The Role of Oxidative Stress and Antioxidants in Liver Diseases
Abstract
:1. Introduction
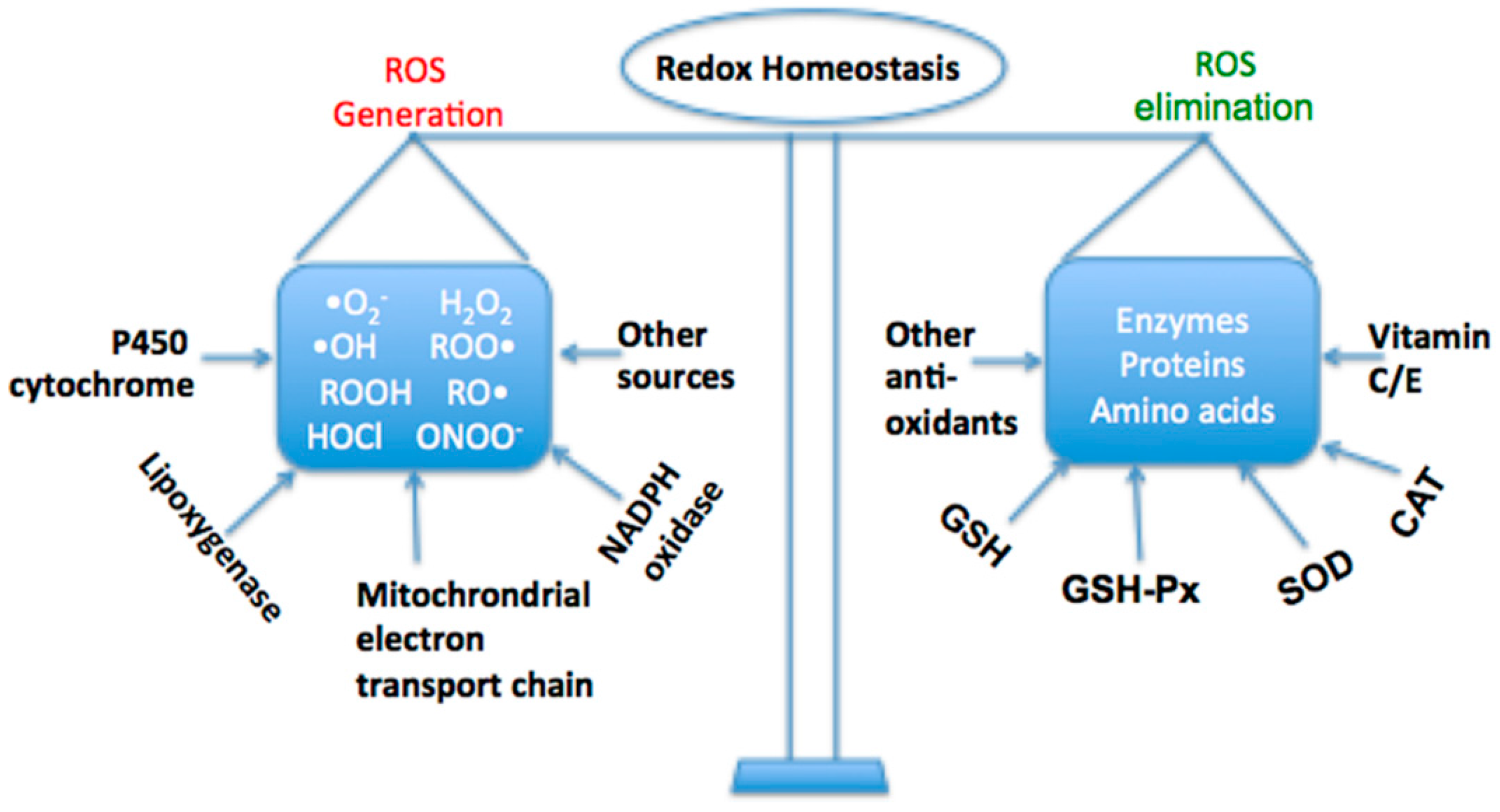
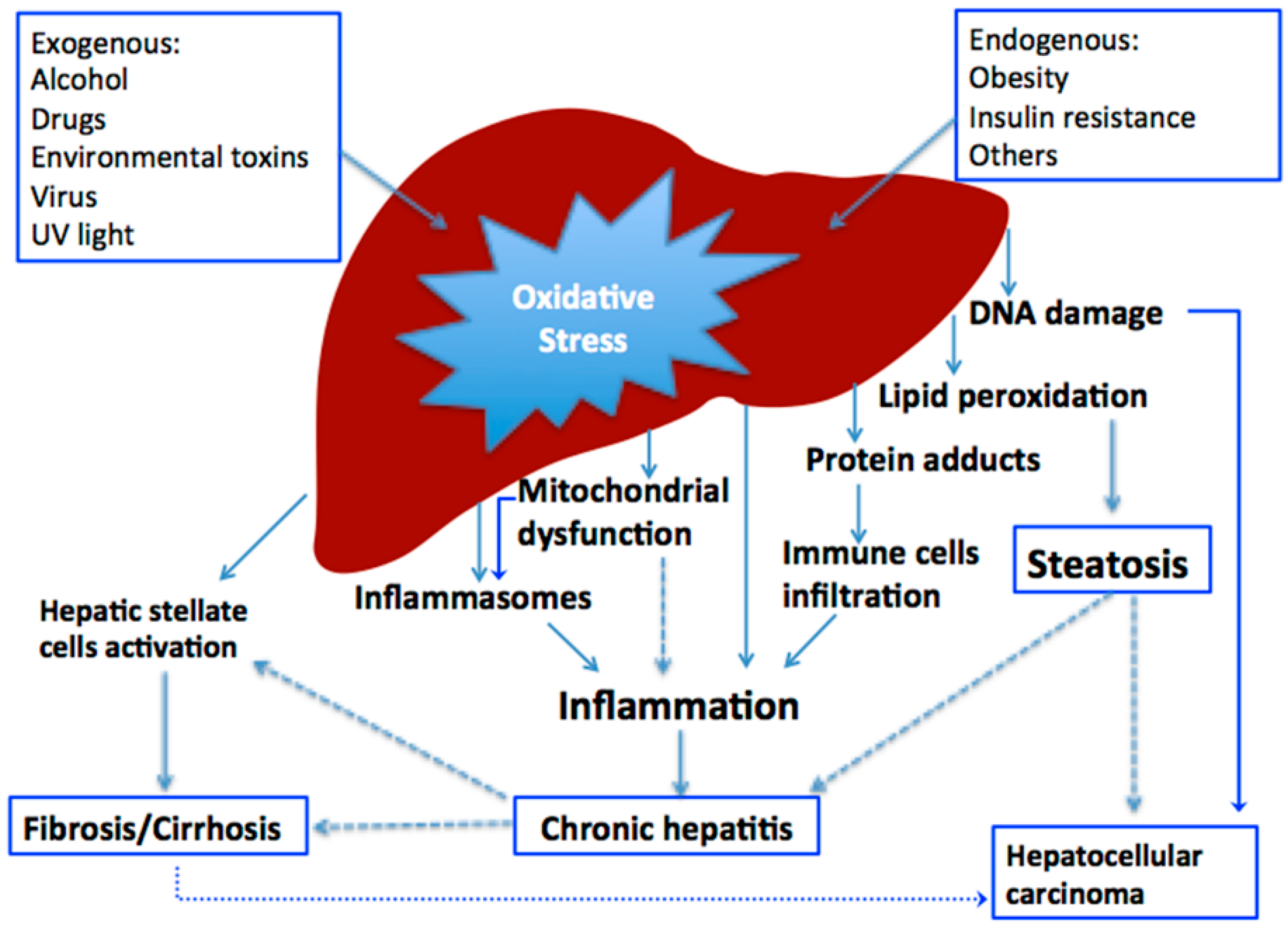
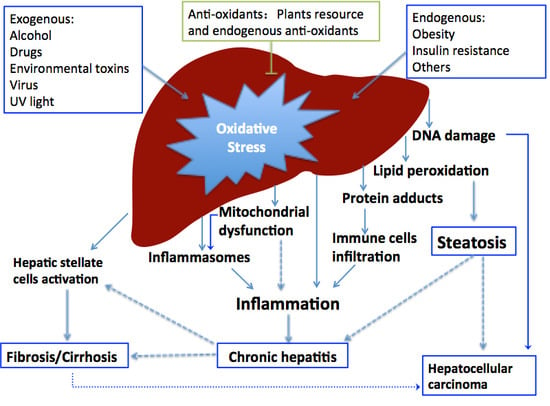
2. Oxidative Stress in Liver Diseases
2.1. Oxidative Stress Caused by Alcohol

2.2. Oxidative Stress Caused by Drugs
2.3. Oxidative Stress Caused by Environmental Pollutants
2.4. Oxidative Stress Caused by Other Factors
3. Antioxidants for Prevention and Treatment of Liver Diseases
3.1. Antioxidants for Prevention and Treatment of Alcoholic Liver Diseases
| Models (Prevent/Treatemnt) | Materials | Effect | Dose (Dose-Effect) | Bioactive Compounds | References |
|---|---|---|---|---|---|
| Rats treated with ethanol diet (Prevent) | Green tea | ↑ Enzymes, non-enzymatic antioxidants; ↓ lipid and protein oxidation | 7 g/L in ethanol Lieber-DeCarli diet | Epicatechin, epicatechin gallate | [84] |
| Rats treated with ethanol (Prevent) | Ziziphus mauritiana leaf | ↓ ALT, AST, ALP, total bilirubin, CAT; ↑ GSH-Px, glutathione reductase and SOD | 200 and 400 mg/kg b.w. (Dose-effect) | Tannins, saponins and phenolic compounds | [42] |
| Rats sub-chronically exposed to ethanol (Prevent) | Amaranthus hypochondriacus seed | ↓ MDA, NADPH; ↑ Cu, Zn-SOD | 140 g/kg in diet | Total phenols | [87] |
| Mice with acute alcohol-induced liver injury (Prevent) | Peduncles of Hoveniadulcis | ↓ ALT, AST, MDA; ↑ SOD, GSH-Px | 100, 350 and 600 mg/kg b.w. (Dose-effect) | Non-starch polysaccharide | [86] |
| Rats treated with ehanol (Prevent) | Methanolic extract from Hammada scoparia leaves | ↓ Aminotransferase, glycogen synthase kinase-3 β, lipid peroxidation; ↑ GSH-Px | 200 mg/kg b.w. | Phenolic compounds | [85] |
| Mice with chronic alcoholic liver damage (Prevent) | Jujube honey | ↓ Lipoprotein oxidation, AST, ALT, MAD, 8-hydroxy-2-deoxyguanosine; ↑ GSH-Px | 27 and 54 g /kg b.w. (Dose-effect) | Phenolic acids | [88] |
| Mice with alcohol-induced hepatotoxicity (Treatment) | Freeze-dried, germinated and fermented mung bean | ↑ Antioxidant levels, NO | 200 and 1000 mg/kg b.w. | [89] | |
| Chronic ethanol exposure in rats (Prevent) | Virgin olive oil | ↓ Transaminases levels, hepatic lipid peroxidation; ↑ GSH-Px, SOD and CAT | 5% (wt/wt) in diet | Tocopherols, chlorophyll, total polyphenols | [90] |
3.2. Antioxidants for Prevention and Treatment of Non-Alcoholic Fatty Liver Diseases
| Models (Prevent/Treatment) | Antioxidant/Plants | Effects | Dose (Dose-Effect) | Bioactive Compounds | References |
|---|---|---|---|---|---|
| Diabetic rats fed on a high fat thermolyzed diet (Prevent) | Omega 3-polyunsaturated fatty acids | ↑ SOD, CAT; ↓ triglycerides, non-esterified fatty acid, lipoperoxidation | 3.0% in diet | Omega 3-polyunsaturated fatty acids | [108] |
| Mice fed with high-fat diet (Prevent and treatment) | Moringa oleifera leaves; haw pectic oligosaccharide; Thymbra spicata | ↑ GSH; ↓ ALT, AST, ALP, lipid peroxidation | 50, 150 and 300 mg/kg b.w. (No dose–effect) | Haw pectic oligosaccharide | [109,110,111] |
| Liver damage in diet-induced atherosclerotic rats (Prevent) | Tulbaghia violacea rhizomes | ↓ LDH, AST, ALT, ALP, bilirubin antioxidation | 100 mg/kg b.w. | [112] | |
| Rabbits with high-fat diet (Prevent) | Apolipoprotein A–I | ↑ SOD, GSH-Px; ↓ iNOS, MDA | 15 mg/kg b.w. | [113] | |
| WeRats fed a high-fat diet (Prevent) | Black cabbage sprout | ↑ SOD, CAT, NADPH, GSH-Px, GRD GST | 250 and 500 mg/kg b.w. (Dose–effect) | [114] |
| Models (Prevent/Treatment) | Materials | Effects | Dose (Dose-Effect) | References |
|---|---|---|---|---|
| Streptozotocin-induced diabetic aged rats (Prevent) | Vitamins C and E | Antioxidation, hepatoprotection | [106] | |
| Streptozotocin-induced diabetic rats (Prevent) | Acai | Antioxidation, hepatoprotection | 2% (w/w) in standard diet | [115] |
| Streptozotocin-induced diabetic rats (Prevent) | Herba bidentis | Antioxidation, hepatoprotection | 5 mL/kg | [116] |
| Streptozotocin-induced diabetic rats (Prevent) | (−)-Epicatechin | Antioxidation | 15 and 30 mg/kg (Dose–effect) | [117] |
| Streptozotocin-induced diabetic rats (Treatment) | Stobadine | 24.7 mg/kg | [118] | |
| Streptozotocin-induced diabetic mice (Prevent) | Terminalia glaucescens leaves | Antioxidation | 100 and 300 mg/kg (Dose–effect) | [119] |
| Streptozotocin-induced diabetic rats (Treatment) | Berberine | Antioxidation | 75, 150 and 300 mg/kg (Dose–effect) | [120] |
| Streptozotocin-induced diabetic rats (Prevent) | Aloe vera leaves | 300 mg/kg | [121] | |
| Streptozotocin-induced diabetic rats (Treatment) | N-Acetylcysteine | Antioxidation | 1.5 g/kg | [122] |
| Streptozotocin-induced diabetic rats (Treatment) | Oroxylum indicum stem bark | Antioxidation | 250 mg/kg | [123] |
| Streptozotocin-induced diabetic rats (Treatment) | Maslinic acid | Antioxidation | 40, 80 and 160 mg/kg (Dose–effect) | [124] |
| Streptozotocin-induced diabetic rats (Treatment) | Resveratrol | Antioxidation | 20 mg/kg | [125] |
| Streptozotocin-nicotinamide induced diabetic rats (Prevent) | Stevia rebaudiana | Antioxidation | [126] |
3.3. Antioxidants for Prevention and Treatment of Liver Diseases Induced by Other Factors
| Models (Prevent/Treatment) | Materials | Effects | Dose (Dose-Effect) | References |
|---|---|---|---|---|
| Paracetamol-induced liver toxicity in mice (Prevent) | Gallic acid | Antioxidation, hepatoprotection | 100 mg/kg | [129] |
| Paracetamol-induced liver toxicity in mice (Prevent) | Sauchinone | Antioxidation, hepatoprotection | 30 mg/kg | [130] |
| Paracetamol-induced liver toxicity in mice (Prevent) | Genistein | Antioxidation, hepatoprotection | 50, 100 and 200 mg/kg (Dose-effect) | [131] |
| Paracetamol-induced liver toxicity in mice (Prevent) | Phyllanthus niruri | Antioxidation, hepatoprotection | 100 mg/kg | [132] |
| Paracetamol-induced liver toxicity in mice (Prevent) | Polyalthia longifolia leaves | Antioxidation, hepatoprotection | 200 mg/kg | [133] |
| Paracetamol-induced liver damage in rats (Prevent) | Boerhaavia diffusa leaves | Antioxidation, hepatoprotection | 100, 200, 300 and 400 mg/kg/day (No dose-effect) | [134] |
| Paracetamol-induced liver damage in rats (Prevent) | Saponarin from Gypsophila trichotoma | Antioxidation, hepatoprotection | 80 mg/kg/week | [135] |
| Lipopolysaccharide-induced liver injury in rats (Prevent) | Carnosic acid | Antioxidation, hepatoprotection | 15, 30 and 60 mg/kg (Dose-effect) | [136] |
| d-Galactosamine-induced liver injury in rats (Prevent) | Combination of selenium, ascorbic acid, β-carotene, and α-tocopherol | Antioxidation, hepatoprotection | [137] | |
| d-Galactosamine-induced liver injury in rats (Prevent) | Leucasaspera | Antioxidation, hepatoprotection | 200 and 400 mg/kg (No dose-effect) | [138] |
| d-Galactosamine-induced liver injury in rats (Prevent) | Swertiamarin from Enicostemma axillare | Antioxidation, hepatoprotection | 100 and 200 mg/kg (No dose-effect) | [139] |
| Lipopolysaccharide/d-galactosamineinduced liver injury in rats (Prevent) | Curcumin | Antioxidation, hepatoprotection | 100 mg/kg | [140] |
| Lipopolysaccharide/d-galactosamineinduced liver injury in rats (Prevent) | betulinic acid | Antioxidation, hepatoprotection | 20 and 50 mg/kg (No dose-effect) | [141] |
| Lipopolysaccharide/d-galactosamine induced hepatitis in rats (Prevent) | Tridaxprocumbens | Antioxidation | 300 mg/kg | [142] |
| Doxorubicin-induced liver injury in rats | N-acetylcysteine | Antioxidation, hepatoprotection | 10 mg/kg | [143] |
| Cisplatin-induced liver injury in rats (Prevent) | Tomato juice | Antioxidation, hepatoprotection | [144] | |
| Tert-butyl hydroperoxide-induced liver injury in rats (Prevent) | Propolis | Antioxidation, hepatoprotection | 50 and 100 mg/kg (No dose-effect) | [145] |
| Tamoxifen-induced liver injury in mice (Prevent) | Catechin | Antioxidation | 40 mg/kg | [146] |
| Hepatic steatosis stimulated with tunicamycin (Treatment) | Melatonin | ↓ ER stress, expression of miR-23a | [147] | |
| Ethionine-induced liver injury in mice (Prevent) | Melatonin | Antioxidation, hepatoprotection | 3 mg/kg | [148] |
| Model (Prevent/Treatment) | Antioxidant/Plant | Effects | Dose/(Dose–Effect) | Bioactive Compounds | References |
|---|---|---|---|---|---|
| CCl4-induced liver damage in rats (Prevent) | Coptidis rhizome and berberine | ↑ SOD; ↓ ALT, AST, Erk1/2 | Berberine: 120 mg/kg b.w. Extract: 800 mg/kg b.w. | Berberine | [10] |
| CCl4-induced liver damage in rats (Prevent) | Friedelin isolated from Azima tetracantha leaves | ↑ SOD, CAT, GSH, GSH-Px; ↓ ALT, AST, LDH | [59] | ||
| CCl4-induced liver damage in rats (Treatment) | N-butanol fraction of Actinidias deliciosa roots | ↑ GSH; ↓ ALT, AST, MDA | (Dose–effect) | Oleanolic acid | [167] |
| CCl4-induced liver damage in rats (Prevent) | Silybum marianum seeds | ↑ GSH; HDL/LDL; hepatoprotection | 100 mg/kg b.w. | [168] | |
| CCl4-induced liver damage in rats (Prevent) | Dioclea reflexa seeds | ↑ SOD, CAT; ↓ Transaminases, MDA | 5 mg/kg (acute) 2.5 mg/kg b.w. (chronic) | [169] | |
| CCl4-induced liver damage in rats (Prevent) | Morus bombycis, 2,5-dihydroxy-4,3′-di (β-d-glucopyranosyloxy)-trans-stilbene | ↓ Lipid peroxidation; hepatoprotection | 100, 300 and 500 mg/kg b.w. (No dose–effect) | [170,171] | |
| CCl4-induced liver damage in rats (Prevent) | Nigella sativa, Urticadioica | ↑ Antioxidant enzyme; ↓ lipid peroxidation; hepatoprotection | Nigella sativa: 0.2 mg/mL Urtica dioica: 0.2 mg/mL | [172] | |
| CCl4-induced liver damage in rats (Prevent) | Pleurotusostreatus (oyster mushroom) | ↑ GSH, CAT, SOD, GSH-Px; ↓ ALT, AST, ALP, MDA | 200 mg/kg b.w. | [173] | |
| CCl4-induced liver damage in rats (Prevent) | Cytisusscoparius | ↑ GSH, CAT, SOD, GSH-Px, GST, GRD; ↓ ALT, AST, LDH | 250 and 500 mg/kg (No dose–effect) | [174] | |
| CCl4-induced liver damage in rats (Prevent) | Ethanol extract of Phellinusmerrillii | ↑ CAT, SOD, GSH-Px; ↓ ALT, AST | 0.5, 1 and 2 g/kg b.w. (No dose–effect) | [175] | |
| CCl4-induced liver damage in rats (Prevent) | Ginkgo biloba | ↑ GSH, SOD, CAT, GSH-Px, GRD, albumin; hepatoprotection | 25 and 50 mg/kg b.w. (No dose–effect) | [176] | |
| CCl4-induced liver damage in mice (Prevent) | Protein isolate from Phyllanthus niruri | ↑ SOD, CAT; ↓ ALT, ALP; lipid peroxidation | 5 mg/kg b.w. | [177] | |
| CCl4-induced liver damage in mice (Prevent) | Kahweol and cafestol (Coffee) | ↓ ALT, AST, cytochrome P450 2E1, lipid peroxidation | Kahweol or cafestol: 10–100 mg/kg b.w. (Dose–effect) | Kahweol and cafestol | [178] |
| CCl4-induced liver damage in rats (Prevent) | Cirsium setidens | ↑ GSH-Px; SOD; hepatoprotection | 500 mg/kg b.w. | [179] | |
| CCl4-induced liver damage in rats (Prevent) | Curcumin and saikosaponin A | ↑ SOD, GSH; ↓ MDA; hepatoprotection | [180] | ||
| CCl4-induced liver damage in rats (Prevent) | Ethanolic extract of Momordica tuberosa tubers | Antioxidation, hepatoprotection | [181] | ||
| CCl4-induced liver damage in rats (Prevent) | Oregano and rosemary | ↓ AST, ALT, ALP; antioxidation | 20 g/kg b.w. | [182] | |
| CCl4-induced liver damage in rats (Prevent) | Enicostemma axillare | Antioxidation, hepatoprotection | 100 and 200 mg/kg b.w. (No dose–effect) | [139] | |
| CCl4-induced liver damage in rats (Prevent) | Ficuscarica leaves and fruits, Morus alba root barks | ↑ CAT, SOD, GSH; ↓ MDA, AST, ALT, ALP | 50 and 150 mg/kg b.w. (No dose–effect) | [183] | |
| CCl4-induced liver damage in rats (Prevent) | Podophyllum hexandrum | ↑ GSH, GSH-Px, GRD, SOD, GST; ↓ AST, ALT, LDH | 20, 30 and 50 mg/kg b.w. (No dose–effect) | [184] | |
| CCl4-induced liver damage in rats (Prevent) | Ficusreligiosa roots | ↑ CAT, GSH-Px, GRD, SOD, GST; ↓ lipid peroxidation; hepatoprotection | [185] | ||
| CCl4-induced liver damage in rats (Prevent) | Dehydroabietylamine, Carthamus tinctorious | ↓ AST, ALT, ALP; antioxidation | [186] | ||
| CCl4-induced liver damage in rats (Prevent) | Artemetin, Vitexglabrata | ↑ SOD, CAT, GSH-Px; ↓ AST, ALT, ALP, lipid peroxidation, TB | [187] | ||
| CCl4-induced liver damage in mice (Prevent) | Blueberry anthocyanins | ↑ SOD, CAT, GRD, glycogen; ↓ AST, ALT, MDA | [188] | ||
| CCl4-induced liver damage in rats (Prevent) | Matricaria chamomilla | ↑ SOD, CAT, GSH-Px, GSH; ↓ AST, ALT, MDA | 50, 100 and 200 mL/kg b.w. (No dose–effect) | [189] | |
| CCl4-induced liver damage in mice (Prevent) | Lysimachia clethroides | ↑ SOD; ↓ AST, ALT, MDA | 150, 300 and 600 mg/kg b.w. (No dose–effect) | [190] | |
| CCl4-induced liver damage in rats (Prevent) | Garcinia indica fruit rind | ↑ SOD, CAT, GRD, GSH-Px, GSH; ↓ AST, ALT, MDA | 400 and 800 mg/kg b.w. (No dose–effect) | [191] | |
| CCl4-induced liver damage in rats (Prevent) | Agaricus blazei | ↑ GSH, GRD; ↓ AST, ALT, MDA | 500 mg/kg b.w. | [192] | |
| CCl4-induced liver damage in rats (Prevent) | Nerium oleander flowers | ↑ SOD; ↓ AST, ALT, ALP, MDA | 100, 200 and 400 mg/kg b.w. (No dose–effect) | [193] | |
| CCl4-induced liver damage in rats (Prevent) | Hybanthus enneaspermus | ↓ AST, ALT, ALP, TB; antioxidation | 200 and 400 mg/kg b.w. (No dose–effect) | [194] | |
| CCl4-induced liver damage in mice (Treatment) | Anthocyanins in black rice bran | ↑ SOD, GSH-Px; hepatoprotection | 200, 400 and 800 mg/kg b.w. (No dose–effect) | [195] | |
| CCl4-induced liver damage in rats (Prevent) | Roureainduta | ↑ SOD, CAT, GSH, GSH-Px; ↓ AST, ALT, total bilirubin; | 500 mg/kg b.w. | [196] | |
| CCl4-induced liver damage in rats (Prevent) | Proanthocyanidins extracted from grape seeds | ↑ SOD, GSH, GSH-Px, CAT; ↓ lipid accumulation, liver injury, DNA damage | 400 mg/kg b.w. | Proanthocyanidins | [197] |
| CCl4-induced liver damage in mice (Prevent) | Veronica ciliata | ↑ SOD, GSH; ↓ ALT, AST, ALP | 150, 300 and 600 mg/kg b.w. (No dose–effect) | [198] | |
| CCl4-induced liver damage in rats (Prevent) | Subereamollis | ↑ SOD, GSH, GSH-Px, CAT; ↓ ALT, AST, ALP, MDA | 100, 200 and 400 mg/kg b.w. (No dose–effect) | [199] | |
| CCl4-induced liver damage in rats (Prevent) | Solanum xanthocarpum leaves | ↑ SOD, CAT, GSH, GST; ↓ ALT, AST, ALP, LDH | 100 and 200 mg/kg b.w. (No dose–effect) | [200] | |
| CCl4-induced liver damage in rats (Prevent) | Allopurinol | Modulation of NF-κB, cytokine production and oxidative stress | 50 mg/kg b.w. | ||
| CCl4 and H2O2 induced liver damage in goat (Prevent) | Ocimumbasilicum, Trigonellafoenum-graecum | Antioxidation | [201] | ||
| TAA-induced liver injury (Prevent) | Genistein | ↑ GSH; ↓ MDA, ALT, AST, TB | 0.5, 1.0 and 2.0 mg/kg b.w. (No dose–effect) | [202] | |
| TAA-Induced liver Cirrhosis in rats (Prevent) | Andrographis paniculata Leaf | Hepato-protection, ↓ ROS, LDH | 250 and 500 mg/kg b.w. (No dose–effect) | [203] | |
| TAA-induced hepatotoxicity in rats (Prevent) | coriander | Antioxidant; ↓ ALT, AST, ALP, TBARS, MPO, NO | Phenolic compounds | [204] | |
| TAA-induced fibrosis in mice (Treatment) | Ger-Gen-Chyn-Lian-Tang | Antioxidant; anti-fibrosis; modulation on TGF-β/TGF-β receptor signaling | 100 and 300 mg/kg b.w. (Dose–effect) | [205] | |
| TAA-induced hepatotoxicity in rats (Treatment) | Trigonella foenum-graecum | Antioxidant; hepato-protection; ↓ ALP, MDA | [206] | ||
| TAA-induced hepatotoxicity in rats (Treatment) | Allopurinol | Regulating cellular redox-sensitive transcription factors | [163] | ||
| Cigarette smoke-induced oxidative damage in liver of rats (Treatment) | Sesbania grandiflora leaves | ↑ SOD, GSH, GSH-Px, CAT, GST, GRD, glucose-6-phosphate dehydrogenase; ↓ AST, ALT, ALP | 1000 mg/kg b.w. | [207] | |
| Cigarette smoking induced oxidative damage in liver of mice (Prevent) | Vitamin E and selenium | ↑ GSH-Px, Se-GSH-Px | [208] | ||
| Atrazine exposure rats (Prevent) | Vitamin E | ↑ SOD, GSH-Px, CAT, GST; ↓ lipid peroxidation | [209] | ||
| Methidathion-induced liver injury in rats (Prevent) | Vitamins C and E | ↓ AST, ALT, ALP, MDA; | Vitamin E: 50 mg/kg b.w.;Vitamin C: 20 mg/kg b.w. | [210] | |
| Pesticide (chlorpyriphos and cypermethrin) induced hepatic damage in mice (Prevent) | Black tea | ↑ SOD, GSH, GSH-Px, CAT, GRD, GST; ↓ AST, ALT, ALP | 200 mg/mL b.w. | [211] | |
| Polychlorinated biphenyls induced hepatic damage in rats (Prevent) | α-Tocopherol | Antioxidation | 50 mg/kg. b.w. | [212] | |
| Aflatoxin-induced hepatic injury in rats (Prevent) | Urticadioica seed | ↑ SOD, GSH-Px, CAT, GRD, GST; ↓ lipid peroxides, hydroxyl radical and hydrogen peroxides | 2 mL/rat/day | [213] | |
| Thioacetamide-induced hepatic damage in rats (Prevent) | eugenol | ↑ COX-2; ↓ AST, ALT, ALP, bilirubin, CYP2E1, lipid peroxidation; antioxidation | 10.7 mg/kg b.w. | [214] | |
| Lead-induced liver damage in rats (Prevent) | Ginger | ↑ SOD, CAT; ↓ MDA | 100 mg/kg b.w. | [215] | |
| Dimethylnitrosamine-induced hepatic damage in rats (Prevent) | Anthocyanins from purple sweet potato | ↑ Nrf2, NADPH, GSH, GST; ↓ yclooxygenase-2, MDA | 50, 100 and 200 mg/kg b.w. (No dose–effect) | Anthocyanins | [151] |
| Cadmium-induced hepatic injury in rats (Prevent) | Heated garlic juice, ascorbic acid | ↑ Nrf2, SOD, CAT; ↓ MDA | Heated garlic juice: 100 mg/kg b.w.; Ascorbic acid: 100 mg/kg b.w. | Ascorbic acid | [152] |
| Potassium bromate-induced hepatotoxicity of rat (Prevent) | Launaea procumbens | ↑ SOD, CAT, GSH, GSH-Px, GRD, GST | 200 mg/kg b.w. | [216] | |
| Dimethylnitrosamine induced liver fibrosis in rats (Prevent) | Platycodi radix root | ↑ Nrf2, heme oxygenase-1, NADPH, NQO1, GST; ↓ ALT, AST; anti-fibrotic action | 200 mg/kg b.w. | Changkil | [153] |
| As2O3-induced hepatotoxicity in cat (Prevent) | Resveratrol | ↑ GSH; ↓ ROS, MDA | 3 mL/kg b.w. | [217] | |
| Sodiumarsenite induced liver damage in rats (Prevent) | Emblica officinalis | Antioxidation | 500 mg in 0.1 mL water, 100 g b.w. | [218] | |
| Trichloroacetic acid induced liver injury in rats (Prevent) | Date palm fruit | ↑ SOD, CAT, GSH-Px; ↓ MDA | 0.5 and 2 g/L b.w. (No dose–effect) | [219] |
| Stress (Prevent/Treatment) | Antioxidant/Plants | Effects | Dose (Dose–Effect) | Bioactive Compounds | References |
|---|---|---|---|---|---|
| Human liver cancer cell line | Morinda pubescens leaves | Antioxidation, cytotoxicity | 25, 50, 100 and 250 μg/mL b.w. (Dose–effect) | Hyoscyamine | [220] |
| Liver cancer of rats (Prevent) | Chlorella vulgaris | Antioxidation, antitumour | 50, 150 and 300 mg/mL b.w. (Dose–effect) | [221] | |
| Hepatocellular carcinoma | Caesalpinia bonducella leaves | ↑ SOD, GSH, CAT; ↓ MDA, AST, ALT, ALP; anticancer | Flavonoids, triterpenoids | [222] | |
| Liver cancer of mice (Prevent) | Pleurotus pulmonarius (edible mushroom) | Antioxidation, anti-tumor | [158] | ||
| Rat with secondary biliary cirrhosis (Prevent) | Silybin | Antioxidation | 0.4 g/kg b.w. | [223] | |
| Cholestatic rats with bile duct ligation (Treatment) | Green tea catechin | Antioxidation, reducing hepatic fibrosis | 50 mg/kg b.w. | [161] | |
| Bile duct-ligated cholestatic rats (Treatment) | Epigallocatechin-3-gallate | Anti-fibrotic effects, ↓ phosphorylation of Smad2/3 and Akt | 5 mg/kg b.w. | [224] | |
| Bile duct-ligated cholestatic rats (Treatment) | Holothuria arenicola | ↑ SOD, GSH, GST, CAT; ↓ MDA, AST, ALT, ALP | 200 mg/kg b.w. | Phenolic compounds, chlorogenic acid, pyrogallol, rutin, coumaric acid | [225] |
| Bile-duct ligated Rats (Treatment) | Garlic | ↑ GSH; ↓ LDH, TB, MDA, MPO; ↓ TNF-α, TGF-β, MMP-13 | [226] | ||
| Bile-duct ligated Rats (Treatment) | thymoquinone | ↑ SOD, GSH-Px; ↓ MDA | 50 mg/kg b.w. | [227] | |
| Bile-duct ligated Rats (Treatment) | N-acetylcysteine | ↑ GSH, CAT; ↓ MDA, ALT | 300 mg/kg b.w. | [228] | |
| Bile-duct ligated Rats (Prevent) | Phaseolus trilobus | ↑ SOD; ↓ AST, ALT, ALP, LDH, TB, TBARS; | 125, 250 and 500 mg/kg b.w. (Dose–effect) | [229] | |
| Bile-duct ligated Rats (Treatment) | Melatonin | ↓ TBARS, MPO | 10 and 100 mg/kg b.w. (Dose–effect) | [230] | |
| Ischemia/reperfusion in obese rats with fatty liver | Melatonin | ↑ Antioxidant enzymes; ↓ AST, ALT, MAD, NOx metabolites | 10 mg/kg b.w. | [231] | |
| Bile-duct ligated Rats (Treatment) | Allopurinol | ↓ ROS, brain edema | 100 mg/kg b.w. | [232] | |
| Restraint stress-induced liver injury in mice (Prevent) | Astragali radix and Salviae radix | Antioxidation, hepatoprotection | 50, 100 and 200 mg/kg b.w. (No dose–effect) | Myelophil | [233] |
4. Current Anti-Oxidative Therapy in Clinical Trials
5. Conclusions and Prospects
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AC | Autoimmune cholestatic liver diseases |
| ADH | Alcohol dehydrogenase |
| AIH | Autoimmune hepatitis |
| ALD | Alcoholic liver disease |
| ALP | Alkaline phosphatase |
| ALT | Alanine transaminase |
| ALDH | Aldehyde dehydrogenases |
| ARE | Antioxidant response element |
| AST | Aspartate aminotransferase |
| BHA | Butylated hydroxyanisole |
| bw | Body weight |
| CAT | Catalase |
| CCl4 | Carbon tetrachloride |
| ER | Endoplasmic reticulum |
| GSH-Px | Glutathione peroxidase |
| GSH | Glutathione |
| GRD | Glutathione reductase |
| GST | Glutathione S-transferase |
| HDL | High density lipoprotein |
| HCV | Hepatitis C virus |
| IL-6 | Interleukin 6 |
| INH | Anti-tuberculosis agent isoniazid |
| iNOS | Inducible nitric oxide synthase (iNOS) |
| INrf2 | Inhibitor of Nrf2 |
| IKKβ | IκB kinase-β |
| IRS | Insulin receptor substrate |
| JNK | c-Jun N-terminal kinases |
| Keap1 | kelch-like ECH-associated protein-1 |
| LDH | lactate dehydrogenase |
| LDL | Low density lipoprotein |
| MDA | Malondialdehyde |
| MEOS | Microsomal ethanol oxidizing system |
| NADPH | Nicotinamide adenine dinucleotide phosphate-oxidase |
| NAFLD | Non-alcoholic fatty liver disease NAFLD |
| NO | Nitric Oxide |
| NQO1 | NAD(P)H Dehydrogenase, Quinone 1 |
| Nrf1 | Nuclear respiratory factor 1 |
| Nrf2 | Erythroid 2-related factor 2 |
| PKC | protein kinase C |
| PPARα | Peroxisome proliferator activated receptor α |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species (ROS) |
| SOD | Superoxide dismutases |
| TAA | Thioacetamide |
| TB | Total bilirubin |
| TBARS | Thiobarbituric acid-reactive substances |
| TNF | Tumor necrosis factor |
References
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M. The evolution of free radicals and oxidative stress. Am. J. Med. 2000, 108, 652–659. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Sanchez-Valle, V.; Chavez-Tapia, N.C.; Uribe, M.; Mendez-Sanchez, N. Role of oxidative stress and molecular changes in liver fibrosis: A review. Curr. Med. Chem. 2012, 19, 4850–4860. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Takahashi, S.; Sasaki, T.; Kumagai, T.; Nagata, K. Progression of alcoholic and non-alcoholic steatohepatitis: Common metabolic aspects of innate immune system and oxidative stress. Drug Metab. Pharmacokinet. 2011, 26, 30–46. [Google Scholar] [CrossRef] [PubMed]
- Cichoz-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Cederbaum, A.I. Oxidative stress and alcoholic liver disease. Semin Liver Dis. 2009, 29, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, N.; Ye, X.; Li, H.; Feng, Y.; Cheung, F.; Nagamatsu, T. Hepatoprotective effect and its possible mechanism of Coptidis rhizoma aqueous extract on carbon tetrachloride-induced chronic liver hepatotoxicity in rats. J. Ethnopharmacol. 2011, 138, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.K.; Jampana, S.C.; Weinman, S.A. Antioxidants as therapeutic agents for liver disease. Liver Int. 2011, 31, 1432–1448. [Google Scholar] [CrossRef] [PubMed]
- Medina, J.; Moreno-Otero, R. Pathophysiological basis for antioxidant therapy in chronic liver disease. Drugs 2005, 65, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Palma, H.E.; Wolkmer, P.; Gallio, M.; Correa, M.M.; Schmatz, R.; Thome, G.R.; Pereira, L.B.; Castro, V.S.; Pereira, A.B.; Bueno, A.; et al. Oxidative stress parameters in blood, liver, and kidney of diabetic rats treated with curcumin and/or insulin. Mol. Cell. Biochem. 2014, 386, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Bosoi, C.R.; Yang, X.; Huynh, J.; Parent-Robitaille, C.; Jiang, W.; Tremblay, M.; Rose, C.F. Systemic oxidative stress is implicated in the pathogenesis of brain edema in rats with chronic liver failure. Free Radic. Biol. Med. 2012, 52, 1228–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Chen, X.; Su, Y.; Paueksakon, P.; Hu, W.; Zhang, M.Z.; Harris, R.C.; Blackwell, T.S.; Zent, R.; Pozzi, A. p47phox contributes to albuminuria and kidney fibrosis in mice. Kidney Int. 2015, 87, 948–962. [Google Scholar] [CrossRef] [PubMed]
- Valente, M.J.; Carvalho, F.; Bastos, M.; de Pinho, P.G.; Carvalho, M. Contribution of oxidative metabolism to cocaine-induced liver and kidney damage. Curr. Med. Chem. 2012, 19, 5601–5606. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Lakshmanan, J. The role of antioxidants and other agents in alleviating hyperglycemia mediated oxidative stress and injury in liver. Food Funct. 2013, 4, 1148–1184. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, A.B.; Gui, M.; Karabulut, E.; Kiran, T.R.; Ocak, S.G.; Otlu, O. Oxidant and antioxidant activity in rabbit livers treated with zoledronic acid. Transplant. Proc. 2010, 42, 3820–3822. [Google Scholar] [CrossRef] [PubMed]
- Mallikarjuna, K.; Shanmugam, K.R.; Nishanth, K.; Wu, M.C.; Hou, C.W.; Kuo, C.H.; Reddy, K.S. Alcohol-induced deterioration in primary antioxidant and glutathione family enzymes reversed by exercise training in the liver of old rats. Alcohol 2010, 44, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Jiang, Y.F.; Ponnusamy, M.; Diallo, M. Role of Nrf2 in chronic liver disease. World J. Gastroenterol. 2014, 20, 13079–13087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.K.; Wu, K.C.; Klaassen, C.D. Genetic activation of Nrf2 protects against fasting-induced oxidative stress in livers of mice. PLoS ONE 2013, 8, e59122. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, C.D.; Reisman, S.A. Nrf2 the rescue: Effects of the antioxidative/electrophilic response on the liver. Toxicol. Appl. Pharmacol. 2010, 244, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Emerit, I.; Huang, C.Y.; Serejo, F.; Filipe, P.; Fernandes, A.; Costa, A.; Freitas, J.; Baptista, A.; Carneiro de Moura, M. Oxidative stress in chronic hepatitis C: A preliminary study on the protective effects of antioxidant flavonoids. Hepatogastroenterology 2005, 52, 530–536. [Google Scholar] [PubMed]
- Esrefoglu, M. Oxidative stress and benefits of antioxidant agents in acute and chronic hepatitis. Hepat. Mon. 2012, 12, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Farias, M.S.; Budni, P.; Ribeiro, C.M.; Parisotto, E.B.; Santos, C.E.; Dias, J.F.; Dalmarco, E.M.; Frode, T.S.; Pedrosa, R.C.; Wilhelm Filho, D. Antioxidant supplementation attenuates oxidative stress in chronic hepatitis C patients. Gastroenterol. Hepatol. 2012, 35, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Groenbaek, K.; Friis, H.; Hansen, M.; Ring-Larsen, H.; Krarup, H.B. The effect of antioxidant supplementation on hepatitis C viral load, transaminases and oxidative status: A randomized trial among chronic hepatitis C virus-infected patients. Eur. J. Gastroenterol. Hepatol. 2006, 18, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Fan, Y.C.; Zhang, F.; Yang, Y.; Zhao, Z.H.; Sun, F.K.; Wang, K. Oxidative stress in chronic hepatitis C patients. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 2013, 27, 47–49. (In Chinese) [Google Scholar] [PubMed]
- Kawanaka, M.; Mahmood, S.; Niiyama, G.; Izumi, A.; Kamei, A.; Ikeda, H.; Suehiro, M.; Togawa, K.; Sasagawa, T.; Okita, M.; et al. Control of oxidative stress and reduction in biochemical markers by vitamin E treatment in patients with nonalcoholic steatohepatitis: A pilot study. Hepatol. Res. 2004, 29, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Feng, Y.B.; Cheung, F.; Chow, O.Y.; Wang, X.B.; Su, W.W.; Tong, Y. A comparative study on the hepatoprotective action of bear bile and coptidis rhizoma aqueous extract on experimental liver fibrosis in rats. BMC Complement. Altern. Med. 2012, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loomba, R.; Wesley, R.; Pucino, F.; Liang, T.J.; Kleiner, D.E.; Lavine, J.E. Placebo in nonalcoholic steatohepatitis: Insight into natural history and implications for future clinical trials. Clin. Gastroenterol. Hepatol. 2008, 6, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Masalkar, P.D.; Abhang, S.A. Oxidative stress and antioxidant status in patients with alcoholic liver disease. Clin. Chim. Acta 2005, 355, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Louvet, A.; Mathurin, P. Alcoholic liver disease: Mechanisms of injury and targeted treatment. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Zima, T.; Kalousova, M. Oxidative stress and signal transduction pathways in alcoholic liver disease. Alcohol. Clin. Exp. Res. 2005, 29, 110S–115S. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jia, Z.; Misra, H.; Li, Y.R. Oxidative stress and redox signaling mechanisms of alcoholic liver disease: Updated experimental and clinical evidence. J. Dig. Dis. 2012, 13, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Jana, S.; Chakraborty, S.; Swarnakar, S. Inflammation and MMPs in alcohol-induced liver diseases and protective action of antioxidants. Indian J. Biochem. Biol. 2013, 50, 377–386. [Google Scholar]
- Gao, B.; Bataller, R. Alcoholic liver disease: Pathogenesis and new therapeutic targets. Gastroenterology 2011, 141, 1572–1585. [Google Scholar] [CrossRef] [PubMed]
- Beier, J.I.; McClain, C.J. Mechanisms and cell signaling in alcoholic liver disease. Biol. Chem. 2010, 391, 1249–1264. [Google Scholar] [CrossRef] [PubMed]
- Diehl, A.M. Recent events in alcoholic liver disease V. Effects of ethanol on liver regeneration. Am. J. Physiol.-Gastrointest. Liver Physiol. 2005, 288, G1–G6. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, K.R.; Mallikarjuna, K.; Reddy, K.S. Effect of alcohol on blood glucose and antioxidant enzymes in the liver and kidney of diabetic rats. Indian J. Pharmacol. 2011, 43, 330–335. [Google Scholar] [PubMed]
- Babczynska, A.; Wilczek, G.; Migula, P. Effects of dimethoate on spiders from metal pollution gradient. Sci. Total Environ. 2006, 370, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Dahiru, D.; Obidoa, O. Evaluation of the antioxidant effects of Ziziphus mauritiana lam. leaf extracts against chronic ethanol-induced hepatotoxicity in rat liver. Afr. J. Tradit. Complement. Altern. Med. 2007, 5, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Das, K.S.; Balakrishnan, V.; Mukherjee, S.; Vasudevan, D.M. Evaluation of blood oxidative stress-related parameters in alcoholic liver disease and non-alcoholic fatty liver disease. Scand. J. Clin. Lab. Investig. 2008, 68, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Videla, L.A.; Rodrigo, R.; Orellana, M.; Fernandez, V.; Tapia, G.; Quinones, L.; Varela, N.; Contreras, J.; Lazarte, R.; Csendes, A.; et al. Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients. Clin. Sci. 2004, 106, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Chen, L.J.; Bair, M.J.; Yao, M.L.; Peng, H.C.; Yang, S.S.; Yang, S.C. Antioxidative status of patients with alcoholic liver disease in southeastern Taiwan. World J. Gastroenterol. 2011, 17, 1063–1070. [Google Scholar] [PubMed]
- Albano, E. Oxidative mechanisms in the pathogenesis of alcoholic liver disease. Mol. Asp. Med. 2008, 29, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Moreno, M.; Gutierrez-Reyes, G. The role of oxidative stress in the development of alcoholic liver disease. Revista de Gastroenterología de México 2014, 79, 135–144. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Videla, L.A. Oxidative stress signaling underlying liver disease and hepatoprotective mechanisms. World J. Hepatol. 2009, 1, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Linares, V.; Alonso, V.; Albina, M.L.; Belles, M.; Sirvent, J.J.; Domingo, J.L.; Sanchez, D.J. Lipid peroxidation and antioxidant status in kidney and liver of rats treated with sulfasalazine. Toxicology 2009, 256, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Mladenovic, D.; Radosavljevic, T.; Ninkovic, M.; Vucevic, D.; Jesic-Vukicevic, R.; Todorovic, V. Liver antioxidant capacity in the early phase of acute paracetamol-induced liver injury in mice. Food Chem. Toxicol. 2009, 47, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Afshari, R.; Farkhondeh, T. Effect of long-term treatment of morphine on enzymes, oxidative stress indices and antioxidant status in male rat liver. Int. J. Clin. Exp. Med. 2014, 7, 1449–1453. [Google Scholar] [PubMed]
- Pieniazek, A.; Czepas, J.; Piasecka-Zelga, J.; Gwozdzinski, K.; Koceva-Chyla, A. Oxidative stress induced in rat liver by anticancer drugs doxorubicin, paclitaxel and docetaxel. Adv. Med. Sci. 2013, 58, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.; Huang, J.; Doan, V.; Lin, X.; Tang, X.; Huang, Y.; Tang, A.; Yang, X.; Huang, R. Hepatoprotective effects of Yulangsan polysaccharide against nimesulide-induced liver injury in mice. J. Ethnopharmacol. 2015, 172, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Zlatkovic, J.; Todorovic, N.; Tomanovic, N.; Boskovic, M.; Djordjevic, S.; Lazarevic-Pasti, T.; Bernardi, R.E.; Djurdjevic, A.; Filipovic, D. Chronic administration of fluoxetine or clozapine induces oxidative stress in rat liver: A histopathological study. Eur. J. Pharm. Sci. 2014, 59, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Stine, J.G.; Chalasani, N. Chronic liver injury induced by drugs: A systematic review. Liver Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Shuhendler, A.J.; Pu, K.; Cui, L.; Uetrecht, J.P.; Rao, J. Real-time imaging of oxidative and nitrosative stress in the liver of live animals for drug-toxicity testing. Nat. Biotechnol. 2014, 32, 373–380. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, A.; Brummell, Z.; Sizer, E.; Auzinger, G.; Heaton, N.; O’Grady, J.G.; Bernal, W.; Hendry, B.M.; Wendon, J.A. Acute kidney injury in patients admitted to a liver intensive therapy unit with paracetamol-induced hepatotoxicity. Nephrol. Dial. Transplant. 2011, 26, 3501–3508. [Google Scholar] [CrossRef] [PubMed]
- Bando, I.; Reus, M.I.; Andres, D.; Cascales, M. Endogenous antioxidant defence system in rat liver following mercury chloride oral intoxication. J. Biochem. Mol. Toxicol. 2005, 19, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Adegbesan, B.O.; Adenuga, G.A. Effect of lead exposure on liver lipid peroxidative and antioxidant defense systems of protein-undernourished rats. Biol. Trace Element Res. 2007, 116, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Pichardo, S.; Jos, A.; Gomez-Amores, L.; Mate, A.; Vazquez, C.M.; Camean, A.M. Antioxidant enzyme activity and lipid peroxidation in liver and kidney of rats exposed to microcystin-LR administered intraperitoneally. Toxicon 2005, 45, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Ozgur, E.; Guler, G.; Seyhan, N. Mobile phone radiation-induced free radical damage in the liver is inhibited by the antioxidants N-acetyl cysteine and epigallocatechin-gallate. Int. J. Radiat. Biol. 2010, 86, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, N.C.; Yurekli, M.; Yildirim, N. Investigation of some antioxidant enzymes activities depending on adrenomedullin treatment and cold stress in rat liver tissue. Turk. J. Biochem. 2010, 35, 138–142. [Google Scholar]
- Jia, X.; Wu, Y.; Liu, P. Effects of flour bleaching agent on mice liver antioxidant status and ATPases. Environ. Toxicol. Pharmacol. 2011, 31, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Syama, S.; Reshma, S.C.; Sreekanth, P.J.; Varma, H.K.; Mohanan, P.V. Effect of zinc oxide nanoparticles on cellular oxidative stress and antioxidant defense mechanisms in mouse liver. Environ. Toxicol. Pharmacol. 2013, 95, 495–503. [Google Scholar] [CrossRef]
- Zhang, X.; Strakovsky, R.; Zhou, D.; Zhang, Y.; Pan, Y.X. A maternal high-fat diet represses the expression of antioxidant defense genes and induces the cellular senescence pathway in the liver of male offspring rats. J. Nutr. 2011, 141, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Dornas, W.C.; de Lima, W.G.; dos Santos, R.C.; Guerra, J.F.; de Souza, M.O.; Silva, M.; Souza e Silva, L.; Diniz, M.F.; Silva, M.E. High dietary salt decreases antioxidant defenses in the liver of fructose-fed insulin-resistant rats. J. Nutr. Biochem. 2013, 24, 2016–2022. [Google Scholar] [CrossRef] [PubMed]
- Messarah, M.; Klibet, F.; Boumendjel, A.; Abdennour, C.; Bouzerna, N.; Boulakoud, M.S.; el Feki, A. Hepatoprotective role and antioxidant capacity of selenium on arsenic-induced liver injury in rats. Exp. Toxicol. Pathol. 2012, 64, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Kaffe, E.T.; Rigopoulou, E.I.; Koukoulis, G.K.; Dalekos, G.N.; Moulas, A.N. Oxidative stress and antioxidant status in patients with autoimmune liver diseases. Redox Rep. 2015, 20, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Madan, K.; Bhardwaj, P.; Thareja, S.; Gupta, S.D.; Saraya, A. Oxidant stress and antioxidant status among patients with nonalcoholic fatty liver disease (NAFLD). J. Clin. Gastroenterol. 2006, 40, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Feagins, L.A.; Flores, A.; Arriens, C.; Park, C.; Crook, T.; Reimold, A.; Brown, G. Nonalcoholic fatty liver disease: A potential consequence of tumor necrosis factor-inhibitor therapy. Eur. J. Gastroenterol. Hepatol. 2015, 27, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Coffin, C.S.; Fraser, H.F.; Panaccione, R.; Ghosh, S. Liver diseases associated with anti-tumor necrosis factor-alpha (TNF-α) use for inflammatory bowel disease. Inflamm. Bowel Dis. 2011, 17, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Frazier, T.H.; Stocker, A.M.; Kershner, N.A.; Marsano, L.S.; McClain, C.J. Treatment of alcoholic liver disease. Ther. Adv. Gastroenterol. 2011, 4, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.X.; Li, M.; Chen, X.; Ni, H.M.; Lin, C.W.; Gao, W.; Lu, B.; Stolz, D.B.; Clemens, D.L.; Yin, X.M. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology 2010, 139, 1740–1752. [Google Scholar] [CrossRef] [PubMed]
- Diehl, A.M.; Chute, J. Underlying potential: Cellular and molecular determinants of adult liver repair. J. Clin. Investig. 2013, 123, 1858–1860. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.F.; Xu, X.R.; Zhang, Y.; Li, D.; Gan, R.Y.; Li, H.B. Phenolic compounds and bioactivities of pigmented rice. Crit. Rev. Food Sci. Nutr. 2013, 53, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xu, B.T.; Gan, R.Y.; Zhang, Y.; Xu, X.R.; Xia, E.Q.; Li, H.B. Total phenolic contents and antioxidant capacities of herbal and tea infusions. Int. J. Mol. Sci. 2011, 12, 2112–2124. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.F.; Xu, X.R.; Guo, Y.J.; Xia, E.Q.; Li, S.; Wu, S.; Chen, F.; Ling, W.H.; Li, H.B. Determination of antioxidant property and their lipophilic and hydrophilic phenolic contents in cereal grains. J. Funct. Foods 2012, 4, 906–914. [Google Scholar] [CrossRef]
- Deng, G.F.; Lin, X.; Xu, X.R.; Gao, L.L.; Xie, J.F.; Li, H.B. Antioxidant capacities and total phenolic contents of 56 vegetables. J. Funct. Foods 2013, 5, 260–266. [Google Scholar] [CrossRef]
- Fu, L.; Xu, B.T.; Xu, X.R.; Gan, R.Y.; Zhang, Y.; Xia, E.Q.; Li, H.B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef]
- Guo, Y.J.; Deng, G.F.; Xu, X.R.; Wu, S.; Li, S.; Xia, E.Q.; Li, F.; Chen, F.; Ling, W.H.; Li, H.B. Antioxidant capacities, phenolic compounds and polysaccharide contents of 49 edible macro-fungi. Food Funct. 2012, 3, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Cheng, K.W.; Wong, C.C.; Fan, K.W.; Chen, F.; Jiang, Y. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 2007, 102, 771–776. [Google Scholar] [CrossRef]
- Li, S.; Li, S.K.; Gan, R.Y.; Song, F.L.; Kuang, L.; Li, H.B. Antioxidant capacities and total phenolic contents of infusions from 223 medicinal plants. Ind. Crops Prod. 2013, 51, 289–298. [Google Scholar] [CrossRef]
- Li, A.N.; Li, S.; Li, H.B.; Xu, D.P.; Xu, X.R.; Chen, F. Total phenolic contents and antioxidant capacities of 51 edible and wild flowers. J. Funct. Foods 2014, 6, 319–330. [Google Scholar] [CrossRef]
- Augustyniak, A.; Waszkiewicz, E.; Skrzydlewska, E. Preventive action of green tea from changes in the liver antioxidant abilities of different aged rats intoxicated with ethanol. Nutrition 2005, 21, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Bourogaa, E.; Nciri, R.; Mezghani-Jarraya, R.; Racaud-Sultan, C.; Damak, M.; el Feki, A. Antioxidant activity and hepatoprotective potential of Hammada scoparia against ethanol-induced liver injury in rats. J. Physiol. Biochem. 2013, 69, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhu, P.; Jiang, C.; Ma, L.; Zhang, Z.; Zeng, X. Preliminary characterization, antioxidant activity in vitro and hepatoprotective effect on acute alcohol-induced liver injury in mice of polysaccharides from the peduncles of Hovenia dulcis. Food Chem. Toxicol. 2012, 50, 2964–2970. [Google Scholar] [CrossRef] [PubMed]
- Lopez, V.R.L.; Razzeto, G.S.; Gimenez, M.S.; Escudero, N.L. Antioxidant properties of Amaranthus hypochondriacus seeds and their effect on the liver of alcohol-treated rats. Plant Foods Hum. Nutr. 2011, 66, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Du, B.; Wang, Y.; Gao, H.; Cao, W.; Zheng, J.; Feng, F. Antioxidant properties of jujube honey and its protective effects against chronic alcohol-induced liver damage in mice. Food Funct. 2014, 5, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Mohd Ali, N.; Mohd Yusof, H.; Long, K.; Yeap, S.K.; Ho, W.Y.; Beh, B.K.; Koh, S.P.; Abdullah, M.P.; Alitheen, N.B. Antioxidant and hepatoprotective effect of aqueous extract of germinated and fermented mung bean on ethanol-mediated liver damage. BioMed Res. Int. 2013, 2013, 693613. [Google Scholar] [CrossRef] [PubMed]
- Kasdallah-Grissa, A.; Nakbi, A.; Koubaa, N.; El-Fazaa, S.; Gharbi, N.; Kamoun, A.; Hammami, M. Dietary virgin olive oil protects against lipid peroxidation and improves antioxidant status in the liver of rats chronically exposed to ethanol. Nutr. Res. 2008, 28, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Li, G.L.; Ye, Y.; Kang, J.J.; Yao, X.Y.; Zhang, Y.Z.; Jiang, W.; Gao, M.; Dai, Y.D.; Xin, Y.Q.; Wang, Q.; et al. l-Theanine prevents alcoholic liver injury through enhancing the antioxidant capability of hepatocytes. Food Chem. Toxicol. 2012, 50, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Shalini, S.; Bansal, M.P. Influence of vitamin E on alcohol-induced changes in antioxidant defenses in mice liver. Toxicol. Mech. Methods 2010, 20, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Altavilla, D.; Marini, H.; Seminara, P.; Squadrito, G.; Minutoli, L.; Passaniti, M.; Bitto, A.; Calapai, G.; Calo, M.; Caputi, A.P.; et al. Protective effects of antioxidant raxofelast in alcohol-induced liver disease in mice. Pharmacology 2005, 74, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Penumathsa, S.V.; Kode, A.; Rajagopalan, R.; Menon, V.P. Changes in activities of MMP in alcohol and thermally oxidized sunflower oil-induced liver damage: NAC antioxidant therapy. Toxicol. Mech. Methods 2006, 16, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Kim, S.J.; Kwon do, Y.; Ahn, C.W.; Kim, Y.S.; Choi, D.W.; Kim, Y.C. Alleviation of alcoholic liver injury by betaine involves an enhancement of antioxidant defense via regulation of sulfur amino acid metabolism. Food Chem. Toxicol. 2013, 62, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Xia, W.; Wu, J.; Yuan, L.; Wu, J.; Tu, D.; Fang, J.; Tan, Z. Betulinic acid prevents alcohol-induced liver damage by improving the antioxidant system in mice. J. Vet. Sci. 2014, 15, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Qiang, X.; Zhang, M.; Ma, D.; Zhao, Z.; Zhou, C.; Liu, X.; Li, R.; Chen, H.; Zhang, Y. Demethyleneberberine, a natural mitochondria-targeted antioxidant, inhibits mitochondrial dysfunction, oxidative stress, and steatosis in alcoholic liver disease mouse model. J. Pharmacol. Exp. Ther. 2015, 352, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Lirussi, F.; Azzalini, L.; Orando, S.; Orlando, R.; Angelico, F. Antioxidant supplements for non-alcoholic fatty liver disease and/or steatohepatitis. Cochrane Database Syst. Rev. 2007, 24, CD004996. [Google Scholar]
- Sattar, N.; Forrest, E.; Preiss, D. Non-alcoholic fatty liver disease. BMJ 2014, 349, 8. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Friso, S.; Choi, S.W. Epigenetic mechanisms underlying the link between non-alcoholic fatty liver diseases and nutrition. Nutrients 2014, 6, 3303–3325. [Google Scholar] [CrossRef] [PubMed]
- Carter-Kent, C.; Zein, N.N.; Feldstein, A.E. Cytokines in the pathogenesis of fatty liver and disease progression to steatohepatitis: Implications for treatment. Am. J. Gastroenterol. 2008, 103, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Are oxidative stress-activated signaling pathways mediators of insulin resistance and β-cell dysfunction? Diabetes 2003, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Sato, F.; Kawamori, R. Metabolic syndrome and NAFLD/NASH. Nihon Rinsho 2006, 64, 449–452. [Google Scholar] [PubMed]
- Bogdanova, K.; Poczatkova, H.; Uherkova, L.; Riegrova, D.; Rypka, M.; Feher, J.; Marchesini, G.; Vesely, J. Non-alcoholic fatty liver disease (NAFLD)—A novel common aspect of the metabolic syndrome. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2006, 150, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martinez, D.; Ruperez, F.J.; Ugarte, P.; Barbas, C. Tocopherol fate in plasma and liver of streptozotocin-treated rats that orally received antioxidants and Spirulina extracts. Int. J. Vitam. Nutr. Res. 2007, 77, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Naziroglu, M.; Butterworth, P.J.; Sonmez, T.T. Dietary vitamin C and E modulates antioxidant levels in blood, brain, liver, muscle, and testes in diabetic aged rats. Int. J. Vitam. Nutr. Res. 2011, 81, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Gezginci-Oktayoglu, S.; Basaraner, H.; Yanardag, R.; Bolkent, S. The effects of combined treatment of antioxidants on the liver injury in STZ diabetic rats. Dig. Dis. Sci. 2009, 54, 538–546. [Google Scholar] [CrossRef] [PubMed]
- De Assis, A.M.; Rech, A.; Longoni, A.; Rotta, L.N.; Denardin, C.C.; Pasquali, M.A.; Souza, D.O.; Perry, M.L.S.; Moreira, J.C. Omega 3-polyunsaturated fatty acids prevent lipoperoxidation, modulate antioxidant enzymes, and reduce lipid content but do not alter glycogen metabolism in the livers of diabetic rats fed on a high fat thermolyzed diet. Mol. Cell. Biochem. 2012, 361, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Li, T.P.; Liu, Y.H.; Dong, Y.P.; Li, S.H.; Zhu, R.G. Anti-fat deposition and antioxidant effects of haw pectic oligosaccharide in the liver of high-fat-fed mice. CyTA-J. Food 2014, 12, 27–31. [Google Scholar] [CrossRef]
- Akkol, E.K.; Avci, G.; Kucukkurt, I.; Keles, H.; Tamer, U.; Ince, S.; Yesilada, E. Cholesterol-reducer, antioxidant and liver protective effects of Thymbra spicata L. var. spicata. J. Ethnopharmacol. 2009, 126, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Sikder, K.; Ghosh, S.; Fromenty, B.; Dey, S. Moringa oleifera Lam. leaf extract prevents early liver injury and restores antioxidant status in mice fed with high-fat diet. Indian J. Exp. Biol. 2012, 50, 404–412. [Google Scholar] [PubMed]
- Olorunnisola, O.S.; Bradley, G.; Afolayan, A.J. Protective effect of Tulbaghia violacea Harv. on aortic pathology, tissue antioxidant enzymes and liver damage in diet-induced atherosclerotic rats. Int. J. Mol. Sci. 2012, 13, 12747–12760. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, W.; Wang, B.; Zhu, H.; Ye, L.; Feng, M. Antioxidant effect of apolipoprotein A–I on high-fat diet-induced non-alcoholic fatty liver disease in rabbits. Acta Biochim. Biophys. Sin. 2013, 45, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Melega, S.; Canistro, D.; de Nicola, G.R.; Lazzeri, L.; Sapone, A.; Paolini, M. Protective effect of Tuscan black cabbage sprout extract against serum lipid increase and perturbations of liver antioxidant and detoxifying enzymes in rats fed a high-fat diet. Br. J. Nutr. 2013, 110, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Guerra, J.F.; Magalhaes, C.L.; Costa, D.C.; Silva, M.E.; Pedrosa, M.L. Dietary acai modulates ROS production by neutrophils and gene expression of liver antioxidant enzymes in rats. J. Clin. Biochem. Nutr. 2011, 49, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Ma, Y.; Xing, X.; Huang, C.; Li, L.; Gui, G.; Liu, Q.; Xue, S. Antioxidant and hepatoprotective effect of different extracts of guizhencao (herba bidentis bipinnatae) against liver injury in hyperlipidemia rats. J. Tradit. Chin. Med. 2013, 33, 518–523. [Google Scholar] [CrossRef]
- Quine, S.D.; Raghu, P.S. Effects of (−)-epicatechin, a flavonoid on lipid peroxidation and antioxidants in streptozotocin-induced diabetic liver, kidney and heart. Pharmacol. Rep. 2005, 57, 610–615. [Google Scholar] [PubMed]
- Cumaoglu, A.; Cevik, C.; Rackova, L.; Ari, N.; Karasu, C. Effects of antioxidant stobadine on protein carbonylation, advanced oxidation protein products and reductive capacity of liver in streptozotocin-diabetic rats: Role of oxidative/nitrosative stress. Biofactors 2007, 30, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Njomen, G.B.; Kamgang, R.; Oyono, J.L.; Njikam, N. Antioxidant potential of the methanol-methylene chloride extract of Terminalia glaucescens leaves on mice liver in streptozotocin-induced stress. Indian J. Pharmacol. 2008, 40, 266–270. [Google Scholar] [PubMed]
- Zhou, J.Y.; Zhou, S.W. Protective effect of berberine on antioxidant enzymes and positive transcription elongation factor b expression in diabetic rat liver. Fitoterapia 2011, 82, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Ramachandraiahgari, R.M.Y.; Somesula, S.R.; Adi, P.J.; Mannur, I.S.; Enamala, M.; Matcha, B. Protective role of ethanolic extract of aloe vera antioxidant properties on liver and kidney of streptozotocin-induced diabetic rats. Dig. J. Nanomater. Biostruct. 2012, 7, 175–184. [Google Scholar]
- Lei, S.; Liu, Y.; Liu, H.; Yu, H.; Wang, H.; Xia, Z. Effects of N-acetylcysteine on nicotinamide dinucleotide phosphate oxidase activation and antioxidant status in heart, lung, liver and kidney in streptozotocin-induced diabetic rats. Yonsei Med. J. 2012, 53, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Kakkar, P. Modulation of liver function, antioxidant responses, insulin resistance and glucose transport by Oroxylum indicum stem bark in STZ induced diabetic rats. Food Chem. Toxicol. 2013, 62, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Mkhwanazi, B.N.; Serumula, M.R.; Myburg, R.B.; van Heerden, F.R.; Musabayane, C.T. Antioxidant effects of maslinic acid in livers, hearts and kidneys of streptozotocin-induced diabetic rats: Effects on kidney function. Ren. Fail. 2014, 36, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Sadi, G.; Bozan, D.; Yildiz, H.B. Redox regulation of antioxidant enzymes: Post-translational modulation of catalase and glutathione peroxidase activity by resveratrol in diabetic rat liver. Mol. Cell. Biochem. 2014, 393, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Ozbayer, C.; Degirmenci, I.; Kurt, H.; Ozden, H.; Civi, K.; Basaran, A.; Gunes, H.V. Antioxidant and free radical-scavenging properties of Stevia rebaudiana (Bertoni) extracts and L-NNA in streptozotocine-nicotinamide induced diabetic rat liver. Turk. Klin. Tip Bilim. Derg. 2011, 31, 51–60. [Google Scholar] [CrossRef]
- Ye, X.; Feng, Y.; Tong, Y.; Ng, K.M.; Tsao, S.; Lau, G.K.; Sze, C.; Zhang, Y.; Tang, J.; Shen, J.; et al. Hepatoprotective effects of Coptidis rhizoma aqueous extract on carbon tetrachloride-induced acute liver hepatotoxicity in rats. J. Ethnopharmacol. 2009, 124, 130–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, F.; Feng, Y.B.; Wang, N.; Yuen, M.F.; Tong, Y.; Wong, V.T. Effectiveness of Chinese herbal medicine in treating liver fibrosis: A systematic review and meta-analysis of randomized controlled trials. Chin. Med. 2012, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasool, M.K.; Sabina, E.P.; Ramya, S.R.; Preety, P.; Patel, S.; Mandal, N.; Mishra, P.P.; Samuel, J. Hepatoprotective and antioxidant effects of gallic acid in paracetamol-induced liver damage in mice. J. Pharmacy Pharmacol. 2010, 62, 638–643. [Google Scholar] [CrossRef]
- Kay, H.Y.; Kim, Y.W.; Ryu, D.H.; Sung, S.H.; Hwang, S.J.; Kim, S.G. Nrf2-mediated liver protection by sauchinone, an antioxidant lignan, from acetaminophen toxicity through the PKC δ-GSK3 β pathway. Br. J. Pharmacol. 2011, 163, 1653–1665. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.J.; Rong, Y.; Li, P.F.; Dong, W.L.; Zhang, D.Y.; Zhang, L.; Cui, M.J. Genistein protection against acetaminophen-induced liver injury via its potential impact on the activation of UDP-glucuronosyltransferase and antioxidant enzymes. Food Chem. Toxicol. 2013, 55, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Sabir, S.M.; Rocha, J.B.T. Water-extractable phytochemicals from Phyllanthus niruri exhibit distinct in vitro antioxidant and in vivo hepatoprotective activity against paracetamol-induced liver damage in mice. Food Chem. 2008, 111, 845–851. [Google Scholar] [CrossRef]
- Jothy, S.L.; Aziz, A.; Chen, Y.; Sasidharan, S. Antioxidant activity and hepatoprotective potential of polyalthia longifolia and cassia spectabilis leaves against paracetamol-induced liver injury. Evid.-Based Complement. Altern. Med. 2012, 2012, 561284. [Google Scholar] [CrossRef] [PubMed]
- Olaleye, M.T.; Akinmoladun, A.C.; Ogunboye, A.A.; Akindahunsi, A.A. Antioxidant activity and hepatoprotective property of leaf extracts of Boerhaavia diffusa Linn against acetaminophen-induced liver damage in rats. Food Chem. Toxicol. 2010, 48, 2200–2205. [Google Scholar] [CrossRef] [PubMed]
- Simeonova, R.; Vitcheva, V.; Kondeva-Burdina, M.; Krasteva, I.; Manov, V.; Mitcheva, M. Hepatoprotective and antioxidant effects of saponarin, isolated from Gypsophila trichotoma Wend. on paracetamol-induced liver damage in rats. BioMed Res. Int. 2013, 2013, 757126. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Liu, Z.; Wang, Y.; Xiao, H.; Wu, W.; Xiao, C.; Liu, X. Carnosic acid attenuates lipopolysaccharide-induced liver injury in rats via fortifying cellular antioxidant defense system. Food Chem. Toxicol. 2013, 53, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Catal, T.; Bolkent, S. Combination of selenium and three naturally occurring antioxidants administration protects d-galactosamine-induced liver injury in rats. Biol. Trace Elem. Res. 2008, 122, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Banu, S.; Bhaskar, B.; Balasekar, P. Hepatoprotective and antioxidant activity of Leucas aspera against d-galactosamine induced liver damage in rats. Pharm. Biol. 2012, 50, 1592–1595. [Google Scholar] [CrossRef] [PubMed]
- Jaishree, V.; Badami, S.; Krishnamurthy, P.T. Antioxidant and hepatoprotective effect of the ethyl acetate extract of Enicostemma axillare (Lam). Raynal against CCl4-induced liver injury in rats. Indian J. Exp. Biol. 2010, 48, 896–904. [Google Scholar] [PubMed]
- Cerny, D.; Lekic, N.; Vanova, K.; Muchova, L.; Horinek, A.; Kmonickova, E.; Zidek, Z.; Kamenikova, L.; Farghali, H. Hepatoprotective effect of curcumin in lipopolysaccharide/-galactosamine model of liver injury in rats: Relationship to HO-1/CO antioxidant system. Fitoterapia 2011, 82, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.-W.; Song, S.-Z.; Wu, Y.-L.; Lian, L.-H.; Wan, Y.; Nan, J.-X. Betulinic acid prevention of d-galactosamine/lipopolysaccharide liver toxicity is triggered by activation of Bcl-2 and antioxidant mechanisms. J. Pharm. Pharmacol. 2011, 63, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, V.; Shivashangari, K.S.; Devaki, T. Effect of Tridax procumbens on liver antioxidant defense system during lipopolysaccharide-induced hepatitis in d-galactosamine sensitised rats. Mol. Cell. Biochem. 2005, 269, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Kockar, M.C.; Naziroglu, M.; Celik, O.; Tola, H.T.; Bayram, D.; Koyu, A. N-Acetylcysteine modulates doxorubicin-induced oxidative stress and antioxidant vitamin concentrations in liver of rats. Cell Biochem. Funct. 2010, 28, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Avci, A.; Cetin, R.; Erguder, I.B.; Devrim, E.; Kilicoglu, B.; Candir, O.; Ozturk, H.S.; Durak, I. Cisplatin causes oxidation in rat liver tissues: Possible protective effects of antioxidant food supplementation. Turk. J. Med. Sci. 2008, 38, 117–120. [Google Scholar]
- Wang, B.J.; Lien, Y.H.; Su, C.L.; Wu, C.P.; Yu, Z.R. Fractionation using supercritical CO2 influences the antioxidant and hepatoprotective activity of propolis against liver damage induced by tert-butyl hydroperoxide. Int. J. Food Sci. Technol. 2006, 41, 68–75. [Google Scholar] [CrossRef]
- Tabassum, H.; Parvez, S.; Rehman, H.; Banerjee, B.D.; Raisuddin, S. Catechin as an antioxidant in liver mitochondrial toxicity: Inhibition of tamoxifen-induced protein oxidation and lipid peroxidation. J. Biochem. Mol. Toxicol. 2007, 21, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kang, H.S.; Lee, J.H.; Park, J.H.; Jung, C.H.; Bae, J.H.; Oh, B.C.; Song, D.K.; Baek, W.K.; Im, S.S. Melatonin ameliorates ER stress-mediated hepatic steatosis through miR-23a in the liver. Biochem. Biophys. Res. Commun. 2015, 458, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, S.M.; Lopez-Ortega, A. Antioxidant activity of melatonin on fatty liver induced by ethionine in mice. Arch. Med. Vet. 2008, 40, 51–57. [Google Scholar]
- Feng, Y.; Wang, N.; Tong, Y.; Tsao, S. Berberine: An old drug but new use for liver diseases. Planta Medica 2012, 78, 1091–1091. [Google Scholar] [CrossRef]
- Feng, Y.B.; Wang, N.; Zhu, M.F.; Zhang, Z.J.; Tong, Y.; Tsao, S. Interdisciplinary approaches in study of Chinese medicines: Case of coptis. Int. J. Mol. Med. 2010, 26, S21–S21. [Google Scholar]
- Hwang, Y.P.; Choi, J.H.; Yun, H.J.; Han, E.H.; Kim, H.G.; Kim, J.Y.; Park, B.H.; Khanal, T.; Choi, J.M.; Chung, Y.C.; et al. Anthocyanins from purple sweet potato attenuate dimethylnitrosamine-induced liver injury in rats by inducing Nrf2-mediated antioxidant enzymes and reducing COX-2 and iNOS expression. Food Chem. Toxicol. 2011, 49, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Lawal, A.O.; Lawal, A.F.; Ologundudu, A.; Adeniran, O.Y.; Omonkhua, A.; Obi, F. Antioxidant effects of heated garlic juice on cadmium-induced liver damage in rats as compared to ascorbic acid. J. Toxicol. Sci. 2011, 36, 549–557. [Google Scholar] [CrossRef]
- Choi, J.H.; Jin, S.W.; Kim, H.G.; Khanal, T.; Hwang, Y.P.; Lee, K.J.; Choi, C.Y.; Chung, Y.C.; Lee, Y.C.; Jeong, H.G. Platycodi Radix attenuates dimethylnitrosamine-induced liver fibrosis in rats by inducing Nrf2-mediated antioxidant enzymes. Food Chem. Toxicol. 2013, 56, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Niture, S.K.; Jain, A.K.; Jaiswal, A.K. Antioxidant-induced modification of INrf2 cysteine 151 and PKC-δ-mediated phosphorylation of Nrf2 serine 40 are both required for stabilization and nuclear translocation of Nrf2 and increased drug resistance. J. Cell Sci. 2009, 122, 4452–4464. [Google Scholar] [CrossRef] [PubMed]
- Cheung, F.; Wang, X.B.; Wang, N.; Yuen, M.F.; Ziea, T.C.; Tong, Y.; Wong, V.T.; Feng, Y.B. Chinese medicines as an adjuvant therapy for unresectable Hepatocellular carcinoma during transarterial chemoembolization: A meta-analysis of randomized controlled trials. Evid.-Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Feng, Y.B. Elaborating the role of natural products-induced autophagy in cancer treatment: Achievements and artifacts in the state of the art. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.Y.; Wang, N.; Tsao, S.W.; Zhang, Z.J.; Feng, Y.B. Suppression of vascular endothelial growth factor via inactivation of eukaryotic elongation factor 2 by alkaloids in coptidis rhizome in Hepatocellular carcinoma. Integr. Cancer Ther. 2014, 13, 425–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.W.; Li, B.; Lai, E.T.; Chen, L.; Huang, J.J.; Cheung, A.L.; Cheung, P.C. Water extract from Pleurotus pulmonarius with antioxidant activity exerts in vivo chemoprophylaxis and chemosensitization for liver cancer. Nutr. Cancer 2014, 66, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Feng, Y.B.; Zhu, M.F.; Tsang, C.M.; Man, K.; Tong, Y.; Tsao, S.W. Berberine induces autophagic cell death and mitochondrial apoptosis in liver cancer cells: The cellular mechanism. J. Cell. Biochem. 2010, 111, 1426–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Zhu, M.F.; Wang, X.B.; Tan, H.Y.; Tsao, S.W.; Feng, Y.B. Berberine-induced tumor suppressor p53 up-regulation gets involved in the regulatory network of MIR-23a in Hepatocellular carcinoma. Biochim. Biophys. Acta 2014, 1839, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Tanaka, Y.; Asagiri, K.; Asakawa, T.; Tanikawa, K.; Kage, M.; Yagi, M. The antioxidant effect of green tea catechin ameliorates experimental liver injury. Phytomedicine 2010, 17, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Spahr, L.; Bresson-Hadni, S.; Amann, P.; Kern, I.; Golaz, O.; Frossard, J.L.; Hadengue, A. Allopurinol, oxidative stress and intestinal permeability in patients with cirrhosis: An open-label pilot study. Liver Int. 2007, 27, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Demirel, U.; Yalniz, M.; Aygun, C.; Orhan, C.; Tuzcu, M.; Sahin, K.; Ozercan, I.H.; Bahcecioglu, I.H. Allopurinol ameliorates thioacetamide-induced acute liver failure by regulating cellular redox-sensitive transcription factors in rats. Inflammation 2012, 35, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Karaaslan, C.; Suzen, S. Antioxidant properties of melatonin and its potential action in diseases. Curr. Top. Med. Chem. 2015, 15, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Wahab, M.H.; Akoul, E.S.; Abdel-Aziz, A.A. Modulatory effects of melatonin and vitamin E on doxorubicin-induced cardiotoxicity in Ehrlich ascites carcinoma-bearing mice. Tumori 2000, 86, 157–162. [Google Scholar] [PubMed]
- Baydas, G.; Canatan, H.; Turkoglu, A. Comparative analysis of the protective effects of melatonin and vitamin E on streptozocin-induced diabetes mellitus. J. Pineal Res. 2002, 32, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Qiu, A.; Guan, J.; Shi, Z. Antioxidant and protective effect of an oleanolic acid-enriched extract of A. deliciosa root on carbon tetrachloride induced rat liver injury. Asia Pac. J. Clin. Nutr. 2007, 16, 169–173. [Google Scholar] [PubMed]
- Shaker, E.; Mahmoud, H.; Mnaa, S. Silymarin, the antioxidant component and Silybum marianum extracts prevent liver damage. Food Chem. Toxicol. 2010, 48, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Iliemene, U.D.; Atawodi, S.E.O. In vivo antioxidant and hepatoprotective effects of methanolic extract of dioclea reflexa seed in rats following acute or chronic liver injury. Bangladesh J. Pharmacol. 2014, 9, 112–117. [Google Scholar] [CrossRef]
- Jin, Y.S.; Lee, M.J.; Han, W.; Heo, S.I.; Sohn, S.I.; Wang, M.H. Antioxidant effects and hepatoprotective activity of 2,5-dihydroxy-4,3′-di(β-d-glucopyranosyloxy)-trans-stilbene Morus bombycis Koidzumi roots on CCl4-induced liver damage. Free Radic. Res. 2006, 40, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.S.; Sa, J.H.; Shim, T.H.; Rhee, H.I.; Wang, M.H. Hepatoprotective and antioxidant effects of Morus bombycis Koidzumi on CCl4-induced liver damage. Biochem. Biophs. Res. Commun. 2005, 329, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Kanter, M.; Coskun, O.; Budancamanak, M. Hepatoprotective effects of Nigella sativa L and Urtica dioica L on lipid peroxidation, antioxidant enzyme systems and liver enzymes in carbon tetrachloride-treated rats. World J. Gastroenterol. 2005, 11, 6684–6688. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, T.; Ramesh, E.; Geraldine, P. Antioxidant activity of the oyster mushroom, Pleurotus ostreatus, on CCl4-induced liver injury in rats. Food Chem. Toxicol. 2006, 44, 1989–1996. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.; Ahamed, K.F.H.N.; Kumar, V.; Mukherjee, K.; Bandyopadhyay, A.; Mukherjee, P.K. Antioxidant effect of Cytisus scoparius against carbon tetrachloride treated liver injury in rats. J. Ethnopharmacol. 2007, 109, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Y.; Peng, W.H.; Sheu, M.J.; Huang, G.J.; Tseng, M.C.; Lai, M.T.; Ho, Y.L.; Chang, Y.S. Hepatoprotective and antioxidant effects of ethanol extract from Phellinus merrillii on carbon tetrachloride-induced liver damage. Am. J. Chin. Med. 2007, 35, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.R.; Panda, V.S. Hepatoprotective effect of Ginkgoselect Phytosome® in rifampicin induced liver injurym in rats: Evidence of antioxidant activity. Fitoterapia 2008, 79, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, R.; Sil, P.C. Protein isolate from the herb, Phyllanthus niruri L. (Euphorblaceae), plays hepatoprotective role against carbon tetrachloride induced liver damage via its antioxidant properties. Food Chem. Toxicol. 2007, 45, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Choi, J.H.; Jeong, H.G. Hepatoprotective and antioxidant effects of the coffee diterpenes kahweol and cafestol on carbon tetrachloride-induced liver damage in mice. Food Chem. Toxicol. 2007, 45, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Heo, S.I.; Li, L.; Lee, M.J.; Wang, M.H. Antioxidant and hepatoprotective activities of Cirsium setidens Nakai against CCl4-induced liver damage. Am. J. Chin. Med. 2008, 36, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Lin, Y.H.; Chu, C.C.; Tsai, Y.H.; Chao, J.C.J. Curcumin or saikosaponin a improves hepatic antioxidant capacity and protects against CCl4-induced liver injury in rats. J. Med. Food 2008, 11, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Deval, R.G.; Lakshmayya; Ramachandra, S.S. Antioxidant and hepatoprotective activity of tubers of Momordica tuberosa Cogn. against CCl4 induced liver injury in rats. Indian J. Exp. Biol. 2008, 46, 510–513. [Google Scholar] [PubMed]
- Botsoglou, N.A.; Taitzoglou, I.A.; Botsoglou, E.; Zervos, I.; Kokoli, A.; Christaki, E.; Nikolaidis, E. Effect of long-term dietary administration of oregano and rosemary on the antioxidant status of rat serum, liver, kidney and heart after carbon tetrachloride-induced oxidative stress. J. Sci. Food Agric. 2009, 89, 1397–1406. [Google Scholar] [CrossRef]
- Singab, A.N.B.; Ayoub, N.A.; Ali, E.N.; Mostafa, N.M. Antioxidant and hepatoprotective activities of Egyptian moraceous plants against carbon tetrachloride-induced oxidative stress and liver damage in rats. Pharm. Biol. 2010, 48, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Ganie, S.A.; Haq, E.; Masood, A.; Hamid, A.; Zargar, M.A. Antioxidant and protective effect of ethyl acetate extract of Podophyllum hexandrum Rhizome on carbon tetrachloride induced rat liver injury. Evid.-Based Complement. Altern. Med. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Gupta, M.; Sharma, S.K. Evaluation of antioxidant potential of Ficus religiosa (Linn.) roots against carbon tetrachloride-induced liver injury. J. Med. Plants Res. 2011, 5, 1582–1588. [Google Scholar]
- Paramesha, M.; Ramesh, C.K.; Krishna, V.; Kumar, Y.S.R.; Parvathi, K.M.M. Hepatoprotective and in vitro antioxidant effect of Carthamus tinctorious L, var Annigeri-2-, an oil-yielding crop, against CCl4-induced liver injury in rats. Pharmacogn. Mag. 2011, 7, 289–297. [Google Scholar] [PubMed]
- Sridevi, V.K.; Chouhan, H.S.; Singh, N.K.; Singh, S.K. Antioxidant and hepatoprotective effects of ethanol extract of Vitex glabrata on carbon tetrachloride-induced liver damage in rats. Nat. Prod. Res. 2012, 26, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, H.N.; Sun, A.D.; Lin, Q.H.; Wang, Y.; Tao, X.Y. Studies of the protective effect and antioxidant mechanism of blueberry anthocyanins in a CC14-induced liver injury model in mice. Food Agric. Immunol. 2012, 23, 352–362. [Google Scholar] [CrossRef]
- Aksoy, L.; Sozbilir, N.B. Effects of Matricaria chamomilla L. on lipid peroxidation, antioxidant enzyme systems, and key liver enzymes in CCl4-treated rats. Toxicol. Environ. Chem. 2012, 94, 1780–1788. [Google Scholar] [CrossRef]
- Wei, J.F.; Li, Y.Y.; Yin, Z.H.; Gong, F.; Shang, F.D. Antioxidant activities in vitro and hepatoprotective effects of Lysimachia clethroides Duby on CCl4-induced acute liver injury in mice. Afr. J. Pharm. Pharmacol. 2012, 6, 743–750. [Google Scholar]
- Panda, V.S.; Ashar, H.D. Antioxidant and hepatoprotective effects of Garcinia indica choisy fruits in carbon tetrachloride-induced liver injury in rats. J. Food Biochem. 2012, 36, 240–247. [Google Scholar] [CrossRef]
- Al-Dbass, A.M.; Al-Daihan, S.K.; Bhat, R.S. Agaricus blazei Murill as an efficient hepatoprotective and antioxidant agent against CCl4-induced liver injury in rats. Saudi J. Biol. Sci. 2012, 19, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Singhal, K.G.; das Gupta, G. Hepatoprotective and antioxidant activity of methanolic extract of flowers of Nerium oleander against CCl4-induced liver injury in rats. Asian Pac. J. Trop. Med. 2012, 5, 677–685. [Google Scholar] [CrossRef]
- Vuda, M.; D’Souza, R.; Upadhya, S.; Kumar, V.; Rao, N.; Kumar, V.; Boillat, C.; Mungli, P. Hepatoprotective and antioxidant activity of aqueous extract of Hybanthus enneaspermus against CCl4-induced liver injury in rats. Exp. Toxicol. Pathol. 2012, 64, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.L.; Zhang, R.F.; Zhang, M.W.; Su, D.X.; Wei, Z.C.; Deng, Y.Y.; Zhang, Y.; Chi, J.W.; Tang, X.J. Hepatoprotective and antioxidant activity of anthocyanins in black rice bran on carbon tetrachloride-induced liver injury in mice. J. Funct. Foods 2013, 5, 1705–1713. [Google Scholar] [CrossRef]
- Kalegari, M.; Gemin, C.A.; Araujo-Silva, G.; Brito, N.J.; Lopez, J.A.; Tozetto Sde, O.; Almeida, M.; Miguel, M.D.; Stien, D.; Miguel, O.G. Chemical composition, antioxidant activity and hepatoprotective potential of Rourea induta Planch. (Connaraceae) against CCl4-induced liver injury in female rats. Nutrition 2014, 30, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Dai, N.; Zou, Y.; Zhu, L.; Wang, H.F.; Dai, M.G. Antioxidant properties of proanthocyanidins attenuate carbon tetrachloride (CCl4)-induced steatosis and liver injury in rats via CYP2E1 regulation. J. Med. Food 2014, 17, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wei, L.; Fu, R.; Ding, L.; Guo, Y.; Tang, L.; Chen, F. Antioxidant and hepatoprotective activity of Veronica ciliata Fisch. extracts against carbon tetrachloride-induced liver injury in mice. Molecules 2014, 19, 7223–7236. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.T.; El-Shitany, N.A.; Shaala, L.A.; Ali, S.S.; Azhar, E.I.; Abdel-Dayem, U.A.; Youssef, D.T. Red sea Suberea mollis sponge extract protects against CCl4-induced acute liver injury in rats via an antioxidant mechanism. Evid.-Based Complement. Altern. Med. 2014, 2014, 745606. [Google Scholar] [CrossRef] [PubMed]
- Jalali Ghassam, B.; Ghaffari, H.; Prakash, H.S.; Kini, K.R. Antioxidant and hepatoprotective effects of Solanum xanthocarpum leaf extracts against CCl4-induced liver injury in rats. Pharm. Biol. 2014, 52, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Meera, R.; Devi, P.; Kameswari, B.; Madhumitha, B.; Merlin, N.J. Antioxidant and hepatoprotective activities of Ocimum basilicum Linn. and Trigonella foenum-graecum Linn. against H2O2 and CCl4 induced hepatotoxicity in goat liver. Indian J. Exp. Biol. 2009, 47, 584–590. [Google Scholar] [PubMed]
- Saleh, D.O.; Abdel Jaleel, G.A.; El-Awdan, S.A.; Oraby, F.; Badawi, M. Thioacetamide-induced liver injury: Protective role of genistein. Can. J. Physiol. Pharmacol. 2014, 92, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Abdulaziz Bardi, D.; Halabi, M.F.; Hassandarvish, P.; Rouhollahi, E.; Paydar, M.; Moghadamtousi, S.Z.; Al-Wajeeh, N.S.; Ablat, A.; Abdullah, N.A.; Abdulla, M.A. Andrographis paniculata leaf extract prevents thioacetamide-induced liver cirrhosis in rats. PLoS ONE 2014, 9, e109424. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, A.H.; Ali, E.M.; Moselhey, S.S.; Tousson, E.; El-Said, K.S. Effect of coriander on thioacetamide-induced hepatotoxicity in rats. Toxicol. Ind. Health 2014, 30, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.Y.; Lee, T.Y.; Huang, T.H.; Wen, C.K.; Chien, R.N.; Chang, H.H. Hepatoprotective effects of Ger-Gen-Chyn-Lian-Tang in thioacetamide-induced fibrosis in mice. J. Chin. Med. Assoc. 2014, 77, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Zargar, S. Protective effect of Trigonella foenum-graecum on thioacetamide induced hepatotoxicity in rats. Saudi J. Biol. Sci. 2014, 21, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, T.; Sureka, C.; Bhuvana, S.; Hazeena Begum, V. Sesbania grandiflora diminishes oxidative stress and ameliorates antioxidant capacity in liver and kidney of rats exposed to cigarette smoke. J. Physiol. Pharmacol. 2010, 61, 467–476. [Google Scholar] [PubMed]
- Ozkan, A.; Fiskin, K.; Ayhan, A.G. Effect of vitamin E and selenium on antioxidant enzymes in brain, kidney and liver of cigarette smoke-exposed mice. Biologia 2007, 62, 360–364. [Google Scholar] [CrossRef]
- Singh, S.; Mondal, P.; Trigun, S.K. Acute liver failure in rats activates glutamine-glutamate cycle but declines antioxidant enzymes to induce oxidative stress in cerebral cortex and cerebellum. PLoS ONE 2014, 9, e95855. [Google Scholar] [CrossRef] [PubMed]
- Sutcu, R.; Altuntas, I.; Yildirim, B.; Karahan, N.; Demirin, H.; Delibas, N. The effects of subchronic methidathion toxicity on rat liver: Role of antioxidant vitamins C and E. Cell biol. Toxicol. 2006, 22, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.M. Protective effect of black tea extract on the levels of lipid peroxidation and antioxidant enzymes in liver of mice with pesticide-induced liver injury. Cell Biochem. Funct. 2006, 24, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Banudevi, S.; Krishnamoorthy, G.; Venkataraman, P.; Vignesh, C.; Aruldhas, M.M.; Arunakaran, J. Role of α-tocopherol on antioxidant status in liver, lung and kidney of PCB exposed male albino rats. Food Chem. Toxicol. 2006, 44, 2040–2046. [Google Scholar] [CrossRef] [PubMed]
- Yener, Z.; Celik, I.; Ilhan, F.; Bal, R. Effects of Urtica dioica L. seed on lipid peroxidation, antioxidants and liver pathology in aflatoxin-induced tissue injury in rats. Food Chem. Toxicol. 2009, 47, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Yogalakshmi, B.; Viswanathan, P.; Anuradha, C.V. Investigation of antioxidant, anti-inflammatory and DNA-protective properties of eugenol in thioacetamide-induced liver injury in rats. Toxicology 2010, 268, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Khaki, A.A.; Khaki, A. Antioxidant effect of ginger to prevents lead-induced liver tissue apoptosis in rat. J. Med. Plants Res. 2010, 4, 1492–1495. [Google Scholar]
- Khan, R.A.; Khan, M.R.; Sahreen, S.; Shah, N.A.; Khan, A.M.; Khan, Y.M.; Bokhari, J.; Rashid, U.; Shabbir, B.A.M.; Saeed, N.; et al. Effect of various fractions of Launaea procumbens on antioxidant enzymes in rats liver: Oxidative stress induced by potassium bromate (KBrO3). Afr. J. Pharm. Pharmacol. 2012, 6, 512–515. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Gao, L.; Cheng, Y.Y.; Jiang, J.; Chen, Y.; Jiang, H.J.; Yu, H.X.; Shan, A.S.; Cheng, B.J. Resveratrol, a natural antioxidant, has a protective effect on liver injury induced by inorganic arsenic exposure. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.; Chattopadhyay, S.; Acharyya, N.; Deb, B.; Hati, A.K. Emblica officinalis (amla) ameliorates arsenic-induced liver damage via DNA protection by antioxidant systems. Mol. Cell. Toxicol. 2014, 10, 75–82. [Google Scholar] [CrossRef]
- El Arem, A.; Saafi, E.B.; Ghrairi, F.; Thouri, A.; Zekri, M.; Ayed, A.; Zakhama, A.; Achour, L. Aqueous date fruit extract protects against lipid peroxidation and improves antioxidant status in the liver of rats subchronically exposed to trichloroacetic acid. J. Physiol. Biochem. 2014, 70, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.J.; Santhi, R.J. Antioxidant and cytotoxic effects of hexane extract of Morinda pubescens leaves in human liver cancer cell line. Asian Pac. J. Trop. Med. 2012, 5, 362–366. [Google Scholar] [CrossRef]
- Mukti, N.A.; Sulaiman, S.; Saad, S.M.; Basari, J.M.H.; Rahman, M.A.; Ngah, W.Z.W.; Yusof, Y.A.M. Chlorella vulgaris exhibited antioxidant and antitumour effects against liver cancer in in vivo and in vitro studies. Sains Malays. 2009, 38, 773–784. [Google Scholar]
- Gupta, M.; Mazumder, U.K.; Kumar, R.S.; Sivakumar, T.; Gomathi, P.; Rajeshwar, Y. Antioxidant defense system induced by a methanol extract of Caesalpinia bonducella in rat liver. Pharm. Biol. 2005, 43, 411–419. [Google Scholar] [CrossRef]
- Serviddio, G.; Bellanti, F.; Stanca, E.; Lunetti, P.; Blonda, M.; Tamborra, R.; Siculella, L.; Vendemiale, G.; Capobianco, L.; Giudetti, A.M. Silybin exerts antioxidant effects and induces mitochondrial biogenesis in liver of rat with secondary biliary cirrhosis. Free Radic. Biol. Med. 2014, 73, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.K.; Zhang, C.X.; Zhao, S.S.; Zhang, S.H.; Zhang, H.; Cai, S.Y.; Shao, R.G.; He, H.W. The anti-fibrotic effects of epigallocatechin-3-gallate in bile duct-ligated cholestatic rats and human hepatic stellate LX-2 cells are mediated by the PI3K/Akt/Smad pathway. Acta Pharmacol. Sin. 2015, 36, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, S.R. Anti-fibrotic effect of Holothuria arenicola extract against bile duct ligation in rats. BMC Complement. Altern Med. 2015, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.F.; Zakaria, S.; Fahmy, A. Aqueous garlic extract alleviates liver fibrosis and renal dysfunction in bile-duct-ligated rats. Z. Naturforsch. C 2014, 69, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Oguz, S.; Kanter, M.; Erboga, M.; Erenoglu, C. Protective effects of thymoquinone against cholestatic oxidative stress and hepatic damage after biliary obstruction in rats. J. Mol. Histol. 2012, 43, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Moreno, M.; Favari, L.; Muriel, P. Antifibrotic and antioxidant effects of N-acetylcysteine in an experimental cholestatic model. Eur. J. Gastroenterol. Hepatol. 2012, 24, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Fursule, R.A.; Patil, S.D. Hepatoprotective and antioxidant activity of Phaseolus trilobus, Ait on bile duct ligation induced liver fibrosis in rats. J. Ethnopharmacol. 2010, 129, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Kongo, M.; Kishikawa, T. Melatonin exerts a therapeutic effect on cholestatic liver injury in rats with bile duct ligation. J. Pineal Res. 2003, 34, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Kireev, R.; Bitoun, S.; Cuesta, S.; Tejerina, A.; Ibarrola, C.; Moreno, E.; Vara, E.; Tresguerres, J.A. Melatonin treatment protects liver of Zucker rats after ischemia/reperfusion by diminishing oxidative stress and apoptosis. Eur. J. Pharmacol. 2013, 701, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Aldaba-Muruato, L.R.; Moreno, M.G.; Hernandez-Mercado, E.; Shibayama, M.; Muriel, P. Secondary biliary cirrhosis in the rat is prevented by decreasing NF-κB nuclear translocation and TGF-β expression using allopurinol, an inhibitor of xanthine oxidase. Can. J. Physiol. Pharmacol. 2012, 90, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Lee, J.S.; Lee, J.S.; Han, J.M.; Son, C.G. Hepatoprotective and antioxidant effects of Myelophil on restraint stress-induced liver injury in BALB/c mice. J. Ethnopharmacol. 2012, 142, 113–120. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087-26124. https://doi.org/10.3390/ijms161125942
Li S, Tan H-Y, Wang N, Zhang Z-J, Lao L, Wong C-W, Feng Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. International Journal of Molecular Sciences. 2015; 16(11):26087-26124. https://doi.org/10.3390/ijms161125942
Chicago/Turabian StyleLi, Sha, Hor-Yue Tan, Ning Wang, Zhang-Jin Zhang, Lixing Lao, Chi-Woon Wong, and Yibin Feng. 2015. "The Role of Oxidative Stress and Antioxidants in Liver Diseases" International Journal of Molecular Sciences 16, no. 11: 26087-26124. https://doi.org/10.3390/ijms161125942