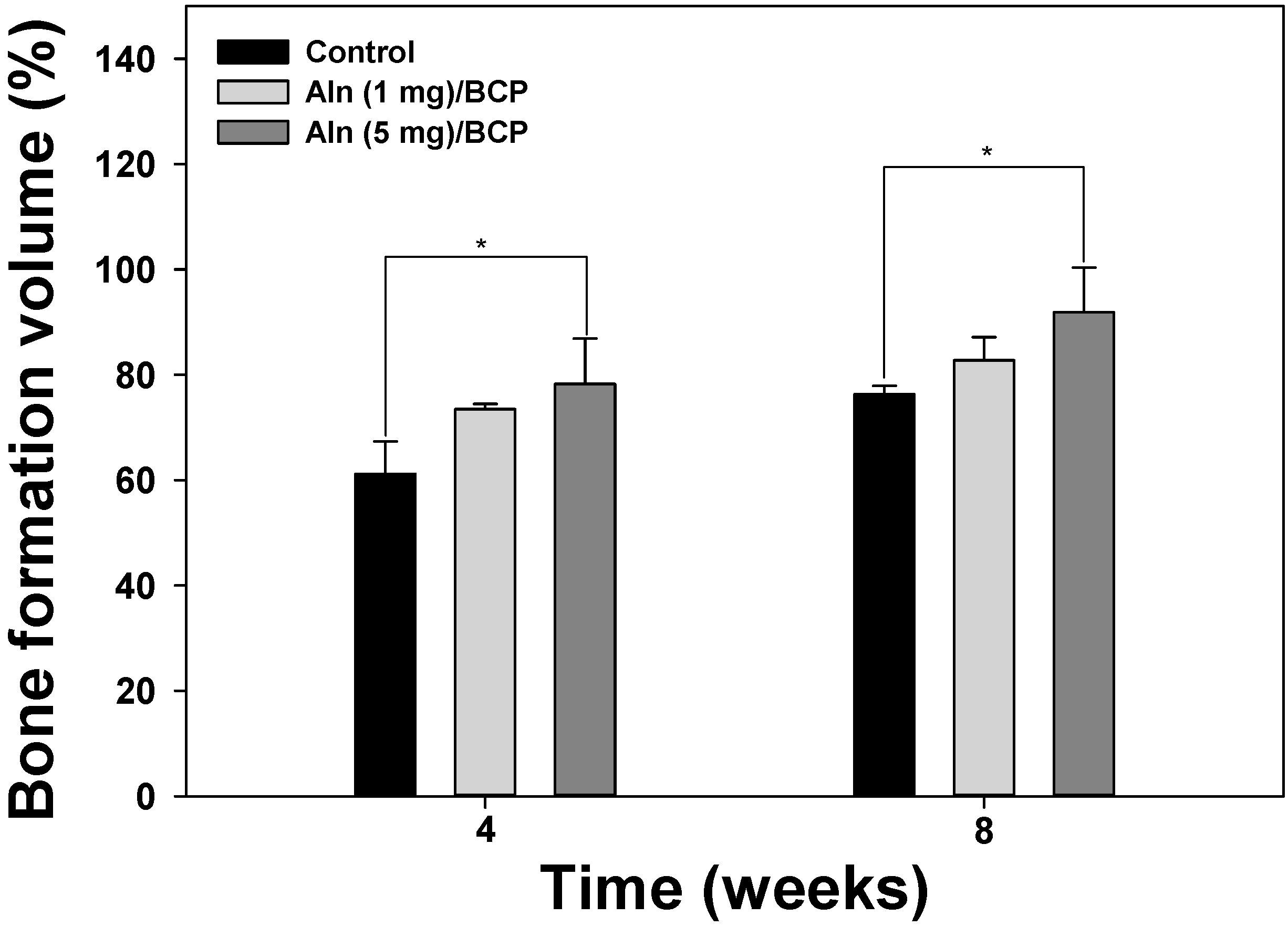
2.6. Histological Evaluation

3. Discussion
4. Experimental Section
4.1. Materials
4.2. Preparation of BCP Scaffold Coated with Alendronate (Aln)
4.3. Characterization of BCP and Aln-Coated BCP
4.4. Release Kinetics of Aln from BCP Scaffold
4.5. Osteogenic Differentiation Conditions
4.6. ALP Activity
4.7. Calcium Content
4.8. Gene Expression
4.9. Rat Tibial Defect and Treatment
- Group I: BCP only
- Group II: Aln (1 mg)/BCP
- Group III: Aln (5 mg)/BCP
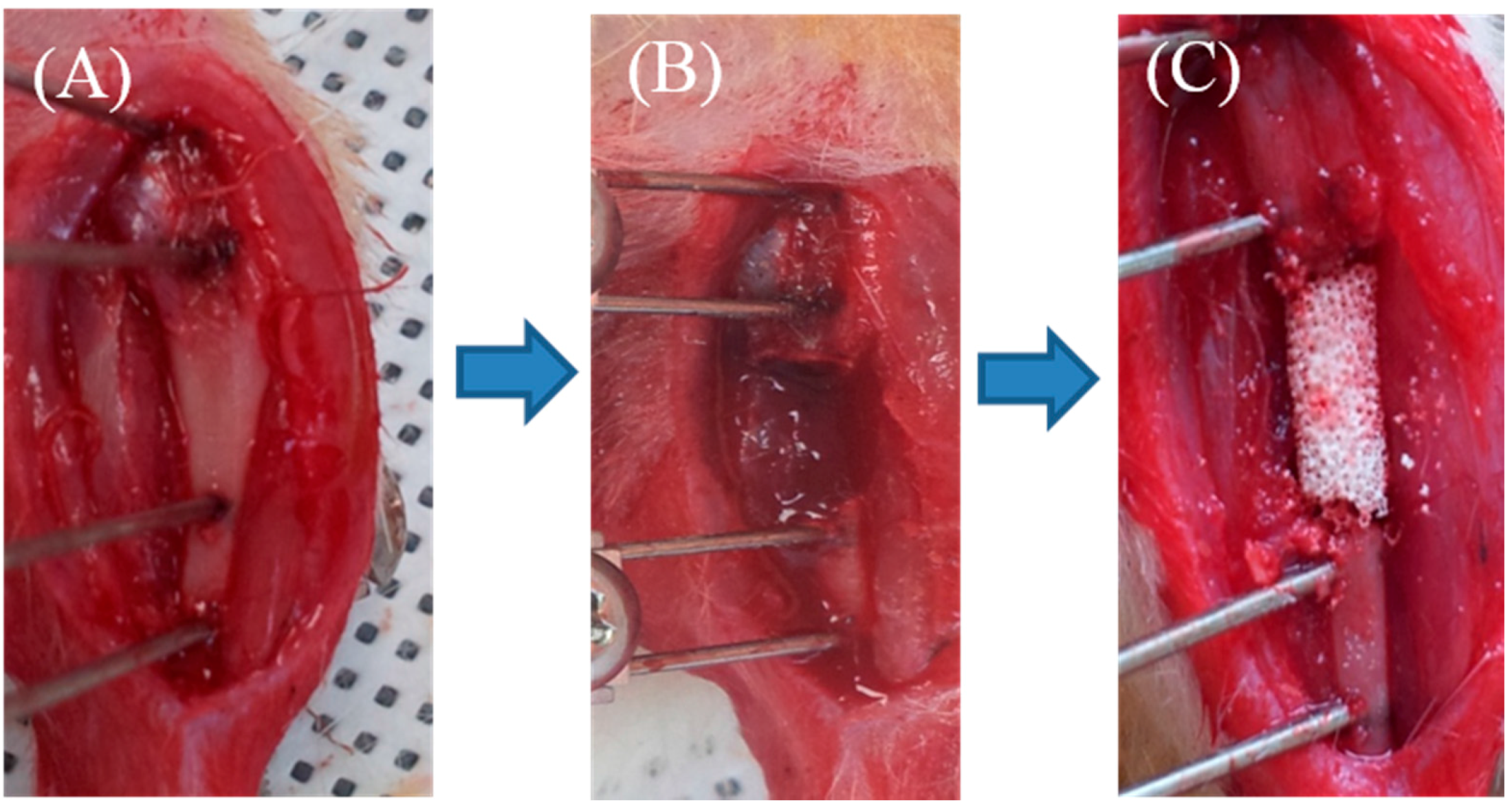
4.10. Analysis of Bone Formation
4.11. Histological Evaluation
4.12. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Long, W.G., Jr.; Einhorn, T.A.; Koval, K.; McKee, M.; Smith, W.; Sanders, R.; Watson, T. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. A critical analysis. J. Bone Jt. Surg. Am. Vol. 2007, 89, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z. Calcium phosphate-based osteoinductive materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Tsai, M.J.; Liao, H.T. Incorporation of biphasic calcium phosphate microparticles in injectable thermoresponsive hydrogel modulates bone cell proliferation and differentiation. Colloids Surf. B Biointerfaces 2013, 110, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Mercier, P.; Bellavance, F.; Cholewa, J.; Djokovic, S. Long-term stability of atrophic ridges reconstructed with hydroxylapatite: A prospective study. J. Oral Maxillofac. Surg. 1996, 54, 960–968, discussion 968–969. [Google Scholar] [CrossRef]
- Okii, N.; Nishimura, S.; Kurisu, K.; Takeshima, Y.; Uozumi, T. In vivo histological changes occurring in hydroxyapatite cranial reconstruction—Case report. Neurol. Medico-Chirur. 2001, 41, 100–104. [Google Scholar] [CrossRef]
- Fuerst, M.; Niggemeyer, O.; Lammers, L.; Schafer, F.; Lohmann, C.; Ruther, W. Articular cartilage mineralization in osteoarthritis of the hip. BMC Musculoskelet. Disord. 2009, 10, 166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiang, Q.; Dong, S.; Li, C.; Zhou, Y. Fabrication and characterization of a recombinant fibronectin/cadherin bio-inspired ceramic surface and its influence on adhesion and ossification in vitro. Acta Biomater. 2010, 6, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Roohani-Esfahani, S.I.; Nouri-Khorasani, S.; Lu, Z.; Appleyard, R.; Zreiqat, H. The influence hydroxyapatite nanoparticle shape and size on the properties of biphasic calcium phosphate scaffolds coated with hydroxyapatite-PCL composites. Biomaterials 2010, 31, 5498–5509. [Google Scholar] [CrossRef] [PubMed]
- Ralston, S.H.; Hacking, L.; Willocks, L.; Bruce, F.; Pitkeathly, D.A. Clinical, biochemical, and radiographic effects of aminohydroxypropylidene bisphosphonate treatment in rheumatoid arthritis. Ann. Rheum. Dis. 1989, 48, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Eggelmeijer, F.; Papapoulos, S.E.; van Paassen, H.C.; Dijkmans, B.A.; Breedveld, F.C. Clinical and biochemical response to single infusion of pamidronate in patients with active rheumatoid arthritis: A double blind placebo controlled study. J. Rheumatol. 1994, 21, 2016–2020. [Google Scholar] [PubMed]
- Rodan, G.A.; Martin, T.J. Therapeutic approaches to bone diseases. Science 2000, 289, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Von Knoch, F.; Jaquiery, C.; Kowalsky, M.; Schaeren, S.; Alabre, C.; Martin, I.; Rubash, H.E.; Shanbhag, A.S. Effects of bisphosphonates on proliferation and osteoblast differentiation of human bone marrow stromal cells. Biomaterials 2005, 26, 6941–6949. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Hisa, I.; Seino, S.; Kaji, H. Alendronate induces mineralization in mouse osteoblastic MC3T3-E1 cells: Regulation of mineralization-related genes. Exp. Clin. Endocrinol. Diabetes: Off. J. German Soc. Endocrinol. German Diabetes Assoc. 2010, 118, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Chen, S.M.; Chen, C.H.; Wang, C.K.; Wang, G.J.; Chang, J.K.; Ho, M.L. The effect of the local delivery of alendronate on human adipose-derived stem cell-based bone regeneration. Biomaterials 2010, 31, 8674–8683. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Yun, Y.P.; Han, C.W.; Kim, M.S.; Kim, S.E.; Bae, M.S.; Kim, G.T.; Choi, Y.S.; Hwang, E.H.; Lee, J.W.; et al. Effect of heparin and alendronate coating on titanium surfaces on inhibition of osteoclast and enhancement of osteoblast function. Biochem. Biophys. Res. Commun. 2011, 413, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Yun, Y.P.; Lee, H.J.; Hwang, Y.S.; Kwon, I.K.; Lee, S.C. In situ fabrication of alendronate-loaded calcium phosphate microspheres: Controlled release for inhibition of osteoclastogenesis. J. Control. Release: Off. J. Control. Release Soc. 2010, 147, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Suh, D.H.; Yun, Y.P.; Lee, J.Y.; Park, K.; Chung, J.Y.; Lee, D.W. Local delivery of alendronate eluting chitosan scaffold can effectively increase osteoblast functions and inhibit osteoclast differentiation. J. Mater. Sci. Mater. Med. 2012, 23, 2739–2749. [Google Scholar] [CrossRef] [PubMed]
- Peter, B.; Pioletti, D.P.; Laib, S.; Bujoli, B.; Pilet, P.; Janvier, P.; Guicheux, J.; Zambelli, P.Y.; Bouler, J.M.; Gauthier, O. Calcium phosphate drug delivery system: Influence of local zoledronate release on bone implant osteointegration. Bone 2005, 36, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Faucheux, C.; Verron, E.; Soueidan, A.; Josse, S.; Arshad, M.D.; Janvier, P.; Pilet, P.; Bouler, J.M.; Bujoli, B.; Guicheux, J. Controlled release of bisphosphonate from a calcium phosphate biomaterial inhibits osteoclastic resorption in vitro. J. Biomed. Mater. Res. A 2009, 89, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Josse, S.; Faucheux, C.; Soueidan, A.; Grimandi, G.; Massiot, D.; Alonso, B.; Janvier, P.; Laib, S.; Pilet, P.; Gauthier, O.; et al. Novel biomaterials for bisphosphonate delivery. Biomaterials 2005, 26, 2073–2080. [Google Scholar] [CrossRef] [PubMed]
- Bucholz, R.W. Nonallograft osteoconductive bone graft substitutes. Clin. Orthop. Related Res. 2002, 395, 44–52. [Google Scholar] [CrossRef]
- Chen, J.; Luo, Y.; Hong, L.; Ling, Y.; Pang, J.; Fang, Y.; Wei, K.; Gao, X. Synthesis, characterization and osteoconductivity properties of bone fillers based on alendronate-loaded poly(epsilon-caprolactone)/hydroxyapatite microspheres. J. Mater. Sci. Mater. Med. 2011, 22, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Boanini, E.; Torricelli, P.; Gazzano, M.; Fini, M.; Bigi, A. The effect of alendronate doped calcium phosphates on bone cells activity. Bone 2012, 51, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Im, G.I.; Qureshi, S.A.; Kenney, J.; Rubash, H.E.; Shanbhag, A.S. Osteoblast proliferation and maturation by bisphosphonates. Biomaterials 2004, 25, 4105–4115. [Google Scholar] [CrossRef] [PubMed]
- Duque, G.; Vidal, C.; Rivas, D. Protein isoprenylation regulates osteogenic differentiation of mesenchymal stem cells: Effect of alendronate, and farnesyl and geranylgeranyl transferase inhibitors. Br. J. Pharmacol. 2011, 162, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, K.; Shimada, A.; Shibata, T.; Wada, S.; Ideno, H.; Nakashima, K.; Amizuka, N.; Noda, M.; Nifuji, A. Alendronate promotes bone formation by inhibiting protein prenylation in osteoblasts in rat tooth replantation model. J. Endocrinol. 2013, 219, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef] [PubMed]
- Jongpaiboonkit, L.; Franklin-Ford, T.; Murphy, W.L. Growth of hydroxyapatite coatings on biodegradable polymer microspheres. ACS Appl. Mater. Interfaces 2009, 1, 1504–1511. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Okazaki, M.; Inoue, M.; Yamaguchi, S.; Kusunose, T.; Toyonaga, T.; Hamada, Y.; Takahashi, J. Hydroxyapatite particles as a controlled release carrier of protein. Biomaterials 2004, 25, 3807–3812. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Yun, Y.P.; Park, K.; Kim, S.E. Gentamicin and bone morphogenic protein-2 (BMP-2)-delivering heparinized-titanium implant with enhanced antibacterial activity and osteointegration. Bone 2012, 50, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, X.; Ren, L.; Yao, Y.; Wang, D.A. In vitro osteogenesis of synovium mesenchymal cells induced by controlled release of alendronate and dexamethasone from a sintered microspherical scaffold. J. Biomater. Sci. Polym. Ed. 2010, 21, 1227–1238. [Google Scholar] [CrossRef] [PubMed]
- Diefenderfer, D.L.; Osyczka, A.M.; Garino, J.P.; Leboy, P.S. Regulation of BMP-induced transcription in cultured human bone marrow stromal cells. J. Bone Jt. Surg. Am. Vol. 2003, 85 (Suppl. 3), 19–28. [Google Scholar]
- Lecanda, F.; Avioli, L.V.; Cheng, S.L. Regulation of bone matrix protein expression and induction of differentiation of human osteoblasts and human bone marrow stromal cells by bone morphogenetic protein-2. J. Cell. Biochem. 1997, 67, 386–396. [Google Scholar] [CrossRef]
- Mashiba, T.; Hirano, T.; Turner, C.H.; Forwood, M.R.; Johnston, C.C.; Burr, D.B. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J. Bone Miner. Res. 2000, 15, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Sama, A.A.; Khan, S.N.; Myers, E.R.; Huang, R.C.; Cammisa, F.P., Jr.; Sandhu, H.S.; Lane, J.M. High-dose alendronate uncouples osteoclast and osteoblast function: A study in a rat spine pseudarthrosis model. Clin. Orthop. Related Res. 2004, 425, 135–142. [Google Scholar] [CrossRef]
- Odvina, C.V.; Zerwekh, J.E.; Rao, D.S.; Maalouf, N.; Gottschalk, F.A.; Pak, C.Y. Severely suppressed bone turnover: A potential complication of alendronate therapy. J. Clin. Endocrinol. Metab. 2005, 90, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Iwata, K.; Li, J.; Follet, H.; Phipps, R.J.; Burr, D.B. Bisphosphonates suppress periosteal osteoblast activity independently of resorption in rat femur and tibia. Bone 2006, 39, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Ozalay, M.; Sahin, O.; Akpinar, S.; Ozkoc, G.; Cinar, M.; Cesur, N. Remodeling potentials of biphasic calcium phosphate granules in open wedge high tibial osteotomy. Arch. Orthop. Trauma Surg. 2009, 129, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Rouvillain, J.L.; Lavalle, F.; Pascal-Mousselard, H.; Catonne, Y.; Daculsi, G. Clinical, radiological and histological evaluation of biphasic calcium phosphate bioceramic wedges filling medial high tibial valgisation osteotomies. Knee 2009, 16, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Yun, Y.P.; Lee, D.W.; Kang, E.Y.; Jeong, W.J.; Lee, B.; Jeong, M.S.; Kim, H.J.; Park, K.; Song, H.R. Alendronate-eluting biphasic calcium phosphate (BCP) scaffolds stimulate osteogenic differentiation. Biomed. Res. Int. 2015, 2015, 320713. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.-W.; Yun, Y.-P.; Kim, S.E.; Song, H.-R. The Effect of Alendronate Loaded Biphasic Calcium Phosphate Scaffolds on Bone Regeneration in a Rat Tibial Defect Model. Int. J. Mol. Sci. 2015, 16, 26738-26753. https://doi.org/10.3390/ijms161125982
Park K-W, Yun Y-P, Kim SE, Song H-R. The Effect of Alendronate Loaded Biphasic Calcium Phosphate Scaffolds on Bone Regeneration in a Rat Tibial Defect Model. International Journal of Molecular Sciences. 2015; 16(11):26738-26753. https://doi.org/10.3390/ijms161125982
Chicago/Turabian StylePark, Kwang-Won, Young-Pil Yun, Sung Eun Kim, and Hae-Ryong Song. 2015. "The Effect of Alendronate Loaded Biphasic Calcium Phosphate Scaffolds on Bone Regeneration in a Rat Tibial Defect Model" International Journal of Molecular Sciences 16, no. 11: 26738-26753. https://doi.org/10.3390/ijms161125982