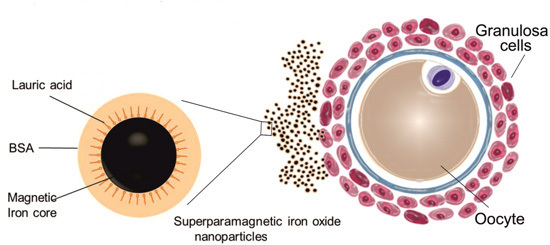
Genotoxicity of Superparamagnetic Iron Oxide Nanoparticles in Granulosa Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Uptake of Iron Oxide Nanoparticles by Granulosa Cells
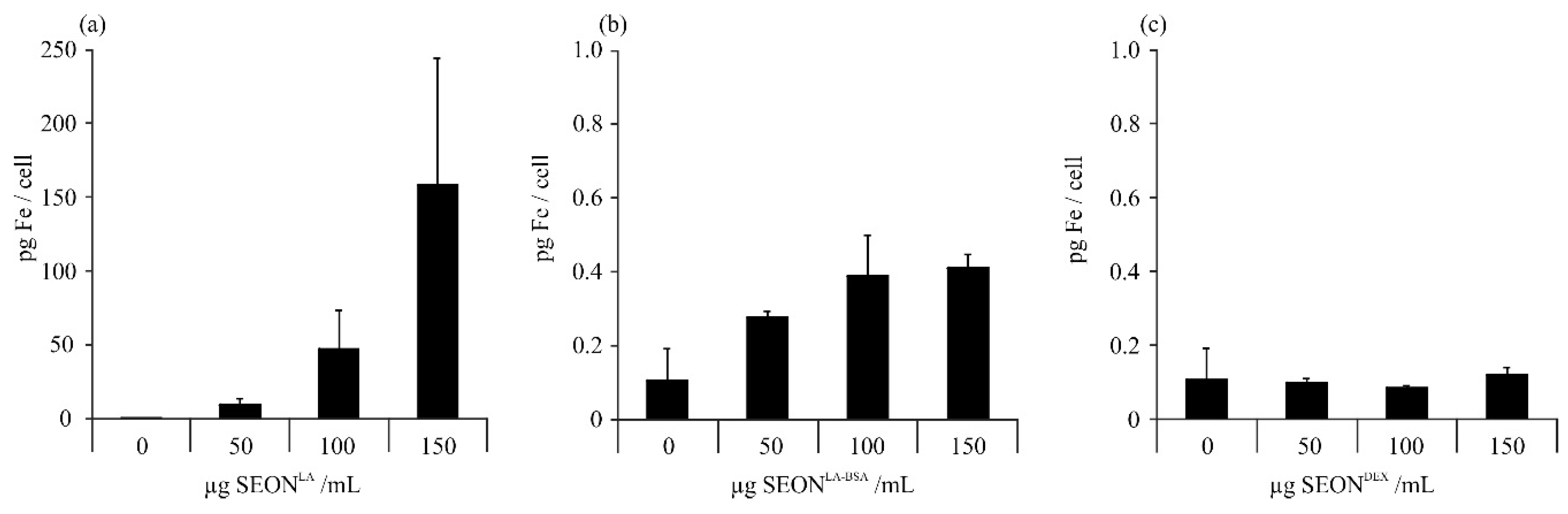
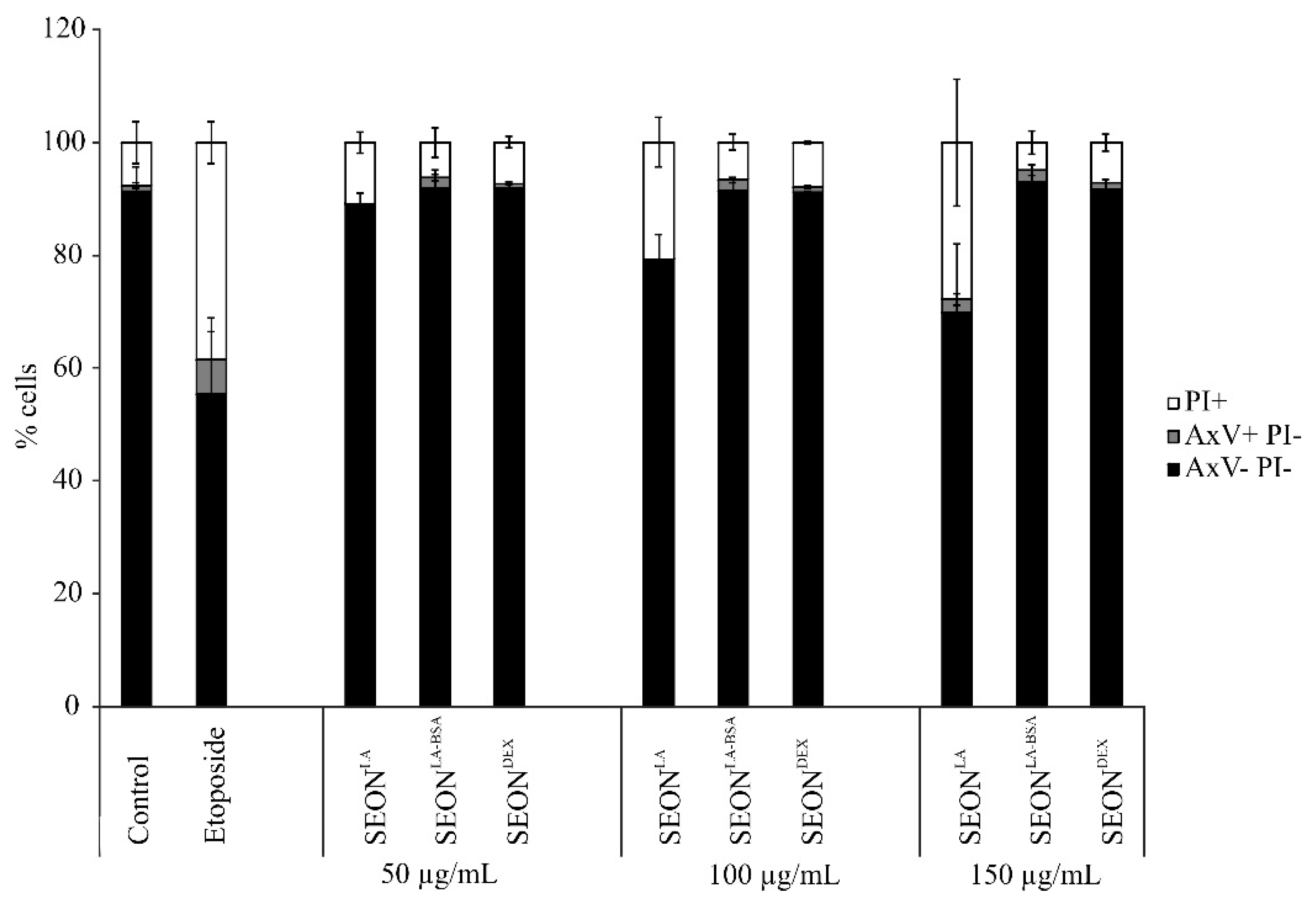
2.2. Viability of Granulosa Cells after Incubation with SPION
2.3. Micronuclei Formation in Granulosa Cells after Incubation with SPION


2.4. DNA Damage in Granulosa Cells after Incubation with SPION

3. Experimental Section
3.1. Nanoparticles
| Physico-Chemical Properties of Nanoparticles | SEONLA | SEONLA-BSA | SEONDEX |
|---|---|---|---|
| Core diameter (TEM) (nm) in H2O | 7.64 ± 1.6 | 7.64 ± 1.6 | 4.3 ± 0.9 |
| Hydrodynamic diameter (DLS) (nm) in RPMI | 46.92 ± 0.1 | 61.7 ± 1.1 | 79 ± 1.3 |
| ζ potential (mV) in RPMI | −15.5 ± 0.8 | −12.9 ± 0.55 | −2.0 ± 0.6 |
| Polydispersity index in RPMI | 0.331 ± 0.019 | 0.346 ± 0.028 | 0.304 ± 0.031 |
3.2. Cell Culture
3.3. Live/Dead Staining Using Flow Cytometry
3.4. Micronuclei Test
3.5. DNA Damage Detection
3.6. Flow Cytometry
3.7. Microwave Plasma–Atomic Emission Spectrometer (MP-AES)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jin, R.; Lin, B.; Li, D.; Ai, H. Superparamagnetic iron oxide nanoparticles for MR imaging and therapy: Design considerations and clinical applications. Curr. Opin. Pharmacol. 2014, 18, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Oliveira, T.R.; Mamani, J.B.; Malheiros, S.M.; Malavolta, L.; Pavon, L.F.; Sibov, T.T.; Amaro, E., Jr.; Tannus, A.; Vidoto, E.L.; et al. Application of hyperthermia induced by superparamagnetic iron oxide nanoparticles in glioma treatment. Int. J. Nanomed. 2011, 6, 591–603. [Google Scholar]
- Alexiou, C.; Tietze, R.; Schreiber, E.; Jurgons, R.; Richter, H.; Trahms, L.; Rahn, H.; Odenbach, S.; Lyer, S. Cancer therapy with drug loaded magnetic nanoparticles—Magnetic drug targeting. J. Magn. Magn. Mater. 2011, 323, 1404–1407. [Google Scholar] [CrossRef]
- Tietze, R.; Lyer, S.; Durr, S.; Struffert, T.; Engelhorn, T.; Schwarz, M.; Eckert, E.; Goen, T.; Vasylyev, S.; Peukert, W.; et al. Efficient drug-delivery using magnetic nanoparticles—Biodistribution and therapeutic effects in tumour bearing rabbits. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Cicha, I.; Lyer, S.; Alexiou, C.; Garlichs, C.D. Nanomedicine in diagnostics and therapy of cardiovascular diseases: Beyond atherosclerotic plaque imaging. Nanotechnol. Rev. 2013, 2, 449–472. [Google Scholar] [CrossRef]
- Lee, E.A.; Yim, H.; Heo, J.; Kim, H.; Jung, G.; Hwang, N.S. Application of magnetic nanoparticle for controlled tissue assembly and tissue engineering. Arch. Pharmacal. Res. 2014, 37, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Tietze, R.; Lyer, S.; Durr, S.; Alexiou, C. Nanoparticles for cancer therapy using magnetic forces. Nanomedicine 2012, 7, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Li, H.; Luo, Z.; Kong, J.; Wan, Y.; Zheng, L.; Zhang, Q.; Niu, H.; Vermorken, A.; van de Ven, W.; et al. Dextran-coated superparamagnetic nanoparticles as potential cancer drug carriers in vivo. Nanoscale 2015, 7, 11155–11162. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Hussain, S.M.; Krestin, G.P. Superparamagnetic iron oxide contrast agents: Physicochemical characteristics and applications in MR imaging. Eur. Radiol. 2001, 11, 2319–2331. [Google Scholar] [CrossRef] [PubMed]
- Zaloga, J.; Janko, C.; Nowak, J.; Matuszak, J.; Knaup, S.; Eberbeck, D.; Tietze, R.; Unterweger, H.; Friedrich, R.P.; Duerr, S.; et al. Development of a lauric acid/albumin hybrid iron oxide nanoparticle system with improved biocompatibility. Int. J. Nanomed. 2014, 9, 4847–4866. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Ji, Z.X.; Xia, T.; Meng, H.; Low-Kam, C.; Liu, R.; Pokhrel, S.; Lin, S.J.; Wang, X.; Liao, Y.P.; et al. Use of Metal Oxide Nanoparticle Band Gap To Develop a Predictive Paradigm for Oxidative Stress and Acute Pulmonary Inflammation. Acs Nano 2012, 6, 4349–4368. [Google Scholar] [CrossRef] [PubMed]
- Emerit, J.; Beaumont, C.; Trivin, F. Iron metabolism, free radicals, and oxidative injury. Biomed. Pharmacother. 2001, 55, 333–339. [Google Scholar] [CrossRef]
- Voinov, M.A.; Sosa Pagan, J.O.; Morrison, E.; Smirnova, T.I.; Smirnov, A.I. Surface-mediated production of hydroxyl radicals as a mechanism of iron oxide nanoparticle biotoxicity. J. Am. Chem. Soc. 2011, 133, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Kryston, T.B.; Georgiev, A.B.; Pissis, P.; Georgakilas, A.G. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat. Res. 2011, 711, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Di Bona, K.R.; Xu, Y.; Ramirez, P.A.; DeLaine, J.; Parker, C.; Bao, Y.; Rasco, J.F. Surface charge and dosage dependent potential developmental toxicity and biodistribution of iron oxide nanoparticles in pregnant CD-1 mice. Reprod. Toxicol. 2014, 50, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Bica, D.; Vekas, L.; Avdeev, M.V.; Marinica, O.; Socoliuc, V.; Balasoiu, M.; Garamus, V.M. Sterically stabilized water based magnetic fluids: Synthesis, structure and properties. J. Magn. Magn. Mater. 2007, 311, 17–21. [Google Scholar] [CrossRef]
- Unterweger, H.; Tietze, R.; Janko, C.; Zaloga, J.; Lyer, S.; Durr, S.; Taccardi, N.; Goudouri, O.M.; Hoppe, A.; Eberbeck, D.; et al. Development and characterization of magnetic iron oxide nanoparticles with a cisplatin-bearing polymer coating for targeted drug delivery. Int. J. Nanomed. 2014, 9, 3659–3676. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, R.P.; Janko, C.; Poettler, M.; Tripal, P.; Zaloga, J.; Cicha, I.; Durr, S.; Nowak, J.; Odenbach, S.; Slabu, I.; et al. Flow cytometry for intracellular SPION quantification: Specificity and sensitivity in comparison with spectroscopic methods. Int. J. Nanomed. 2015, 10, 4185–4201. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Yang, H.; Zhang, S.; Yang, Y.; Zhang, D.; Qi, Y.; Zou, L. Superparamagnetic core/shell GoldMag nanoparticles: Size-, concentration- and time-dependent cellular nanotoxicity on human umbilical vein endothelial cells and the suitable conditions for magnetic resonance imaging. J. Nanobiotechnol. 2015, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Gaihre, B.; Hee Lee, Y.; Khil, M.S.; Yi, H.K.; Kim, H.Y. In-vitro cytotoxicity and cell uptake study of gelatin-coated magnetic iron oxide nanoparticles. J. Microencapsul. 2011, 28, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, L.; Christner, C.; Storck, W.; Schick, I.; Krumbein, I.; Dahring, H.; Haedicke, K.; Heinz-Herrmann, K.; Teichgraber, U.; Reichenbach, J.R.; et al. A plasma protein corona enhances the biocompatibility of Au@Fe3O4 Janus particles. Biomaterials 2015, 68, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Munoz, L.E.; Maueroder, C.; Chaurio, R.; Berens, C.; Herrmann, M.; Janko, C. Colourful death: Six-parameter classification of cell death by flow cytometry—Dead cells tell tales. Autoimmunity 2013, 46, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Makhluf, S.B.D.; Qasem, R.; Rubinstein, S.; Gedanken, A.; Breitbart, H. Loading magnetic nanoparticles into sperm cells does not affect their functionality. Langmuir ACS J. Surf. Colloids 2006, 22, 9480–9482. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, R.C.; Osman, I.F.; Amani, A.; de Matas, M.; Anderson, D. The effect of zinc oxide and titanium dioxide nanoparticles in the Comet assay with UVA photoactivation of human sperm and lymphocytes. Nanotoxicology 2009, 3, 33–39. [Google Scholar] [CrossRef]
- Hsieh, M.S.; Shiao, N.H.; Chan, W.H. Cytotoxic effects of CdSe quantum dots on maturation of mouse oocytes, fertilization, and fetal development. Int. J. Mol. Sci. 2009, 10, 2122–2135. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Bhatia, A.; Dey, P. Spontaneously occurring micronuclei in infiltrating ductal carcinoma of breast: A potential biomarker for aggressive phenotype detection? Diagn. Cytopathol. 2013, 41, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Rao, N.N.; Nair, N.S. Micronuclei in oral squamous cell carcinoma. A marker of genotoxic damage. Indian J. Dent. Res. 2000, 11, 101–106. [Google Scholar] [PubMed]
- Geiser, M.; Rothen-Rutishauser, B.; Kapp, N.; Schurch, S.; Kreyling, W.; Schulz, H.; Semmler, M.; Im Hof, V.; Heyder, J.; Gehr, P. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ. Health Perspect. 2005, 113, 1555–1560. [Google Scholar] [CrossRef] [PubMed]
- Schonn, I.; Hennesen, J.; Dartsch, D.C. Cellular responses to etoposide: Cell death despite cell cycle arrest and repair of DNA damage. Apoptosis 2010, 15, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Huang, X.; Halicka, H.D.; Zhao, H.; Traganos, F.; Albino, A.P.; Dai, W.; Darzynkiewicz, Z. Cytometry of ATM activation and histone H2AX phosphorylation to estimate extent of DNA damage induced by exogenous agents. Cytom. A: J. Int. Soc. Anal. Cytol. 2007, 71, 648–661. [Google Scholar] [CrossRef] [PubMed]
- Wan, R.; Mo, Y.; Feng, L.; Chien, S.; Tollerud, D.J.; Zhang, Q. DNA damage caused by metal nanoparticles: Involvement of oxidative stress and activation of ATM. Chem. Res. Toxicol. 2012, 25, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Zaloga, J.; Stapf, M.; Nowak, J.; Pottler, M.; Friedrich, R.P.; Tietze, R.; Lyer, S.; Lee, G.; Odenbach, S.; Hilger, I.; et al. Tangential flow ultrafiltration allows purification and concentration of lauric acid-/albumin-coated particles for improved magnetic treatment. Int. J. Mol. Sci. 2015, 16, 19291–19307. [Google Scholar] [CrossRef] [PubMed]
- Pavlik, R.; Wypior, G.; Hecht, S.; Papadopoulos, P.; Kupka, M.; Thaler, C.; Wiest, I.; Pestka, A.; Friese, K.; Jeschke, U. Induction of G protein-coupled estrogen receptor (GPER) and nuclear steroid hormone receptors by gonadotropins in human granulosa cells. Histochem. Cell Biol. 2011, 136, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Tolbert, P.E.; Shy, C.M.; Allen, J.W. Micronuclei and other nuclear anomalies in buccal smears—A field-test in snuff users. Am. J. Epidemiol. 1991, 134, 840–850. [Google Scholar] [PubMed]
- Bryce, S.; Avlasevich, S.; Raja, S.; Torous, D.; Bemis, J.; Dertinger, S. Flow Cytometric in vitro Micronucleus Scoring Provides Simultaneous Mode of Action Information. Environ. Mol. Mutagen. 2009, 50, 582. [Google Scholar]
- Santos, M.; Zhang, W.; Rollins, L.; Hsu, M. A Quantitative Approach for Interrogation of H2A.X and ATM DNA Damage Signaling Using Rapid Benchtop Flow Cytometry; Merck Millipore Application Note, Biocompare: South San Francisco, CA, USA, 2014. [Google Scholar]
- Forte, G.; Alimonti, A.; Violante, N.; di Gregorio, M.; Senofonte, O.; Petrucci, F.; Sancesario, G.; Bocca, B. Calcium, copper, iron, magnesium, silicon and zinc content of hair in Parkinson’s disease. J. Trace Elem. Med. Biol. 2005, 19, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Wick, P.; Malek, A.; Manser, P.; Meili, D.; Maeder-Althaus, X.; Diener, L.; Diener, P.A.; Zisch, A.; Krug, H.F.; von Mandach, U. Barrier capacity of human placenta for nanosized materials. Environ. Health Perspect. 2010, 118, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Myllynen, P.K.; Loughran, M.J.; Howard, C.V.; Sormunen, R.; Walsh, A.A.; Vahakangas, K.H. Kinetics of gold nanoparticles in the human placenta. Reprod. Toxicol. 2008, 26, 130–137. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative by Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pöttler, M.; Staicu, A.; Zaloga, J.; Unterweger, H.; Weigel, B.; Schreiber, E.; Hofmann, S.; Wiest, I.; Jeschke, U.; Alexiou, C.; et al. Genotoxicity of Superparamagnetic Iron Oxide Nanoparticles in Granulosa Cells. Int. J. Mol. Sci. 2015, 16, 26280-26290. https://doi.org/10.3390/ijms161125960
Pöttler M, Staicu A, Zaloga J, Unterweger H, Weigel B, Schreiber E, Hofmann S, Wiest I, Jeschke U, Alexiou C, et al. Genotoxicity of Superparamagnetic Iron Oxide Nanoparticles in Granulosa Cells. International Journal of Molecular Sciences. 2015; 16(11):26280-26290. https://doi.org/10.3390/ijms161125960
Chicago/Turabian StylePöttler, Marina, Andreas Staicu, Jan Zaloga, Harald Unterweger, Bianca Weigel, Eveline Schreiber, Simone Hofmann, Irmi Wiest, Udo Jeschke, Christoph Alexiou, and et al. 2015. "Genotoxicity of Superparamagnetic Iron Oxide Nanoparticles in Granulosa Cells" International Journal of Molecular Sciences 16, no. 11: 26280-26290. https://doi.org/10.3390/ijms161125960