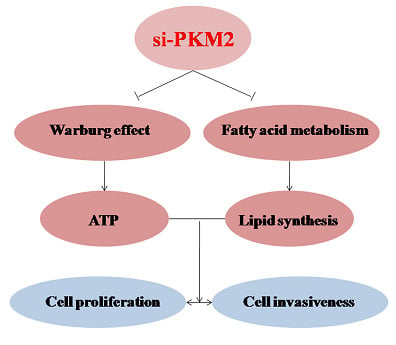
Knockdown of PKM2 Suppresses Tumor Growth and Invasion in Lung Adenocarcinoma
Abstract
:1. Introduction
2. Results
2.1. Correlation between PKM2 Expression and the Clinicopathological Features of Lung Adenocarcinoma
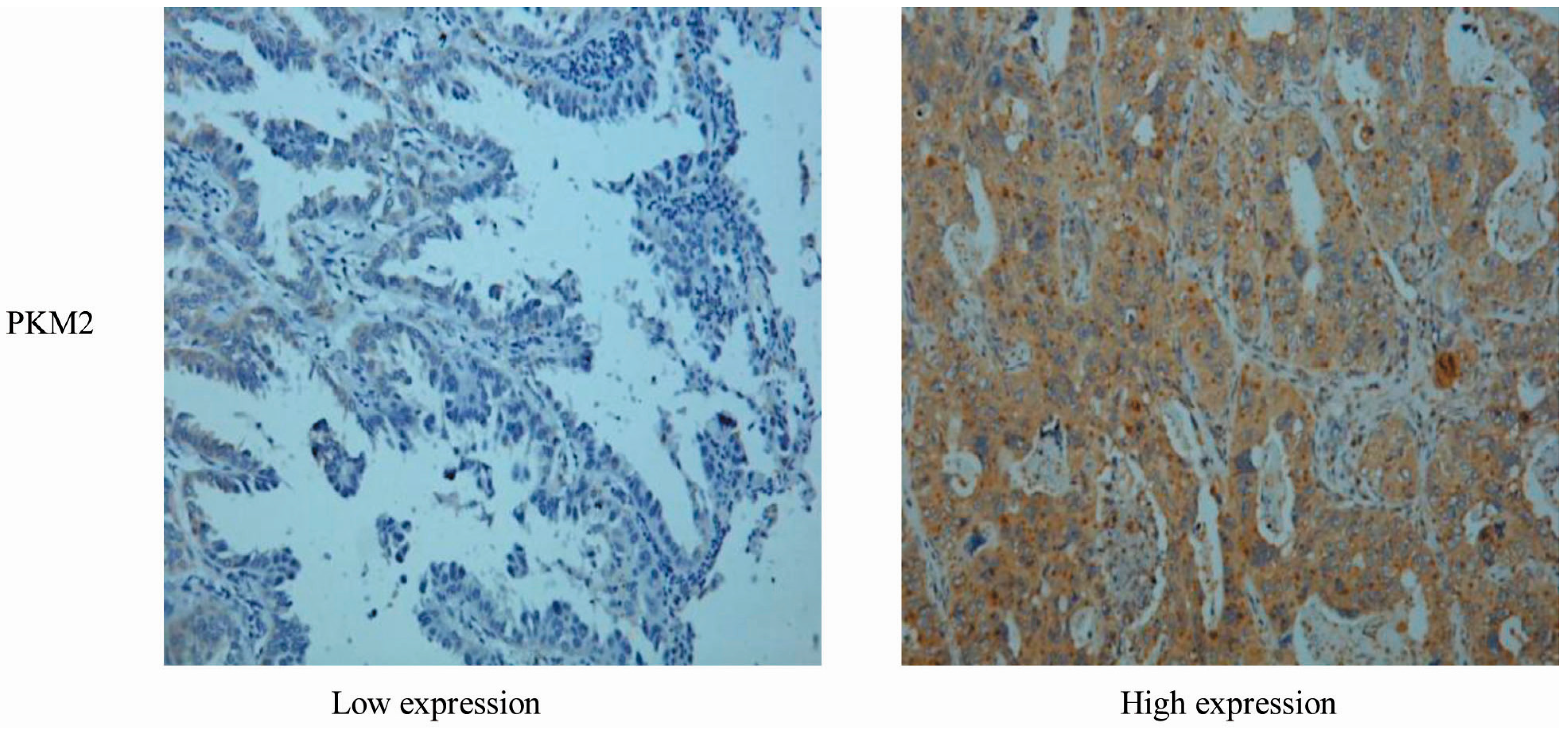
| Characteristic | PKM2 (IHC Score) | ||
|---|---|---|---|
| No. of Patients(n = 65) | |||
| Negative (37) | Positive (28) | p-Value | |
| Age | |||
| <60 years | 18 | 12 | 0.643 |
| ≥60 years | 19 | 16 | |
| Gender | |||
| Male | 23 | 17 | 0.905 |
| Female | 14 | 11 | |
| Tumor Differentiation | |||
| Well | 25 | 10 | 0.011 |
| Poor | 12 | 18 | |
| Tumor Size (cm) | |||
| ≤3 | 25 | 13 | 0.087 |
| >3 | 12 | 15 | |
| Pathological N Stage | |||
| N0 | 23 | 8 | 0.007 |
| N1 | 14 | 20 | |
| Metastasis | |||
| M0 | 24 | 10 | 0.020 |
| M1 | 13 | 18 | |

2.2. Tumor Growth Was Inhibited by PKM2 Gene Silencing in A549 Cells

2.3. PKM2-Knockdown Inhibited the Warburg Effect in Tumors

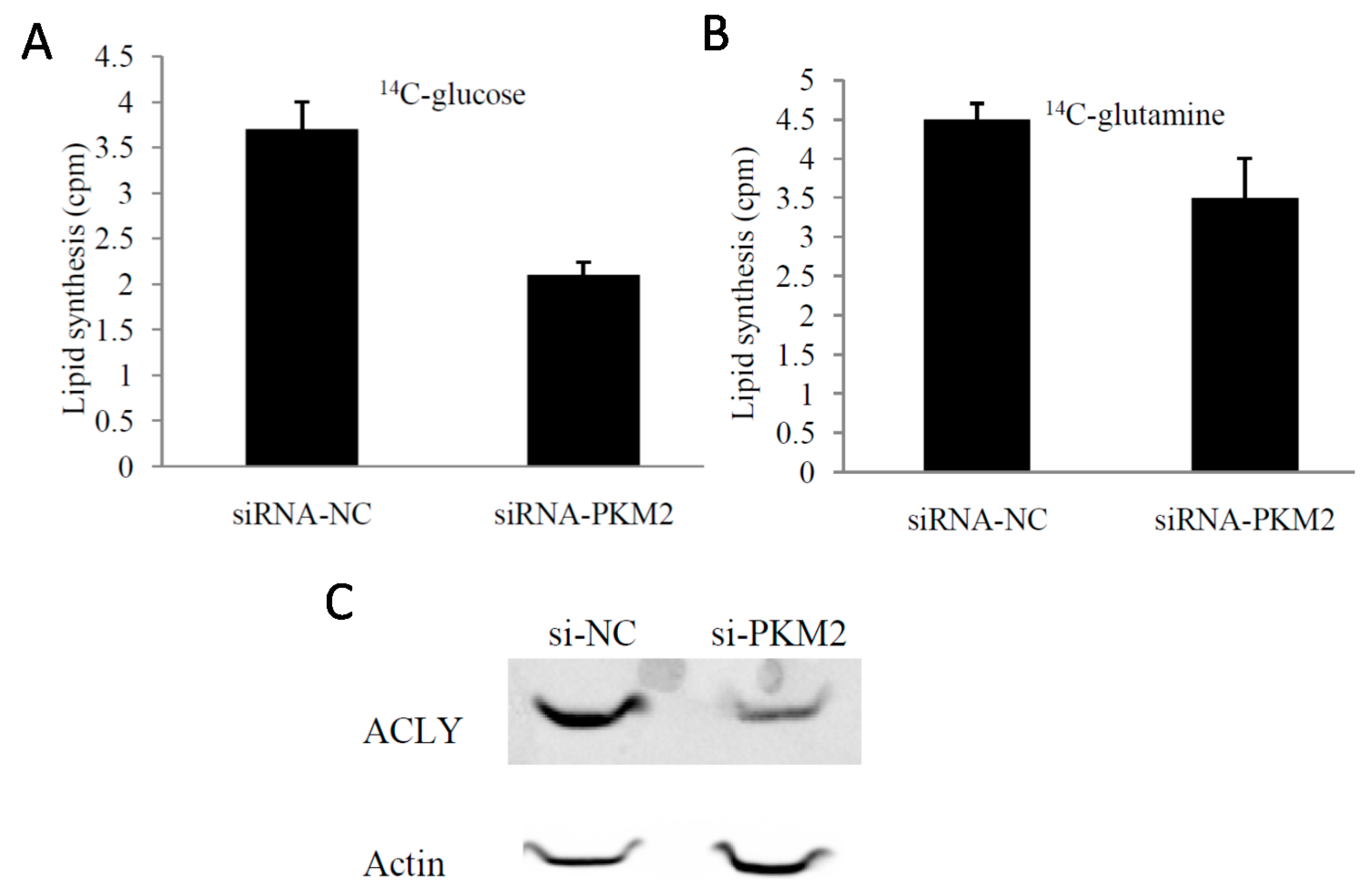
2.4. PKM2-Knockdown Inhibited Fatty Acid Synthesis of Glucose Carbon Source in Tumor Cells
2.5. Effect of PKM2 Downregulationon the A549 Cell Invasiveness

2.6. Discussion
3. Experimental Section
3.1. Study Population
3.2. Immunohistochemical Staining
3.3. Cell Culture and Viability Assay
3.4. Cell Metabolism Assay
3.5. Cell Invasion Assays
3.6. Lipid Synthesis
3.7. Western Blotting
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The Next Generation. Cell 2011, 5, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Bensinger, S.J.; Christofk, H.R. New aspects of the Warburg effect in cancer cell biology. Semin. Cells. Dev. Biol. 2012, 23, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Carins, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia inducible factor 1 and cancer pathogenesis. IUBMB Life 2008, 60, 591–597. [Google Scholar] [CrossRef] [PubMed]
- EI Mjiyad, N.; Caro-Maldonado, A.; Ramirez-Peinado, S.; Munoz-Pinedo, C. Sugar-free approaches to cancer cell killing. Oncogene 2011, 30, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef]
- Sheng, S.L.; Liu, J.J.; Dai, Y.H.; Sun, X.G.; Xiong, X.P.; Huang, G. Knockdown of lactate dehydrogenase A suppresses tumor growth and metastasis of human hepatocellular carcinoma. FEBS J. 2012, 279, 3898–3910. [Google Scholar] [CrossRef] [PubMed]
- Rui-hui, X.U.; Helene, P.; Yan, Z.; Jennifer, S.C.; Li, F.; Kapil, N.; Keating, M.G.; Huang, P. Inhibition of glycolysis in cancer cells: A novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res. 2005, 65, 613–621. [Google Scholar]
- Zhou, X.; Chen, R.H.; Xie, W.H.; Ni, Y.C.; Liu, J.J.; Huang, G. Relationship between 18F-FDG accumulation and lactate dehydrogenase A expression in lung adenocarcinomas. J. Nucl. Med. 2014, 55, 1766–1771. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, E.C.; Rathmell, J.C. New roles for pyruvate kinase M2: Working out the Warburg effect. Trends Biochem. Sci. 2008, 33, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Dombrauckas, J.D.; Santarsiero, B.D.; Mesecar, A.D. Structural basis for tumor pyruvate kinase M2 allosteric regulation and catalysis. Biochemistry 2005, 44, 9417–9429. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Inoue, H.; Tanaka, T. The M1- and M2-type isozymes of rat pyruvate kinase are produced from the same gene by alternative RNA splicing. J. Biol. Chem. 1986, 261, 13807–13812. [Google Scholar] [PubMed]
- Christofk, H.R.; Vander Heiden, M.G.; Harris, M.H.; Ramanathan, A.; Gerszten, R.E.; Wei, R.; Fleming, M.D.; Schreiber, S.L.; Cantley, L.C. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 2008, 452, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.; De Melo, J.; Tang, D. PKM2, a central point of regulation in cancer metabolism. Int. J. Cell Biol. 2013, 2013. [Google Scholar] [CrossRef]
- Bonuccelli, G.; Whitaker-Menezes, D.; Castello-Cros, R.; Pavlides, S.; Pestell, R.G.; Fatatis, A.; Witkiewicz, A.K.; Vander Heiden, M.G.; Migneco, G.; Chiavarina, B.; et al. The reverse Warburg effect: Glycolysis inhibitors prevent the tumor promoting effects of caveolin-1 deficient cancer associated fibroblasts. Cell Cycle 2010, 9, 1960–1971. [Google Scholar] [CrossRef] [PubMed]
- Hathurusinghe, H.R.; Coonetilleke, K.S.; Siriwardena, A.K. Current status of tumor M2 pyruvate kinase (tumor M2-PK) as a biomarker of gastrointestinal malignancy. Ann. Surg. Oncol. 2007, 14, 2714–2720. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.F.; Li, X.B.; Sun, H.; Zhang, B.; Han, Y.S.; Jiang, Y.; Wu, G.H. Pyruvate kinase type M2 is upregulated in colorectal cancer and promotes proliferation and migration of colon cancer cells. IUBMB Life 2012, 64, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y. TNM classification for lung cancer. Ann. Thorac. Cardiovasc. Surg. 2012, 9, 343–350. [Google Scholar]
- Yang, W.; Xia, Y.; Hawke, D.; Li, X.; Liang, J.; Xing, D.; Lu, Z. PKM2 phosphorylates histone H3 and promotes gene transcription and tumorigenesis. Cell 2012, 150, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Vesselle, H.; Freeman, J.D.; Wiens, L.; Stern, J.; Nguyen, H.Q.; Hawes, S.E.; Wood, D.E. Fluorodeoxyglucose uptake of primary non-small cell lung cancer at positron emission tomography: New contrary data on prognostic role. Clin. Cancer Res. 2007, 13, 3105–3106. [Google Scholar] [CrossRef] [PubMed]
- Starska, K.; Forma, E.; Jóźwiak, P.; Bryś, M.; Lewy-Trenda, I.; Brzezińska-Błaszczyk, E.; Krześlak, A. Gene and protein expression of glucose transporter 1 and glucose transporter 3 in human laryngeal cancer-the relationship with regulatory hypoxia-inducible factor-1α expression, tumor invasiveness, and patient prognosis. Tumour Biol. 2014, 36, 2309–2321. [Google Scholar] [CrossRef] [PubMed]
- Miao, P.; Sheng, S.; Sun, X.; Liu, J.; Huang, G. Lactate dehydrogenase A in cancer: A promising target for diagnosis and therapy. IUBMB Life 2013, 65, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zheng, Y.; Xia, Y.; Ji, H.; Chen, X.; Guo, F.; Lu, Z. ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat. Cell Biol. 2012, 14, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Xia, Y.; Cao, Y.; Zheng, Y.; Bu, W.; Zhang, L.; You, M.J.; Koh, M.Y.; Cote, G.; Aldape, K. EGFR-induced and PKC monoubiquitylation-dependent NF-B activation up-regulates PKM2 expression and promotes tumorigenesis. Mol. Cell 2012, 48, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Beckner, M.E.; Fellows-Mayle, W.; Zhang, Z.; Agostino, N.R.; Kant, J.A.; Day, B.W.; Pollack, I.F. Identification of ATP citrate lyaseas a positive regulator of glycolytic function in glioblastomas. Int. J. Cancer 2010, 126, 2282–2295. [Google Scholar] [PubMed]
- Hatzivassiliou, G.; Zhao, F.P.; Bauer, D.E.; Andreadis, C.; Shaw, A.N.; Dhanak, D.; Hingorani, S.R.; Tuveson, D.A.; Thompson, C.B. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell 2005, 8, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Graph Pad Prism, version 5.00; Software for Biostatistics, GraphPad software: San Diego, CA, USA, 2007.
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Zhu, A.; Zhang, L.; Zhang, J.; Zhong, Z.; Wang, F. Knockdown of PKM2 Suppresses Tumor Growth and Invasion in Lung Adenocarcinoma. Int. J. Mol. Sci. 2015, 16, 24574-24587. https://doi.org/10.3390/ijms161024574
Sun H, Zhu A, Zhang L, Zhang J, Zhong Z, Wang F. Knockdown of PKM2 Suppresses Tumor Growth and Invasion in Lung Adenocarcinoma. International Journal of Molecular Sciences. 2015; 16(10):24574-24587. https://doi.org/10.3390/ijms161024574
Chicago/Turabian StyleSun, Hong, Anyou Zhu, Lunjun Zhang, Jie Zhang, Zhengrong Zhong, and Fengchao Wang. 2015. "Knockdown of PKM2 Suppresses Tumor Growth and Invasion in Lung Adenocarcinoma" International Journal of Molecular Sciences 16, no. 10: 24574-24587. https://doi.org/10.3390/ijms161024574