Identification of Specific Variations in a Non-Motile Strain of Cyanobacterium Synechocystis sp. PCC 6803 Originated from ATCC 27184 by Whole Genome Resequencing
Abstract
:1. Introduction
2. Results and Discussion
2.1. Overview
2.2. Chromosome Variations Shared with Other Strains
2.3. Variations in Plasmids
| Event | Effect | Locus | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| # | M | Start | End | Size | Nucl Change | Ref→mut | AA Change | Result | Locus | Gene Name | Product |
| Chromosome | |||||||||||
| 1 | I | 386410 | 386411 | 102 | - | - | - | 34 additional AAs | slr1084 | - | Hypothetical protein |
| 2 | I | 781625 | 781626 | 154 | - | - | - | 5'extension of reading frame | IGR slr2030-slr2031 | - | - |
| 3 | S | 831647 | 831647 | 1 | C→T | - | - | Possible effect on infA promoter | IGR adk-infA | - | - |
| 4 | S | 943495 | 943495 | 1 | G→A | GTC→ATC | V→I | AA 'change | slr1834 | psaA | P700 apoprotein subunit Ia |
| 5 | S | 1012958 | 1012958 | 1 | G→T | - | - | - | IGR ssl3177-sll1633 | - | - |
| 6 | D | 1200294 | 1201476 | 1183 | - | - | - | ISY203b missing | sll1780 | - | Transposase |
| 7 | S | 1364187 | 1364187 | 1 | A→G | TTG→CTG | L→L | –silent- | sll0838 | pyrF | Orotidine 5' monophosphate decarboxylase |
| 8 | I | 1765792 | 1765793 | 1 | *→T | AAT→AAA | N→K | Frameshift | sll1895 | - | Hypothetical protein |
| 9 | S | 1819782 | 1819782 | 1 | A→G | TCT→TCC | S→S | –silent- | sll1867 | psbA3 | Photosystem II D1 protein |
| 10 | S | 1819788 | 1819788 | 1 | A→G | CTT→CTC | L→L | –silent- | sll1867 | psbA3 | Photosystem II D1 protein |
| 11 | D | 2048412 | 2049594 | 1183 | - | - | - | ISY203e missing | slr1635 | - | Transposase |
| 12 | S | 2092571 | 2092571 | 1 | A→T | TTA→TAA | L→* | New stop codon | sll0422 | - | Asparaginase |
| 13 | S | 2198893 | 2198893 | 1 | T→C | TTA→TTG | L→L | –silent- | sll0142 | - | Probable cation efflux system protein |
| 14 | D | 2204584 | 2204584 | 1 | G→* | GGT→GTT | G→V | Frameshift | slr0162 | gspF,pilC | A part of pilC, pilin biogenesis protein, required for twitching motility |
| 15 | S | 2235441 | 2235441 | 1 | A→G | GGT→GGC | G→G | –silent- | sll1851 | - | Hypothetical protein, no conserved domains |
| 16 | S | 2272418 | 2272418 | 1 | C→A | CCC→ACC | P→T | AA change | slr0322 | pilL-C | Homologous to the C-terminal of CheA-like protein, essential for motility, thick pili biosynthesis and transformation competency. |
| 17 | D | 2272927 | 2273907 | 981 | - | - | - | delete 327 AAs | slr0322 | pilL-C | As above |
| 18 | S | 2301721 | 2301721 | 1 | A→G | AAG→GAG | K→E | AA change | slr0168 | - | Hypothetical protein, no conserved domains |
| 19 | I | 2350285 | 2350286 | 1 | *→A | - | - | - | IGR sml0001-slr0363 | - | - |
| 20 | I | 2360245 | 2360246 | 1 | *→C | GCG→GCC | A→A | Frameshift | slr0364/slr0366 | - | Hypothetical protein, no conserved domains |
| 21 | D | 2409244 | 2409244 | 1 | C→* | GGA→GAT | G→D | Frameshift | sll0762 | - | Hypothetical protein, no conserved domains |
| 22 | D | 2419399 | 2419399 | 1 | T→* | AAT→ATG | N→M | Frameshift | sll0751(ycf22);sll0752 | ycf22 | Hypothetical protein YCF22 |
| 23 | I | 2544044 | 2544045 | 1 | *→C | AGG→GAG | R→E | Frameshift | ssl0787/ssl0788 | - | Hypothetical protein, no conserved domains |
| 24 | S | 2602717 | 2602717 | 1 | C→A | CAC→CAA | H→Q | AA change | slr0468 | - | Hypothetical protein, no conserved domains |
| 25 | S | 2602734 | 2602734 | 1 | T→A | ATT→AAT | I→N | AA change | slr0468 | - | Hypothetical protein, no conserved domains |
| 26 | S | 2748897 | 2748897 | 1 | C→T | - | - | - | IGR slr0210-ssr0332 | - | - |
| 27 | S | 3014665 | 3014665 | 1 | T→C | ACT→ACC | T→T | –silent- | slr0302 | pleD | PleD-like protein |
| 28 | S | 3096187 | 3096187 | 1 | T→C | ATA→ACA | I→T | AA change | ssr1175(transposase) | - | Located in a mobile element(ISY100v1) |
| 29 | S | 3098707 | 3098707 | 1 | T→C | TGT→CGT | C→R | AA change | ssr1176(transposase) | - | Located in a mobile element(ISY100v3) |
| 30 | S | 3110189 | 3110189 | 1 | G→A | - | - | - | IGR sll0665-sll0666 | - | Located in a mobile element(ISY523) |
| 31 | S | 3110343 | 3110343 | 1 | G→T | CCA→CAA | P→Q | AA change | sll0665 | - | Transposase |
| 32 | S | 3142651 | 3142651 | 1 | A→G | CTT→CTC | L→L | –silent- | sll0045 | spsA | Sucrose phosphate synthase |
| 33 | D | 3260096 | 3260096 | 1 | C→* | - | - | - | IGR sll0528-sll0529 | - | - |
| 34 | D | 3400331 | 3401513 | 1183 | - | - | - | ISY203g missing | sll1474 | - | Transposase |
| pSYSM | |||||||||||
| 35 | D | 117269 | 118451 | 1183 | - | - | - | ISY203j missing | sll5131 | - | Transposase |
| pSYSX | |||||||||||
| 36 | S | 4241 | 4241 | 1 | C→G | ATC→ATG | I→M | AA change | slr6004 | - | Hypothetical protein, no conserved domains |
| 37 | S | 4253 | 4253 | 1 | C→T | CCC→CCT | P→P | –silent- | slr6004 | - | Hypothetical protein, no conserved domains |
| 38 | S | 4295 | 4295 | 1 | T→C | TCT→TCC | S→S | –silent- | slr6004 | - | Hypothetical protein, no conserved domains |
| 39 | S | 82405 | 82405 | 1 | A→G | AAC→AGC | N→S | AA change | ssr6089 | Hypothetical protein, no conserved domains | |
| pCB2.4 | |||||||||||
| 40 | D | 1211 | 1211 | 1 | A→* | CAG→CGG | Q→R | Frameshift | MYO_820 | - | Hypothetical protein, no conserved domains |
| Variation | Strains Reported in Literatures and This Work | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| # | Event | GT-Kazusa [10,11] | GT-S [10] | GT-I [11] | GT-O1 [13] | GT-O2 [13] | GT-G | PCC-P [11] | PCC-N [11] | PCC-M [12] |
| 1 | I | - | - | √ | √ | √ | √ | √ | √ | √ |
| 2 | I | - | - | - | - | - | √ | √ | √ | √ |
| 3 | S | - | - | - | - | - | √ | √ | √ | √ |
| 4 | S | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 5 | S | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 6 | D | - | √ | √ | √ | √ | √ | √ | √ | √ |
| 7 | S | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 8 | I | - | - | - | - | - | √ | - | - | - |
| 9 | S | √ | √ | - | √ | √ | √ | - | - | - |
| 10 | S | √ | √ | - | √ | √ | √ | - | - | - |
| 11 | D | - | - | √ | √ | √ | √ | √ | √ | √ |
| 12 | S | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 13 | S | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 14 | D | - | √ | √ | √ | √ | √ | √ | √ | √ |
| 15 | S | - | - | - | - | - | √ | - | - | - |
| 16 | S | - | - | - | - | - | √ | - | - | - |
| 17 | D | - | - | - | - | - | √ | - | - | - |
| 18 | S | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 19 | I | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 20 | I | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 21 | D | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 22 | D | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 23 | I | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 24 | S | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 25 | S | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 26 | S | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 27 | S | - | - | - | - | - | √ | √ | √ | √ |
| 28 | S | √ | √ | √ | √ | √ | √ | √ | √ | - |
| 29 | S | - | - | - | - | - | √ | √ | √ | √ |
| 30 | S | √ | √ | - | √ | √ | √ | - | - | √ |
| 31 | S | √ | √ | - | √ | √ | √ | - | - | - |
| 32 | S | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 33 | D | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 34 | D | - | √ | √ | √ | √ | √ | √ | √ | √ |
| 35 | D | NIa | NI | NI | - | - | √ | NI | NI | √ |
| 36 | S | NI | NI | NI | - | - | √ | NI | NI | - |
| 37 | S | NI | NI | NI | - | - | √ | NI | NI | - |
| 38 | S | NI | NI | NI | - | - | √ | NI | NI | - |
| 39 | S | NI | NI | NI | √ | √ | √ | NI | NI | - |
| 40 | D | NI | NI | NI | - | - | √ | NI | NI | - |
2.4. Novel Variations in GT-G
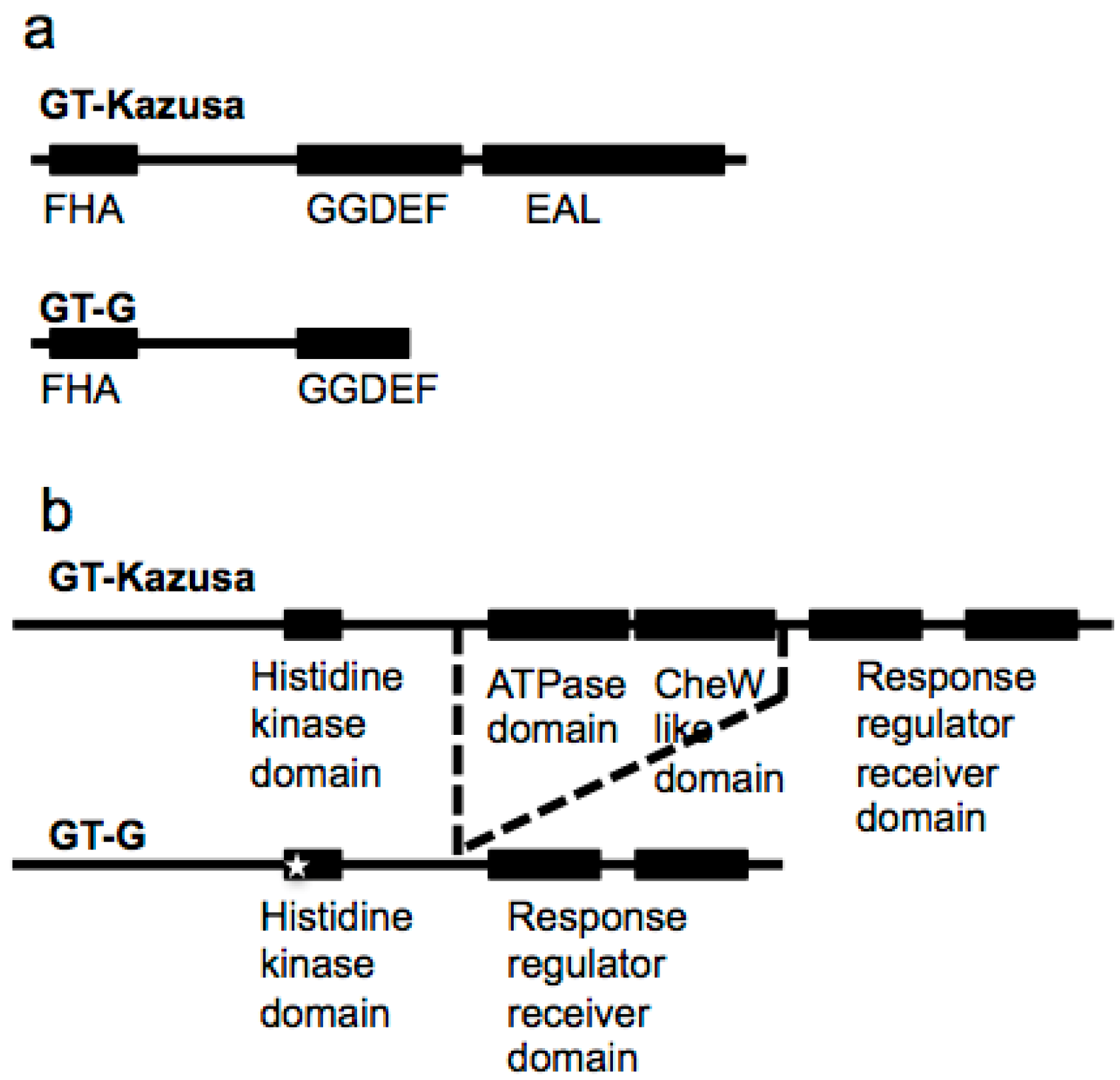
2.5. Phylogenetic Relationships
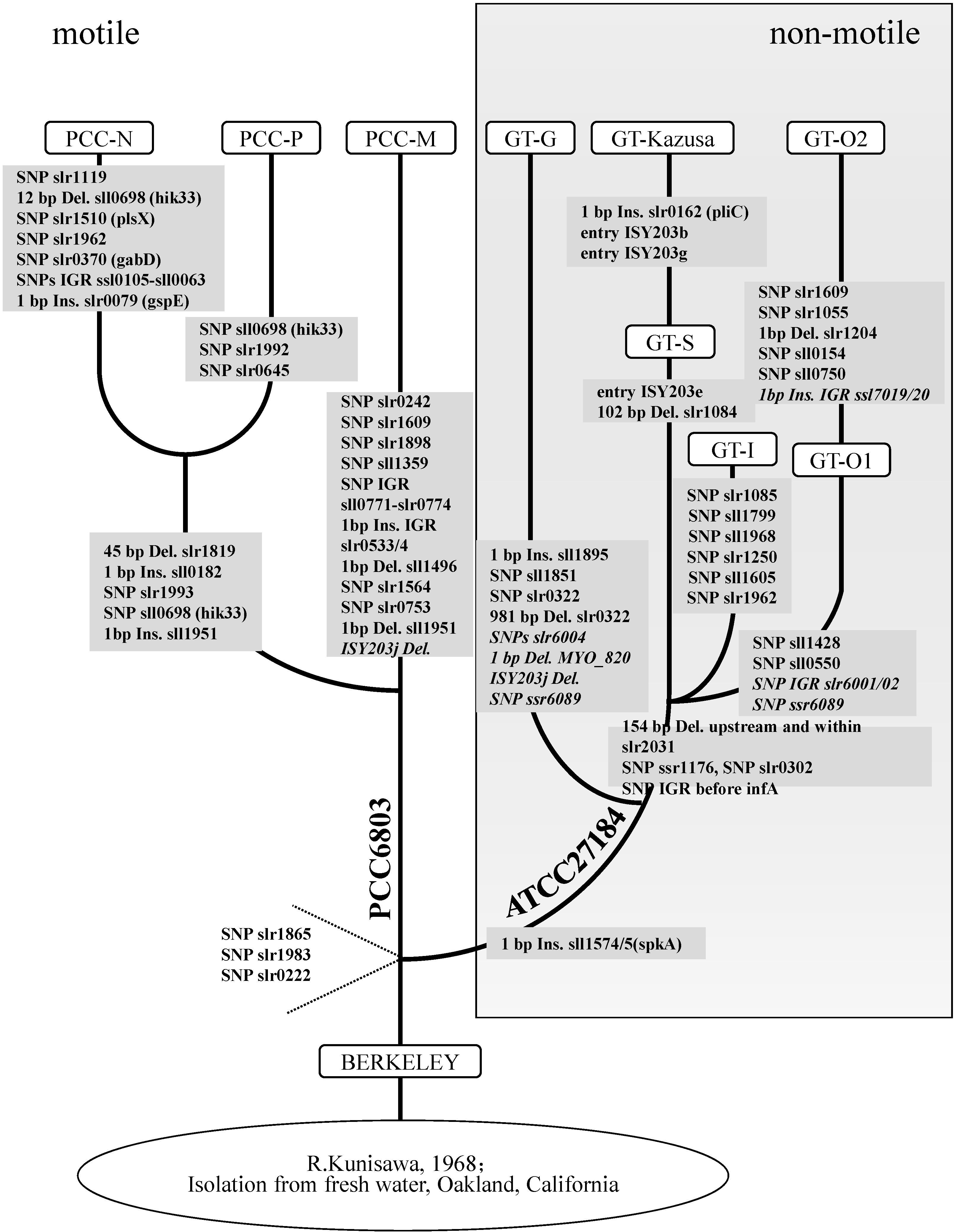
3. Experimental Section
3.1. Strain and DNA Extraction
3.2. Sequencing Methods and Data Analysis
3.3. Mutation Verification
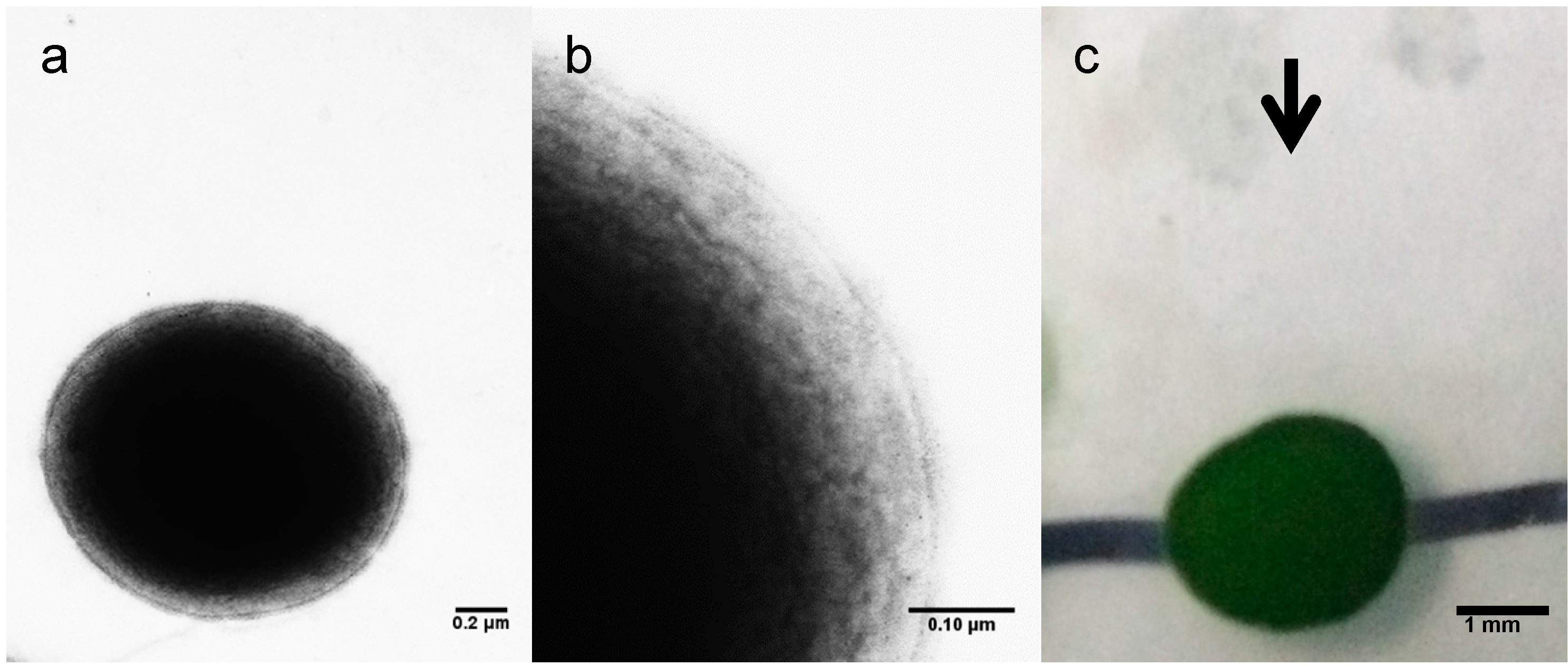
3.4. Electron Microscopy and Motility Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Oliver, J.W.; Machado, I.M.; Yoneda, H.; Atsumi, S. Cyanobacterial conversion of carbon dioxide to 2,3-butanediol. Proc. Natl. Acad. Sci. USA 2013, 110, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Osanai, T.; Oikawa, A.; Numata, K.; Kuwahara, A.; Iijima, H.; Doi, Y.; Saito, K.; Hirai, M.Y. Pathway-level acceleration of glycogen catabolism by a response regulator in the cyanobacterium Synechocystis species PCC 6803. Plant Physiol. 2014, 164, 1831–1841. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.X.; Li, H.C.; Wei, Y.P.; Chu, W.Y.; Chong, Y.L.; Long, X.H.; Liu, Z.P.; Qin, S.; Shao, H.B. Signal transduction pathways in Synechocystis sp. PCC 6803 and biotechnological implications under abiotic stress. Crit. Rev. Biotechnol. 2015, 35, 269–280. [Google Scholar] [CrossRef] [PubMed]
- McKinlay, J.B.; Harwood, C.S. Photobiological production of hydrogen gas as a biofuel. Curr. Opin. Biotechnol. 2010, 21, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, M.; Tabata, S. Synechocystis sp PCC 6803—A useful tool in the study of the genetics of cyanobacteria. Photosynth. Res. 2001, 70, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [PubMed]
- Williams, J.G.K. Construction of specific mutations in photosystem II photosynthetic reaction center by genetic engineering methods in Synechocystis 6803. Methods Enzymol. 1988, 167, 766–778. [Google Scholar]
- Kaneko, T.; Tanaka, A.; Sato, S.; Kotani, H.; Sazuka, T.; Miyajima, N.; Sugiura, M.; Tabata, S. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. I. Sequence features in the 1 Mb region from map positions 64% to 92% of the genome. DNA Res. 1995, 2, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Sato, S.; Kotani, H.; Tanaka, A.; Asamizu, E.; Nakamura, Y.; Miyajima, N.; Hirosawa, M.; Sugiura, M.; Sasamoto, S.; et al. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996, 3, 109–136. [Google Scholar] [CrossRef] [PubMed]
- Tajima, N.; Sato, S.; Maruyama, F.; Kaneko, T.; Sasaki, N.V.; Kurokawa, K.; Ohta, H.; Kanesaki, Y.; Yoshikawa, H.; Tabata, S.; et al. Genomic structure of the cyanobacterium Synechocystis sp. PCC 6803 strain GT-S. DNA Res. 2011, 18, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Kanesaki, Y.; Shiwa, Y.; Tajima, N.; Suzuki, M.; Watanabe, S.; Sato, N.; Ikeuchi, M.; Yoshikawa, H. Identification of substrain-specific mutations by massively parallel whole-genome resequencing of Synechocystis sp. PCC 6803. DNA Res. 2012, 19, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Trautmann, D.; Voss, B.; Wilde, A.; Al-Babili, S.; Hess, W.R. Microevolution in cyanobacteria: Re-sequencing a motile substrain of Synechocystis sp. PCC 6803. DNA Res. 2012, 19, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.N.; Crawford, T.S.; Jeffs, A.; Stockwell, P.A.; Eaton-Rye, J.J.; Summerfield, T.C. Whole genome re-sequencing of two ‘wild-type’ strains of the model cyanobacterium Synechocystis sp. PCC 6803. N. Z. J. Bot. 2014, 52, 36–47. [Google Scholar] [CrossRef]
- Tillich, U.M.; Wolter, N.; Franke, P.; Duehring, U.; Frohme, M. Screening and genetic characterization of thermo-tolerant Synechocystis sp. PCC 6803 strains created by adaptive evolution. BMC Biotechnol. 2014, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, J.; Kanesaki, Y.; Iwata, N.; Asakura, R.; Funamizu, K.; Tasaki, R.; Agatsuma, M.; Tahara, H.; Matsuhashi, A.; Yoshikawa, H.; et al. Genomic analysis of parallel-evolved cyanobacterium Synechocystis sp. PCC 6803 under acid stress. Photosynth. Res. 2015, 125, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, G.; Qin, C.; Wang, Y.; Wei, D. Slr0643, an S2P homologue, is essential for acid acclimation in the cyanobacterium Synechocystis sp. PCC 6803. Microbiology 2012, 158, 2765–2780. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Chen, G.; Wang, Y.; Ding, Q.; Wei, D. Sll0528, a site-2-protease, is critically involved in cold, salt and hyperosmotic stress acclimation of cyanobacterium Synechocystis sp. PCC 6803. Int. J. Mol. Sci. 2014, 15, 22678–22693. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Chen, G.; Ren, D. Construction of sll0862 or slr0643 disrupted mutants and their phenotype analysis in Synechocystis sp. PCC 6803. Wei Sheng Wu Xue Bao 2009, 49, 1470–1476. (In Chinese) [Google Scholar] [PubMed]
- Chen, G.; Zhang, X. New insights into S2P signaling cascades: Regulation, variation, and conservation. Protein Sci. 2010, 19, 2015–2030. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [PubMed]
- Koboldt, D.C.; Chen, K.; Wylie, T.; Larson, D.E.; McLellan, M.D.; Mardis, E.R.; Weinstock, G.M.; Wilson, R.K.; Ding, L. VarScan: Variant detection in massively parallel sequencing of individual and pooled samples. Bioinformatics 2009, 25, 2283–2285. [Google Scholar] [CrossRef] [PubMed]
- Koboldt, D.C.; Zhang, Q.; Larson, D.E.; Shen, D.; McLellan, M.D.; Lin, L.; Miller, C.A.; Mardis, E.R.; Ding, L.; Wilson, R.K. VarScan 2: Somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 2012, 22, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Kamei, A.; Yuasa, T.; Orikawa, K.; Geng, X.X.; Ikeuchi, M. A eukaryotic-type protein kinase, SpkA, is required for normal motility of the unicellular cyanobacterium Synechocystis sp strain PCC 6803. J. Bacteriol. 2001, 183, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Panichkin, V.B.; Arakawa-Kobayashi, S.; Kanaseki, T.; Suzuki, I.; Los, D.A.; Shestakov, S.V.; Murata, N. Serine/threonine protein kinase SpkA in Synechocystis sp. strain PCC 6803 is a regulator of expression of three putative pil4 operons, formation of thick pili, and cell motility. J. Bacteriol. 2006, 188, 7696–7699. [Google Scholar] [CrossRef] [PubMed]
- Mitschke, J.; Georg, J.; Scholz, I.; Sharma, C.M.; Dienst, D.; Bantscheff, J.; Voss, B.; Steglich, C.; Wilde, A.; Vogel, J.; Hess, W.R. An experimentally anchored map of transcriptional start sites in the model cyanobacterium Synechocystis sp. PCC 6803. Proc. Natl. Acad. Sci. USA 2011, 108, 2124–2129. [Google Scholar] [CrossRef] [PubMed]
- Bhaya, D.; Bianco, N.R.; Bryant, D.; Grossman, A. Type IV pilus biogenesis and motility in the cyanobacterium Synechocystis sp. PCC 6803. Mol. Microbiol. 2000, 37, 941–951. [Google Scholar] [CrossRef] [PubMed]
- de Alda, J.; Houmard, J. Genomic survey of cAMP and cGMP signalling components in the cyanobacterium Synechocystis PCC 6803. Microbiology 2000, 146, 3183–3194. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, L.; Wang, J.; Qiao, J.; Zhang, W. Proteomic analysis reveals resistance mechanism against biofuel hexane in Synechocystis sp. PCC 6803. Biotechnol. Biofuels 2012, 5, 68. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, S.; Geng, X.; Ikeuchi, M. pilG gene cluster and split pilL genes involved in pilus biogenesis, motility and genetic transformation in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 2002, 43, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wallis, J.W.; McLellan, M.D.; Larson, D.E.; Kalicki, J.M.; Pohl, C.S.; McGrath, S.D.; Wendl, M.C.; Zhang, Q.; Locke, D.P.; et al. BreakDancer: An algorithm for high-resolution mapping of genomic structural variation. Nat. Methods 2009, 6, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Cyanobase. Available online: http://genome.microbedb.jp/cyanobase/Synechocystis (accessed on 10 October 2015).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Q.; Chen, G.; Wang, Y.; Wei, D. Identification of Specific Variations in a Non-Motile Strain of Cyanobacterium Synechocystis sp. PCC 6803 Originated from ATCC 27184 by Whole Genome Resequencing. Int. J. Mol. Sci. 2015, 16, 24081-24093. https://doi.org/10.3390/ijms161024081
Ding Q, Chen G, Wang Y, Wei D. Identification of Specific Variations in a Non-Motile Strain of Cyanobacterium Synechocystis sp. PCC 6803 Originated from ATCC 27184 by Whole Genome Resequencing. International Journal of Molecular Sciences. 2015; 16(10):24081-24093. https://doi.org/10.3390/ijms161024081
Chicago/Turabian StyleDing, Qinglong, Gu Chen, Yuling Wang, and Dong Wei. 2015. "Identification of Specific Variations in a Non-Motile Strain of Cyanobacterium Synechocystis sp. PCC 6803 Originated from ATCC 27184 by Whole Genome Resequencing" International Journal of Molecular Sciences 16, no. 10: 24081-24093. https://doi.org/10.3390/ijms161024081






