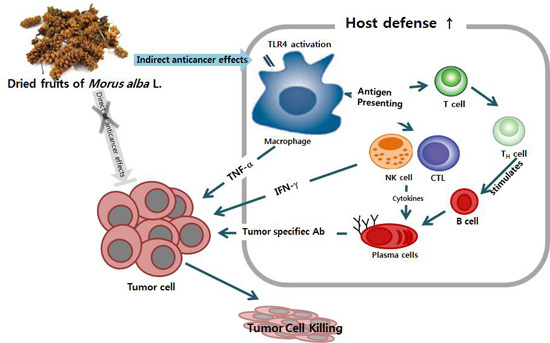
Improved Chemotherapeutic Activity by Morus alba Fruits through Immune Response of Toll-Like Receptor 4
Abstract
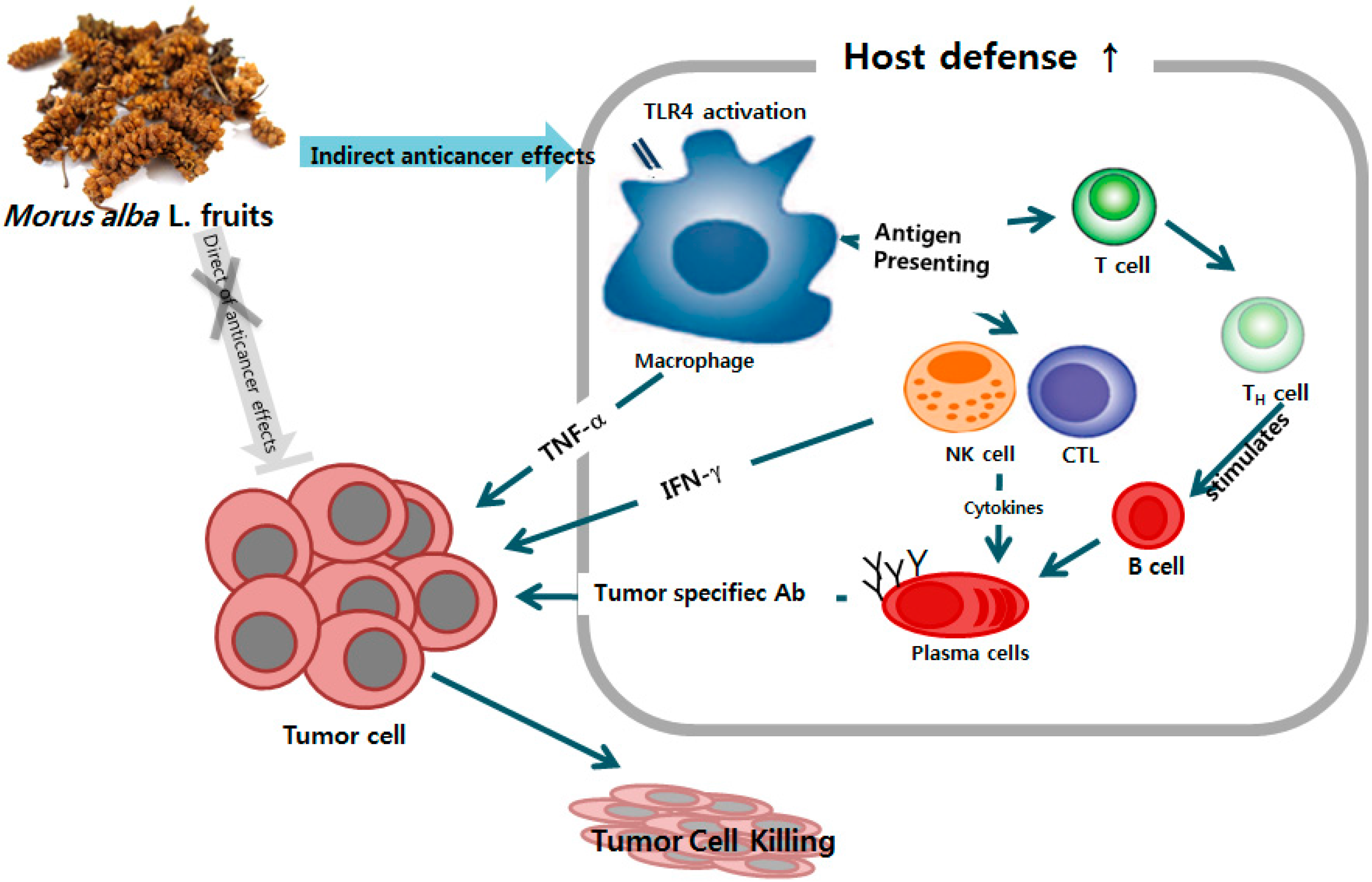
:1. Introduction
2. Results
2.1. Mechanisms of Macrophage Stimulation by M. alba L. Fruit Extract (MFE)
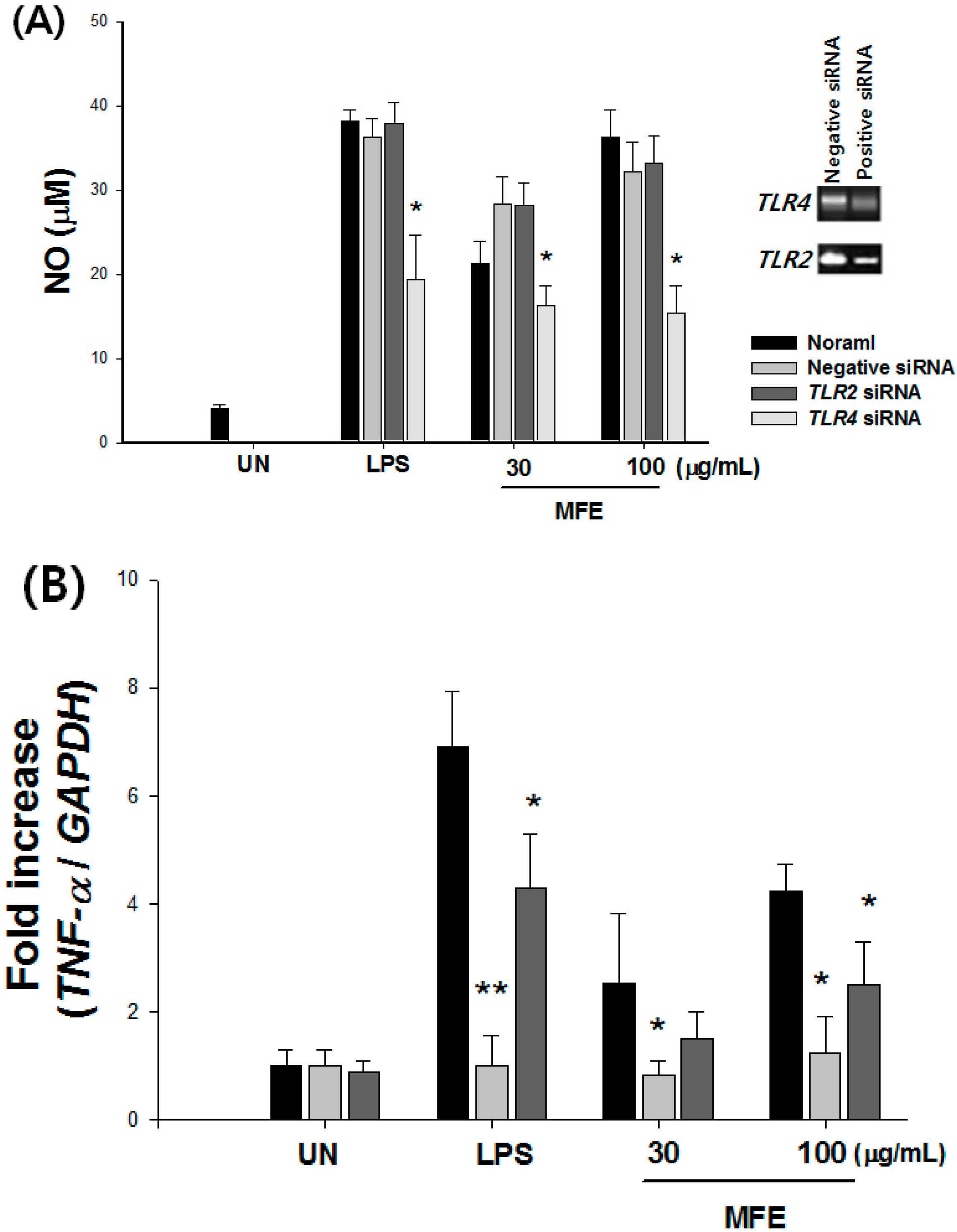
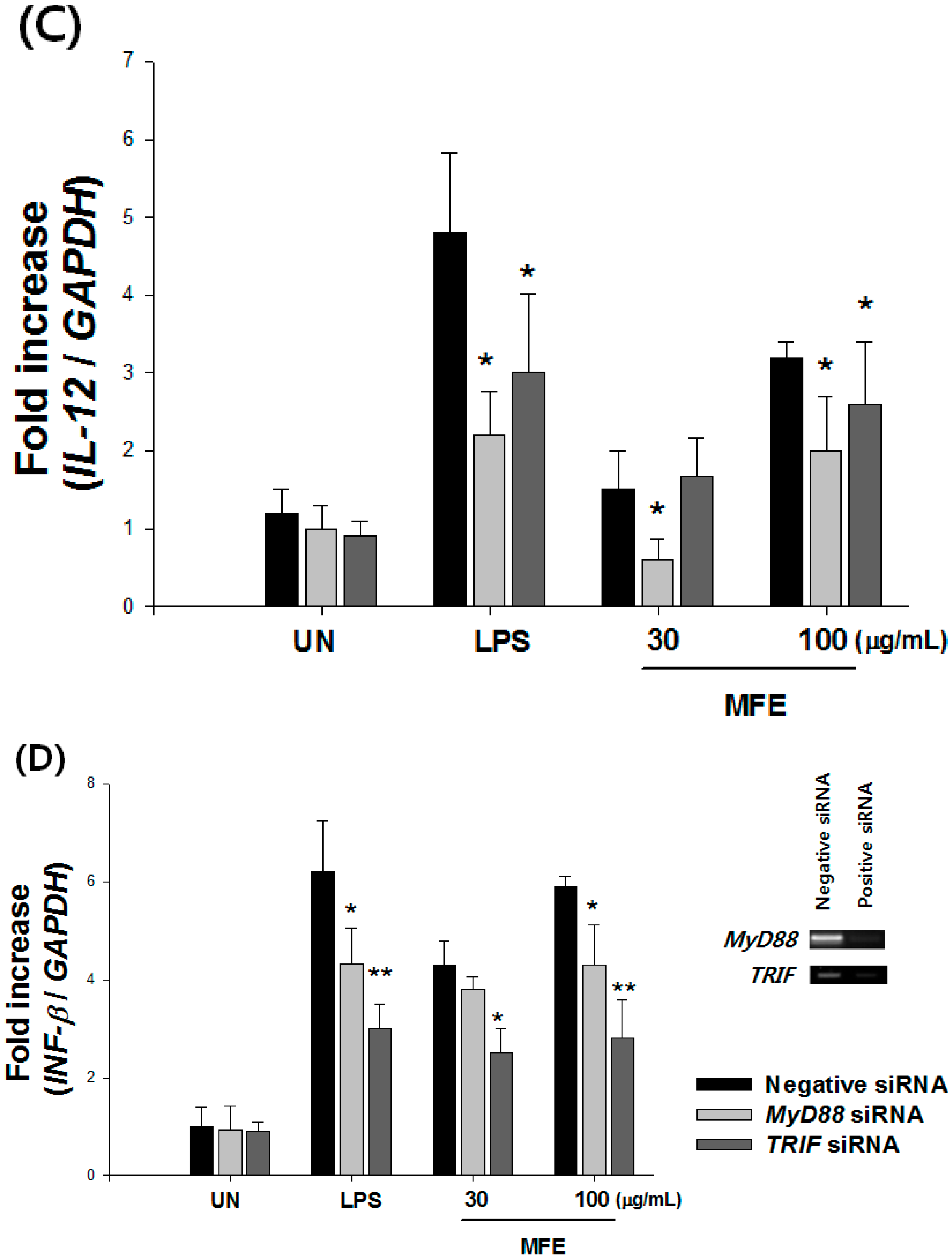
| NO (%) | TNF-α (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ab Free | TLR2 Ab | TLR4 Ab | TLR6 Ab | Ab Free | TLR2 Ab | TLR4 Ab | TLR6 Ab | ||
| Untreated | 100.0 ± 2.4 | 100.0 ± 3.3 | 100.0 ± 2.6 | 100.0 ± 2.4 | 100.0 ± 3.2 | 100.0 ± 3.6 | 100.0 ± 2.6 | 100.0 ± 1.4 | |
| MFE (μg/mL) | 10 | 140.4 ± 22.4 | 138.3 ± 22.7 | 115.7 ± 12.6 * | 126.2 ± 19.4 | 131.9 ± 8.0 | 121.6 ± 5.3 | 90.6 ± 4.3 * | 131.3 ± 7.8 |
| 30 | 312.9 ± 12.3 | 280.4 ± 13.5 | 231.6 ± 13.4 * | 277.8 ± 14.6 | 160.4 ± 12.2 | 150.1 ± 3.4 | 102.7 ± 4.6 * | 150.5 ± 14.5 | |
| 100 | 466.4 ± 10.3 | 392.4 ± 12.5 | 340.4 ± 8.3 * | 340.1 ± 7.3 | 172.9 ± 3.2 | 159.6 ± 24.6 | 122.7 ± 5.3 * | 167.2 ± 18.3 | |

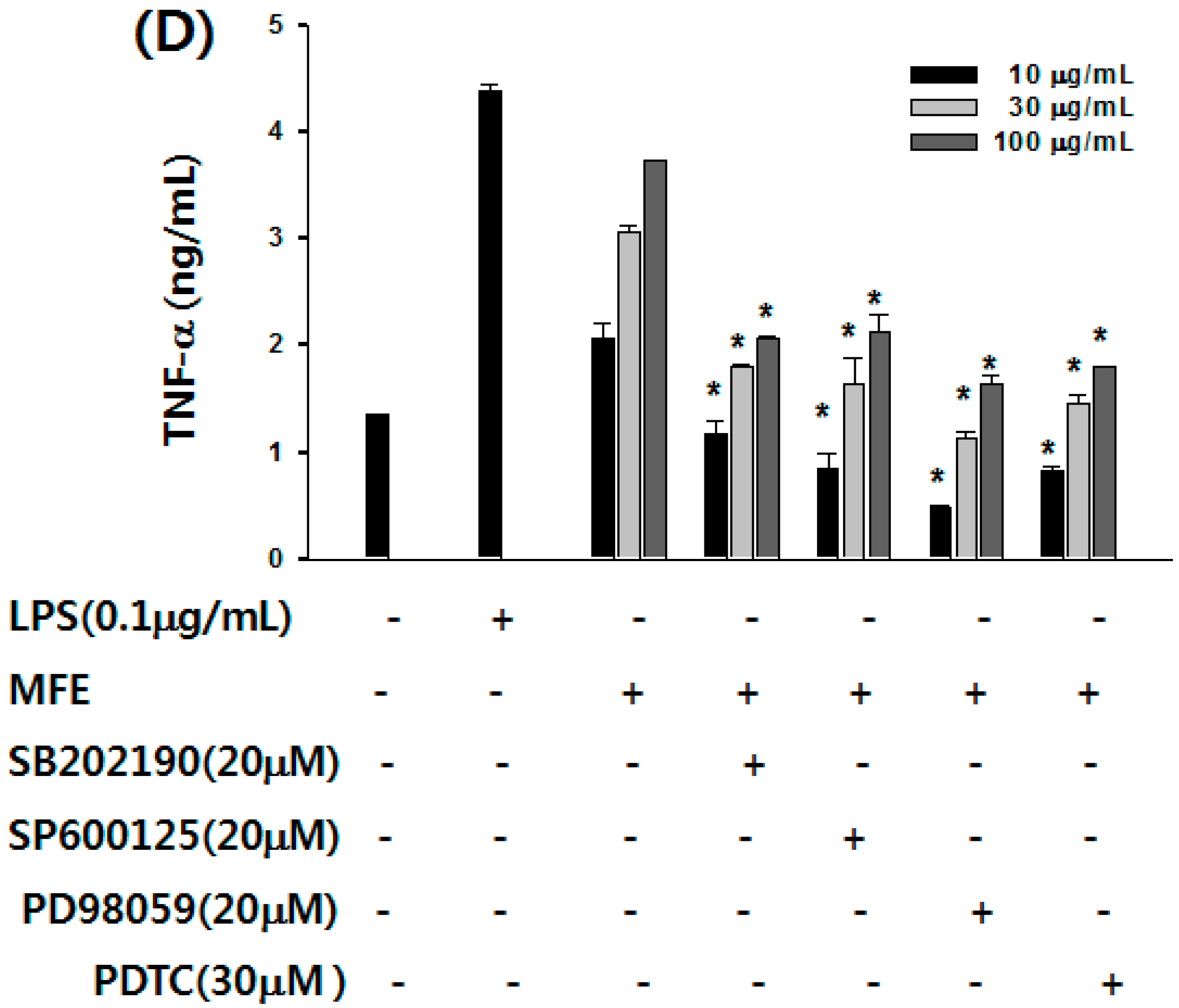
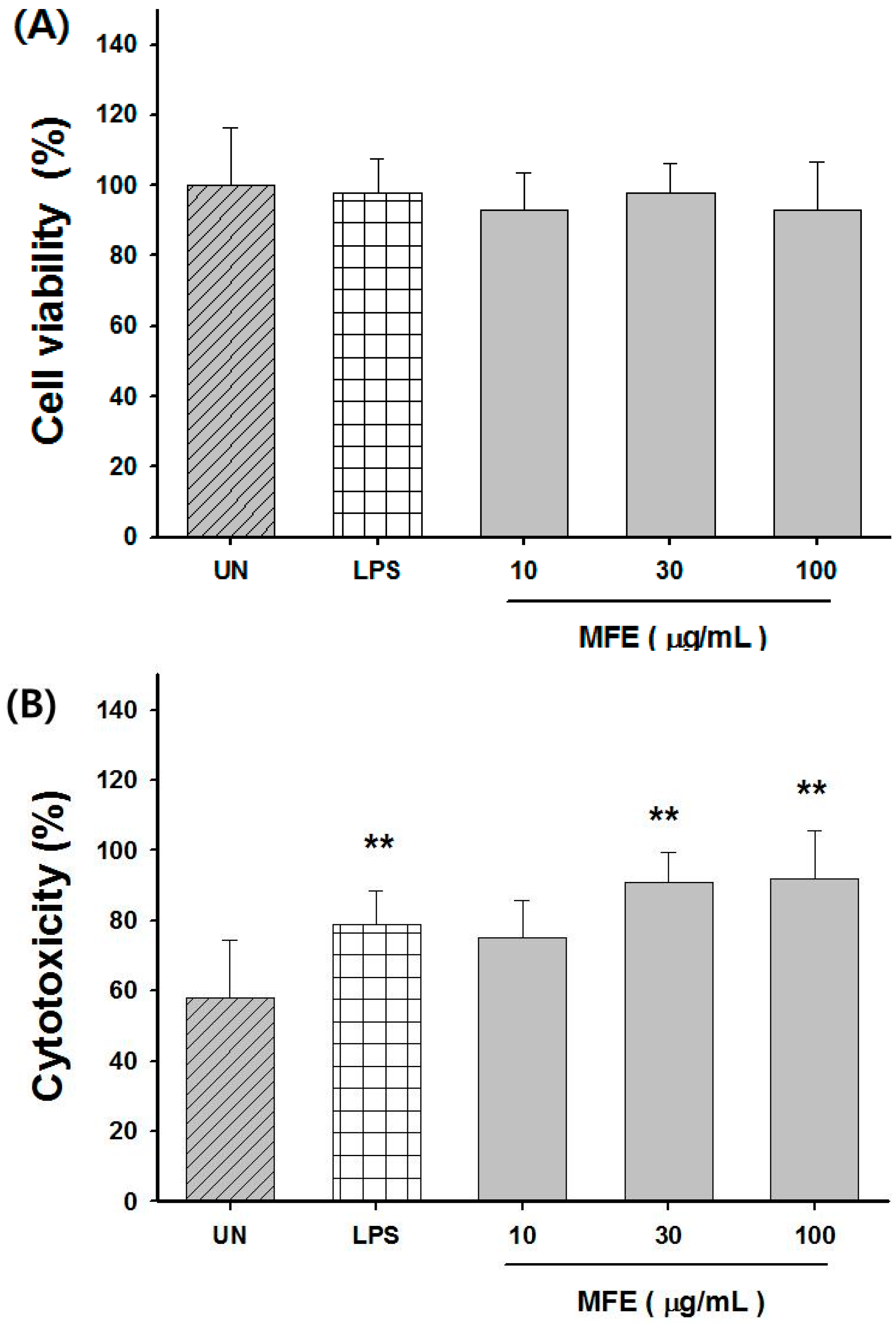
2.2. Macrophage Activation of MFE for Tumor Cytotoxicity
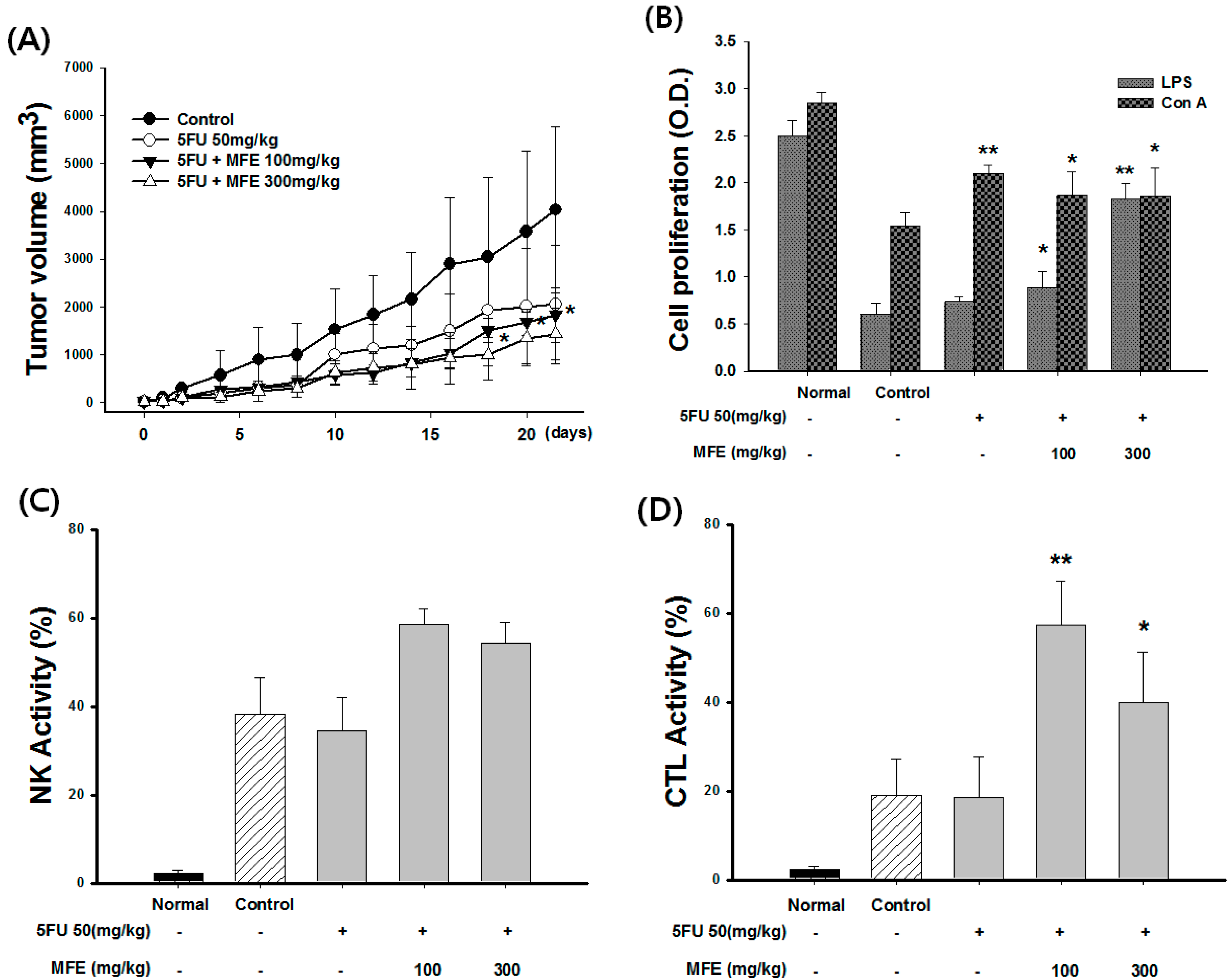
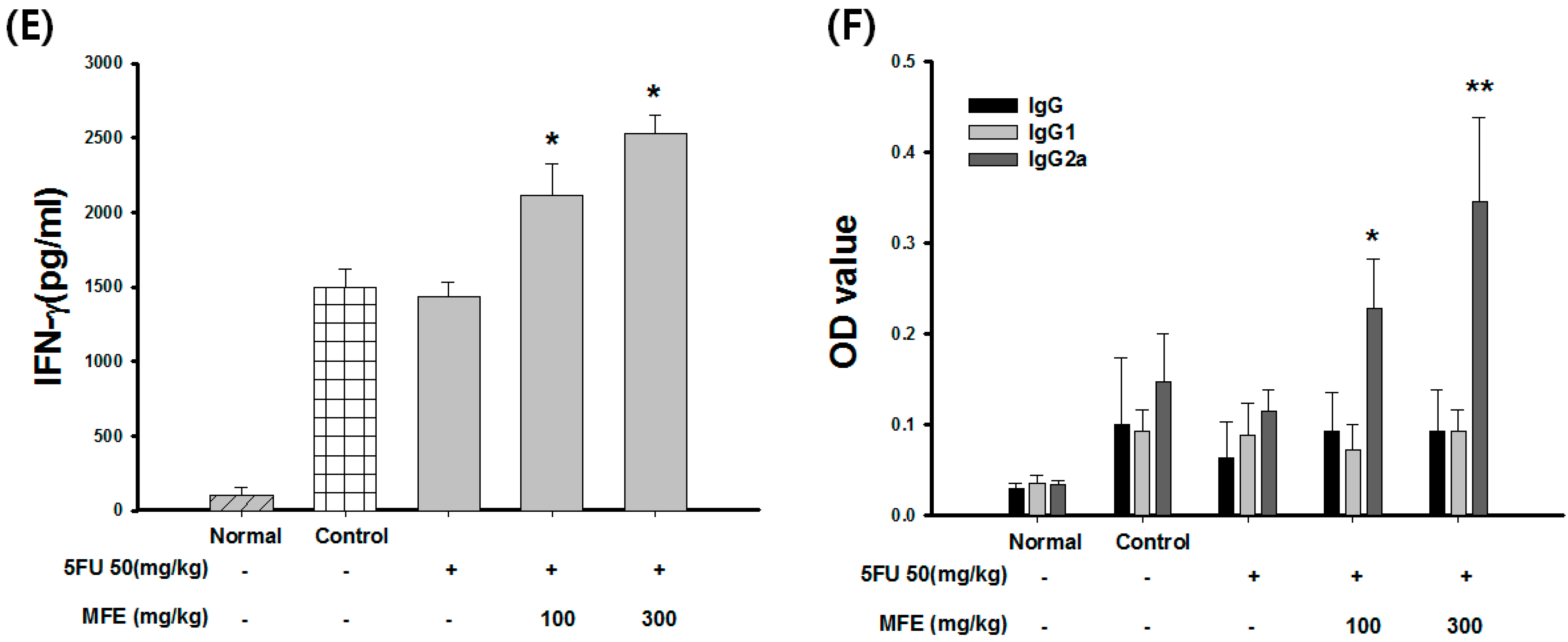
2.3. Enhancement of Chemotherapeutic Effects by MFE in Xenograft Mice
| Group | Dose (mg/kg) | Leukocyte (103/mL) | Weight of Spleen (g) | Weight of Tumor (g) | Inhibition Rate of Tumor (%) |
|---|---|---|---|---|---|
| Control | - | 12.3 ± 1.35 | 0.45 ± 0.04 | 5.81 ± 1.35 | - |
| 5-FU | 50 | 8.32 ± 1.35 | 0.32 ± 0.23 | 4.62 ± 1.24 | 21 |
| 5-FU + MFE | 50 + 100 | 12.5 ± 0.65 * | 0.43 ± 0.20 | 4.26 ± 0.65 | 29 |
| 50 + 300 | 14.2 ± 0.95 * | 0.52 ± 0.08 * | 3.75 ± 0.95 | 42 |
3. Discussion
4. Experimental Section
4.1. Chemical and Reagents
4.2. Cell Culture and Animal Care
4.3. Extract of Morus alba Fruits
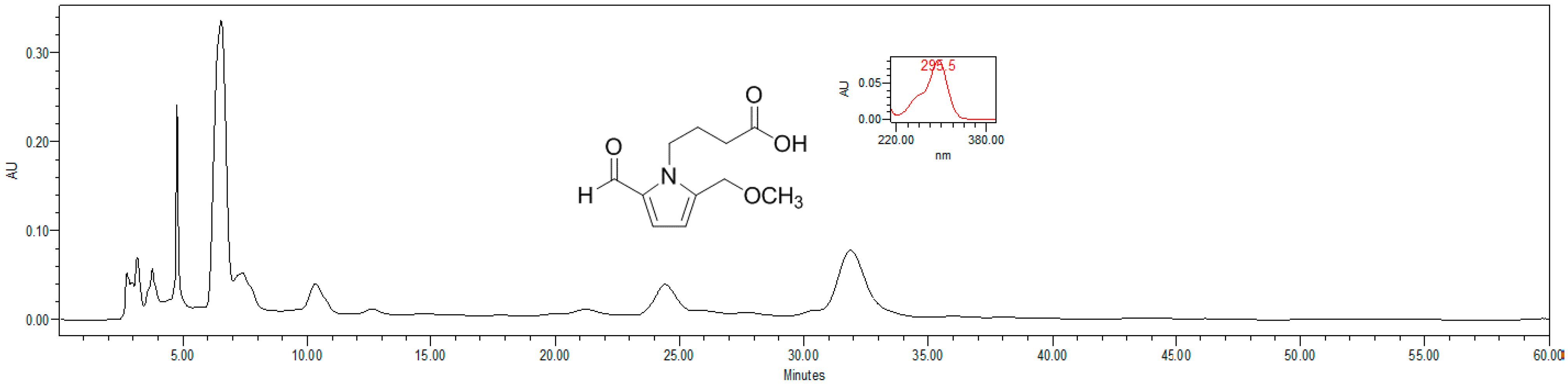
4.4. HPLC Estimation of Pyrrole Alkaloid
4.5. Proliferation Assay
4.6. NO and Cytokine Assays
4.7. Antibody Inhibition Experiments
4.8. siRNA Preparation and Transfection
4.9. Western Blots
4.10. Macrophage-Mediated Cytotoxicity
4.11. Treatment and Drug Administration
4.12. Evaluation of Tumor Volume, Leukocyte Count, Tumor Weight, and Organ Weight
4.13. Assay of NK Cells and CTL Activity
4.14. Measurement of Antigen-Specific Antibody
4.15. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhao, H.Y.; Huang, H.; Hu, Z.H.; Huang, Y.; Lin, S.X.; Tian, Y.; Lin, T.Y. Evaluations of biomarkers associated with sensitivity to 5-fluorouracil and taxanes for recurrent/advanced breast cancer patients treated with capecitabine-based first-line chemotherapy. Anti-Cancer Drugs 2012, 23, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Bremnes, R.M.; Al-Shibli, K.; Donnem, T.; Sirera, R.; Al-Saad, S.; Andersen, S.; Stenvold, H.; Camps, C.; Busund, L.T. The role of tumor-infiltrating immune cells and chronic inflammation at the tumor site on cancer development, progression, and prognosis: Emphasis on non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Salagianni, M.; Baxevanis, C.N.; Papamichail, M.; Perez, S.A. New insights into the role of NK cells in cancer immunotherapy. Oncoimmunology 2012, 1, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Adams, S. Toll-like receptor agonists in cancer therapy. Immunotherapy 2009, 1, 949–964. [Google Scholar] [CrossRef] [PubMed]
- Kaczanowska, S.; Joseph, A.M.; Davila, E. TLR agonists: Our best frenemy in cancer immunotherapy. J. Leukoc. Biol. 2013, 93, 847–863. [Google Scholar] [CrossRef]
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.H.; Kim, Y.K. Promoting effect and recovery activity from physical stress of the fruit of morus alba. Biofactors 2004, 21, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Ju, M.S.; Shim, J.S.; Kim, M.C.; Lee, S.H.; Huh, Y.; Kim, S.Y.; Oh, M.S. Mulberry fruit protects dopaminergic neurons in toxin-induced Parkinson’s disease models. Br. J. Nutr. 2010, 104, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.H.; Hur, J.Y.; Kim, H.B.; Ryu, J.H.; Kim, S.Y. Neuroprotective effects of the cyanidin-3-o-β-d-glucopyranoside isolated from mulberry fruit against cerebral ischemia. Neurosci. Lett. 2006, 391, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Khan, H.; Shah, M.; Khan, R.; Khan, F. Chemical composition and antioxidant activity of certain morus species. J. Zhejiang Univ. Sci. B 2010, 11, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Ercisli, S.; Orhan, E. Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits. Food Chem. 2007, 103, 1380–1384. [Google Scholar] [CrossRef]
- Pawlowska, A.M.; Oleszek, W.; Braca, A. Quali-quantitative analyses of flavonoids of Morus nigra L. and Morus alba L. (Moraceae) fruits. J. Agric. Food Chem. 2008, 56, 3377–3380. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Li, R.; Zheng, N.; Xu, L.; Liang, T.; He, Q. Anti-diabetic effect of ramulus mori polysaccharides, isolated from Morus alba L., on STZ-diabetic mice through blocking inflammatory response and attenuating oxidative stress. Int. Immunopharmacol. 2013, 16, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Park, G.S.; Lee, M.H.; Chang, I.A.; Kim, Y.C.; Kim, S.Y.; Lee, J.Y.; Yun, Y.G.; Park, H. Toll-like receptor 4-mediated immunoregulation by the aqueous extract of mori fructus. Phytother. Res. 2009, 23, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Chang, B.Y.; Jo, Y.H.; Lee, S.H.; Han, S.B.; Hwang, B.Y.; Kim, S.Y.; Lee, M.K. Macrophage activating activity of pyrrole alkaloids from morus alba fruits. J. Ethnopharmacol. 2013, 145, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.; Zhang, Q.; Wang, L.; Wang, C.; Zhang, M.; Xu, X.; Zhou, H.; Li, X.; Xu, Q.; He, F.; et al. OM85-BV induced the productions of IL-1β, IL-6, and TNF-α via TLR4- and TLR2-mediated ERK1/2/NF-κB pathway in RAW264.7 cells. J. Interferon Cytokine Res. 2014, 34, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Feng, Y.; Li, J.; Mao, R.; Zou, Y.; Feng, W.; Zheng, D.; Wang, W.; Chen, Y.; Yang, L.; et al. Schisandra polysaccharide evokes immunomodulatory activity through TLR 4-mediated activation of macrophages. Int. J. Biol. Macromol. 2014, 65, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; He, Y.; Sun, X.; Li, Q.; Wang, W.; Zhao, A.; Di, W. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J. Ovarian Res. 2014, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Li, T.T.; Ogino, S.; Qian, Z.R. Toll-like receptor signaling in colorectal cancer: Carcinogenesis to cancer therapy. World J. Gastroenterol. 2014, 20, 17699–17708. [Google Scholar] [PubMed]
- Nebl, T.; de Veer, M.J.; Schofield, L. Stimulation of innate immune responses by malarial glycosylphosphatidylinositol via pattern recognition receptors. Parasitology 2005, 130, S45–S62. [Google Scholar] [CrossRef] [PubMed]
- Sender, V.; Stamme, C. Lung cell-specific modulation of LPS-induced TLR4 receptor and adaptor localization. Commun. Integr. Biol. 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Liu, S.Q.; Zhang, W.Q.; Luo, G.A. Structural features of a pectic polysaccharide from mulberry leaves. J. Asian Nat. Prod. Res. 2008, 10, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Samavati, V.; Yarmand, M.S. Statistical modeling of process parameters for the recovery of polysaccharide from morus alba leaf. Carbohydr. Polym. 2013, 98, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Synytsya, A.; Kim, H.B.; Choi, D.J.; Lee, S.; Lee, J.; Kim, W.J.; Jang, S.; Park, Y.I. Purification, characterization and immunomodulating activity of a pectic polysaccharide isolated from korean mulberry fruit oddi (Morus alba L.). Int. Immunopharmacol. 2013, 17, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [PubMed]
- Bryant, C.E.; Symmons, M.; Gay, N.J. Toll-like receptor signalling through macromolecular protein complexes. Mol. Immunol. 2015, 63, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Almog, T.; Kandel-Kfir, M.; Shaish, A.; Dissen, M.; Shlomai, G.; Voronov, E.; Apte, R.N.; Harats, D.; Kamari, Y. Knockdown of interleukin-1alpha does not attenuate lps-induced production of interleukin-1beta in mouse macrophages. Cytokine 2015, 73, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, K.; Feng, W.; Wu, X.; Li, H. TLR4-MyD88/Mal-NF-kB axis is involved in infection of HSV-2 in human cervical epithelial cells. PLoS ONE 2013, 8, e80327. [Google Scholar] [CrossRef] [PubMed]
- Iliev, D.B.; Hansen, T.; Jorgensen, S.M.; Krasnov, A.; Jorgensen, J.B. CpG- and LPS-activated MAPK signaling in in vitro cultured salmon (Salmo salar) mononuclear phagocytes. Fish Shellfish Immunol. 2013, 35, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Lien, J.C.; Chang, C.W.; Chang, C.H.; Kuo, S.C.; Huang, T.F. Yuwen02f1 suppresses LPS-induced endotoxemia and adjuvant-induced arthritis primarily through blockade of ROS formation, NFκB and MAPK activation. Biochem. Pharmacol. 2013, 85, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Kim, S.N.; Oh, J.S.; Lee, S.; Kim, Y.K. Anti-mitotic potential of 7-diethylamino-3(2′-benzoxazolyl)-coumarin in 5-fluorouracil-resistant human gastric cancer cell line SNU620/5-FU. Biochem. Biophys. Res. Commun. 2012, 418, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Gravalos, C.; Garcia-Escobar, I.; Garcia-Alfonso, P.; Cassinello, J.; Malon, D.; Carrato, A. Adjuvant chemotherapy for stages II, III and IV of colon cancer. Clin. Transl. Oncol. 2009, 11, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.; Asif, H.M.; Nazar, H.M.; Akhtar, N.; Rehman, J.U.; Rehman, R.U. Medicinal plants combating against cancer—A green anticancer approach. Asian Pac. J. Cancer Prev. 2014, 15, 4385–4394. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Sun, H.X.; Ye, Y.P. Immunomodulatory and antitumor activity of triterpenoid fractions from the rhizomes of astilbe chinensis. J. Ethnopharmacol. 2008, 119, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Alderson, K.L.; Sondel, P.M. Clinical cancer therapy by NK cells via antibody-dependent cell-mediated cytotoxicity. J. Biomed. Biotechnol. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Tittarelli, A.; Janji, B.; van Moer, K.; Noman, M.Z.; Chouaib, S. The selective degradation of synaptic connexin-43 by hypoxia-induced autophagy impairs natural killer cell-mediated tumor cell killing. J. Biol. Chem. 2015. [Google Scholar] [CrossRef] [PubMed]
- Burkholder, B.; Huang, R.Y.; Burgess, R.; Luo, S.; Jones, V.S.; Zhang, W.; Lv, Z.Q.; Gao, C.Y.; Wang, B.L.; Zhang, Y.M.; et al. Tumor-induced perturbations of cytokines and immune cell networks. Biochim. Biophys. Acta 2014, 1845, 182–201. [Google Scholar] [CrossRef] [PubMed]
- Riether, C.; Schurch, C.M.; Ochsenbein, A.F. Regulation of hematopoietic and leukemic stem cells by the immune system. Cell Death Differ. 2015, 22, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Zhang, Y.M.; Zhang, X.M.; Tao, J. Effect of TACI signaling on humoral immunity and autoimmune diseases. J. Immunol. Res. 2015, 2015, 247426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Nie, S.; Huang, D.; Li, W.; Xie, M. Immunomodulatory effect of ganoderma atrum polysaccharide on CT26 tumor-bearing mice. Food Chem. 2013, 136, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.X.; Ye, Y.P.; Pan, H.J.; Pan, Y.J. Adjuvant effect of panax notoginseng saponins on the immune responses to ovalbumin in mice. Vaccine 2004, 22, 3882–3889. [Google Scholar] [CrossRef] [PubMed]
- Huber, V.C.; McKeon, R.M.; Brackin, M.N.; Miller, L.A.; Keating, R.; Brown, S.A.; Makarova, N.; Perez, D.R.; Macdonald, G.H.; McCullers, J.A. Distinct contributions of vaccine-induced immunoglobulin G1 (IgG1) and IgG2a antibodies to protective immunity against influenza. Clin. Vaccine Immunol. 2006, 13, 981–990. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, B.Y.; Kim, S.B.; Lee, M.K.; Park, H.; Kim, S.Y. Improved Chemotherapeutic Activity by Morus alba Fruits through Immune Response of Toll-Like Receptor 4. Int. J. Mol. Sci. 2015, 16, 24139-24158. https://doi.org/10.3390/ijms161024139
Chang BY, Kim SB, Lee MK, Park H, Kim SY. Improved Chemotherapeutic Activity by Morus alba Fruits through Immune Response of Toll-Like Receptor 4. International Journal of Molecular Sciences. 2015; 16(10):24139-24158. https://doi.org/10.3390/ijms161024139
Chicago/Turabian StyleChang, Bo Yoon, Seon Beom Kim, Mi Kyeong Lee, Hyun Park, and Sung Yeon Kim. 2015. "Improved Chemotherapeutic Activity by Morus alba Fruits through Immune Response of Toll-Like Receptor 4" International Journal of Molecular Sciences 16, no. 10: 24139-24158. https://doi.org/10.3390/ijms161024139