Evaluating Osteogenic Potential of Ligamentum Flavum Cells Cultivated in Photoresponsive Hydrogel that Incorporates Bone Morphogenetic Protein-2 for Spinal Fusion
Abstract
:1. Introduction
2. Results
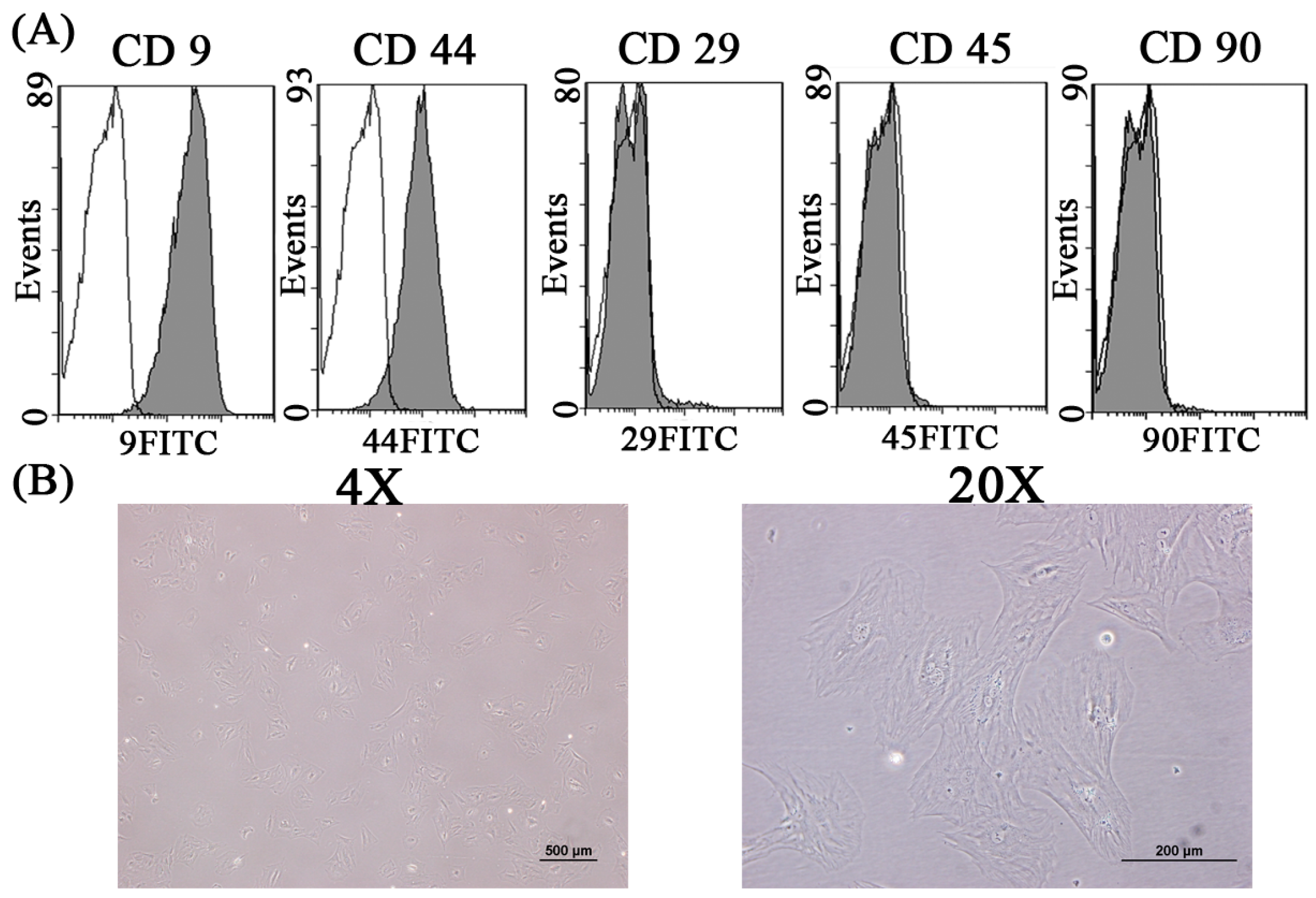
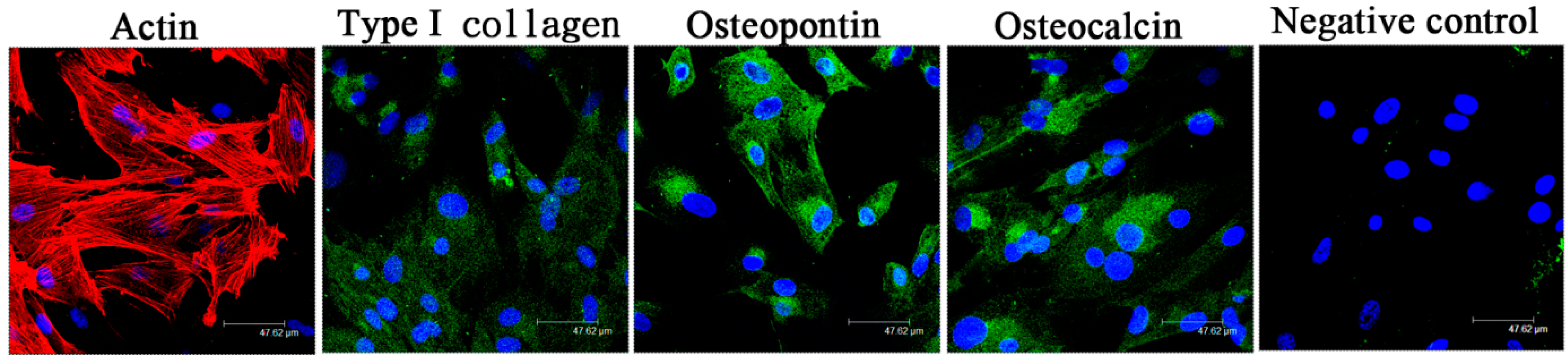
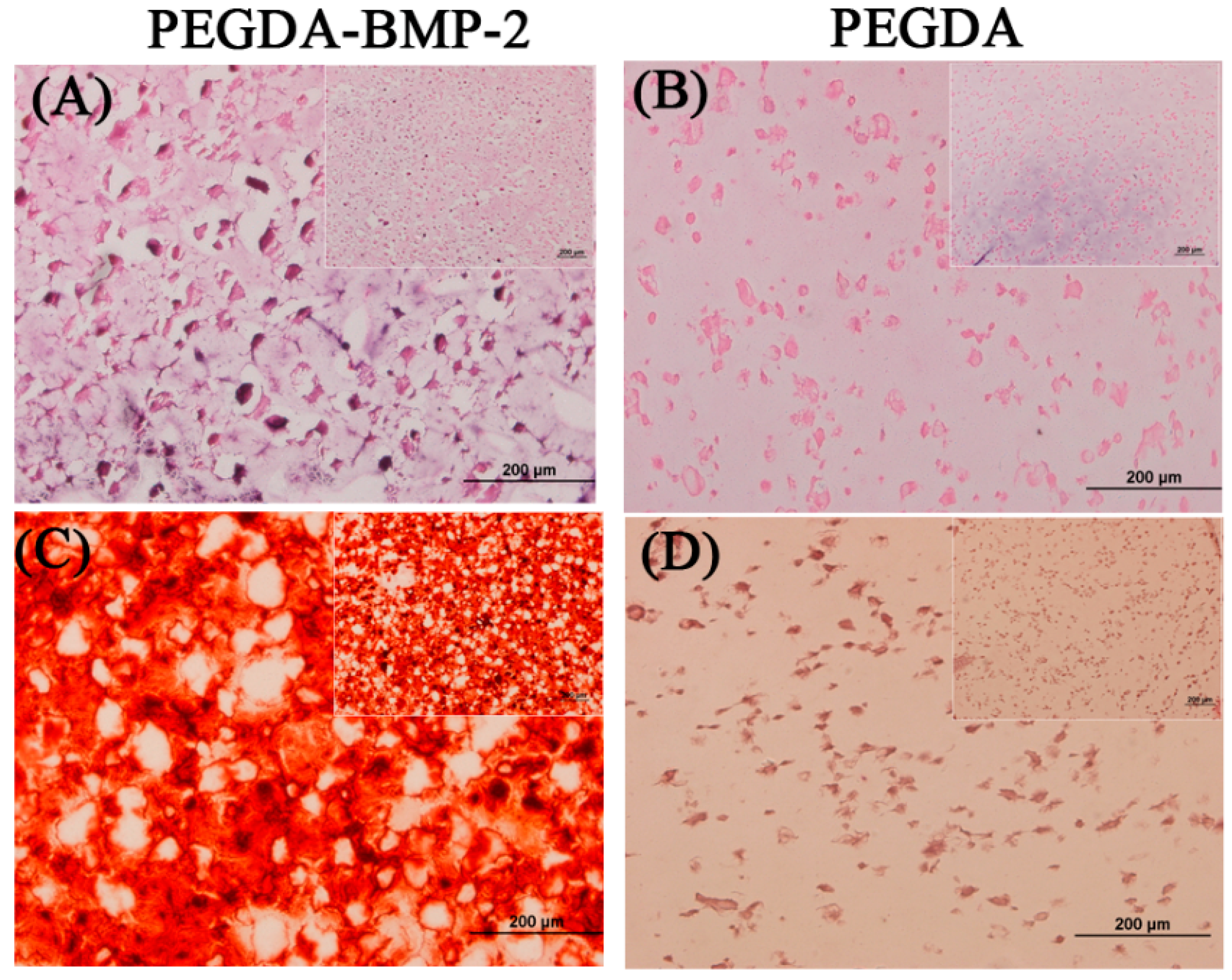
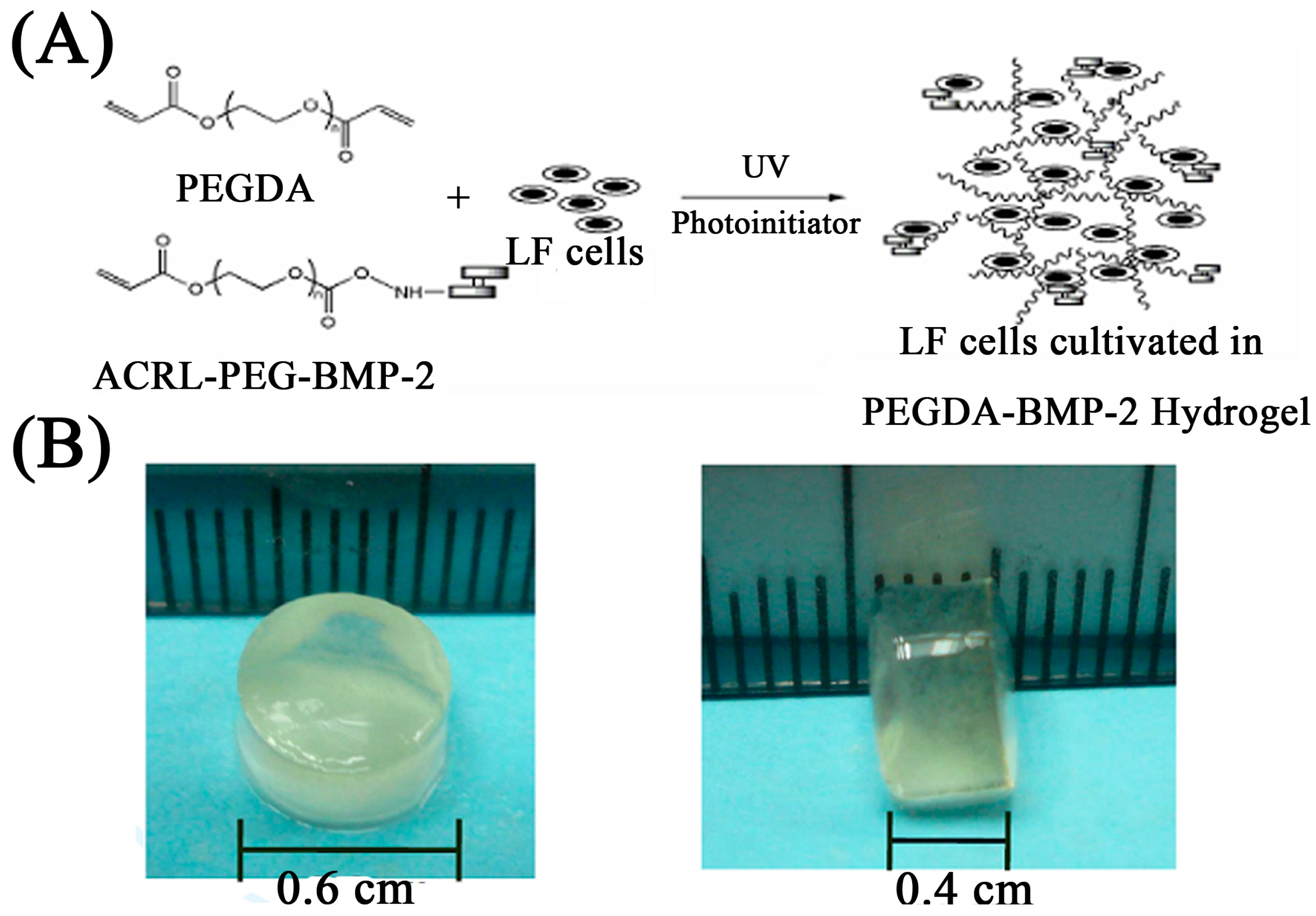
2.1. The in Vitro Study of Ligamentum Flavum Cells
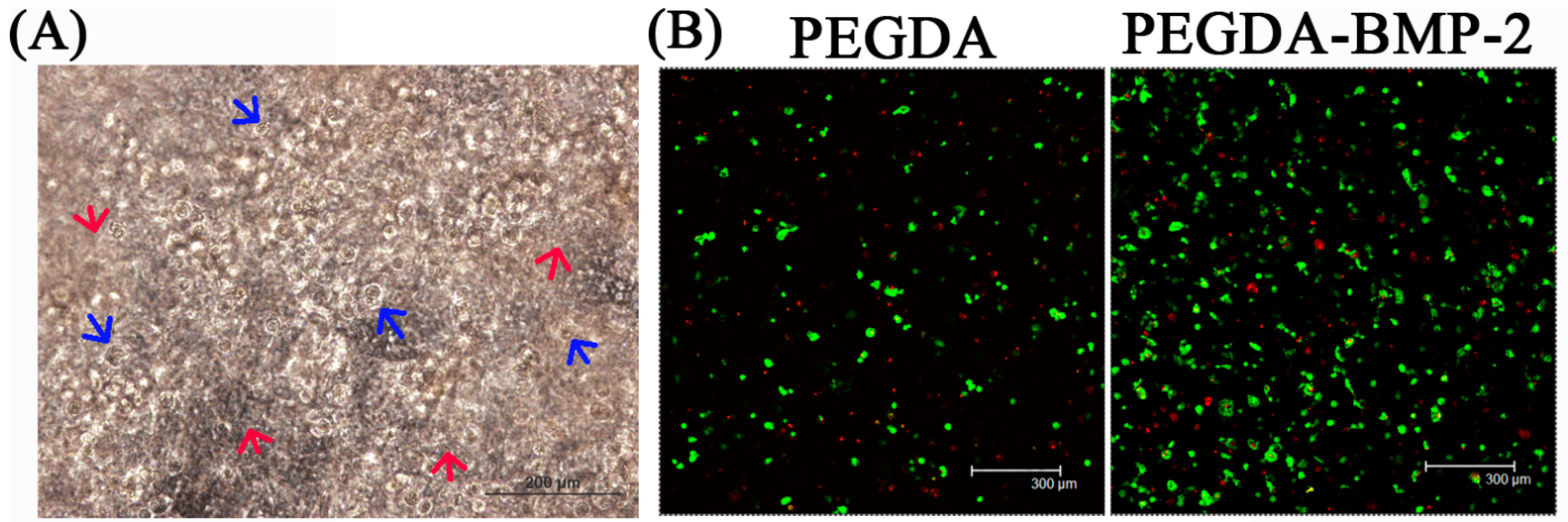

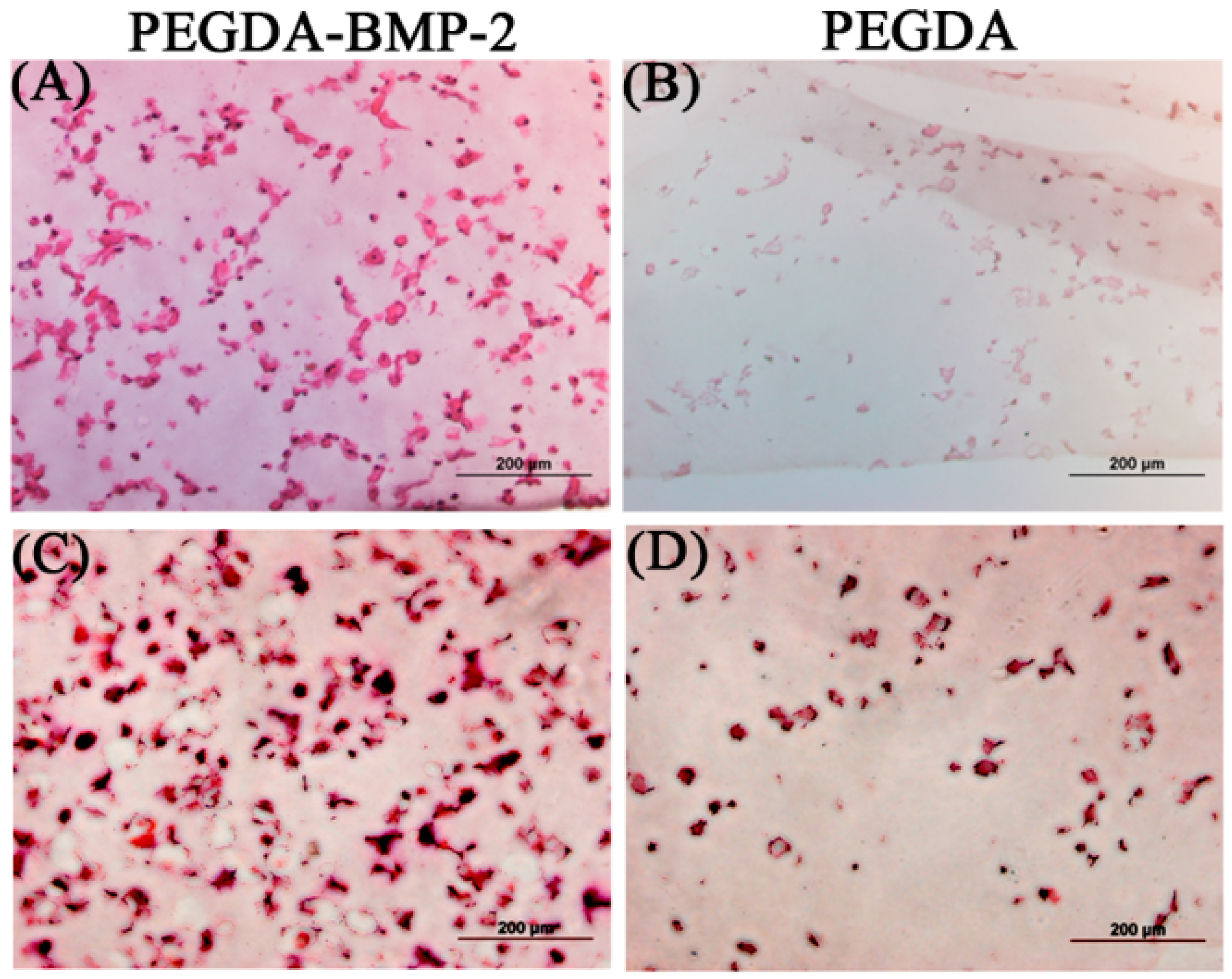
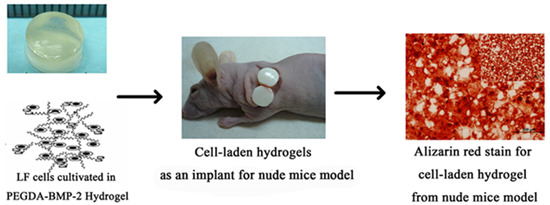
2.2. The in Vivo Study of LF Cells on Hydrogels
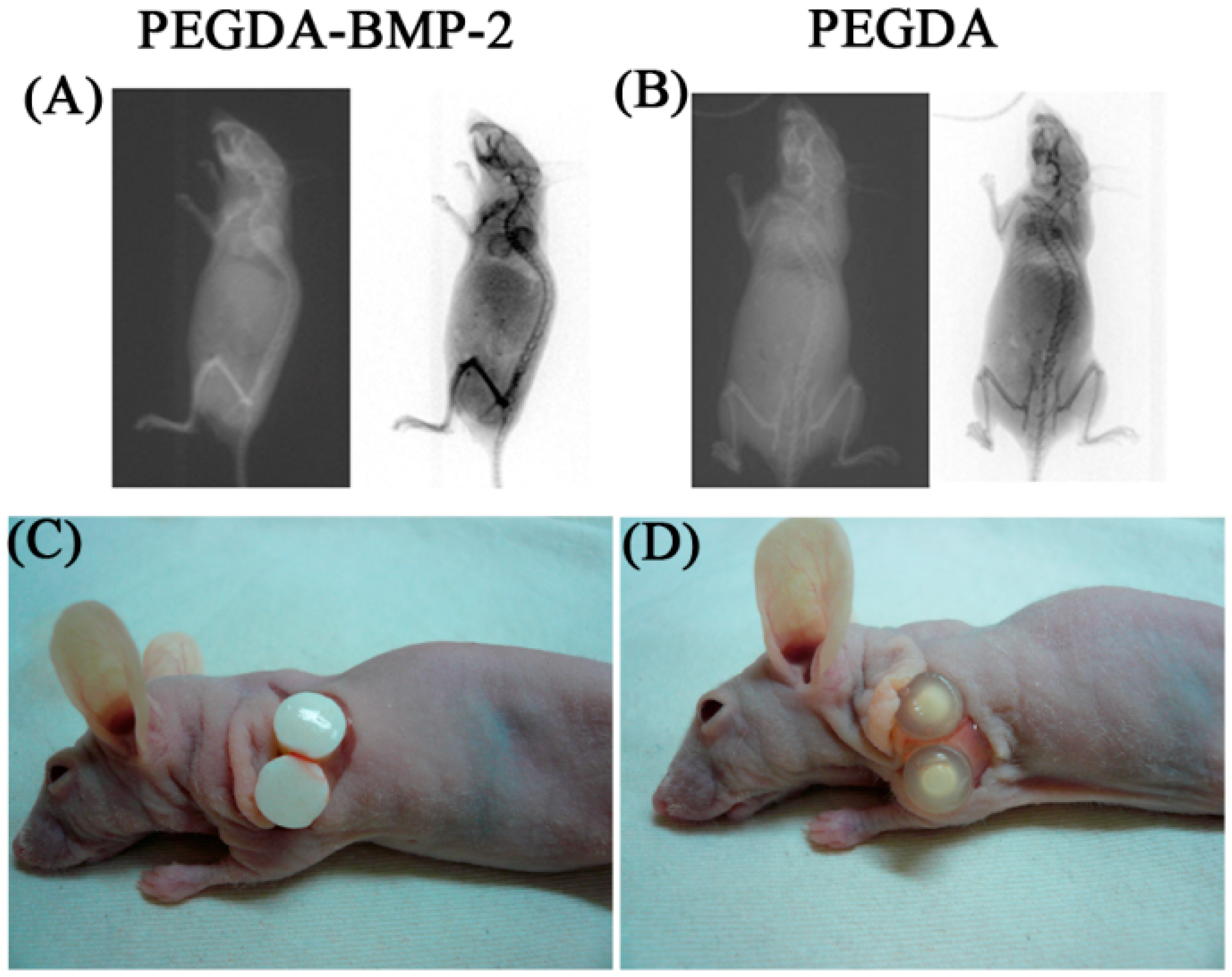

3. Discussion
4. Experimental Section
4.1. Isolation of Ligamentum Flavum Cells
4.2. Identification of Ligamentum Flavum Cells
4.3. Conjugation of Polyethylene Glycol Tethered BMP-2
4.4. Gel Fabrication and LF Cells Photo-Encapsulation
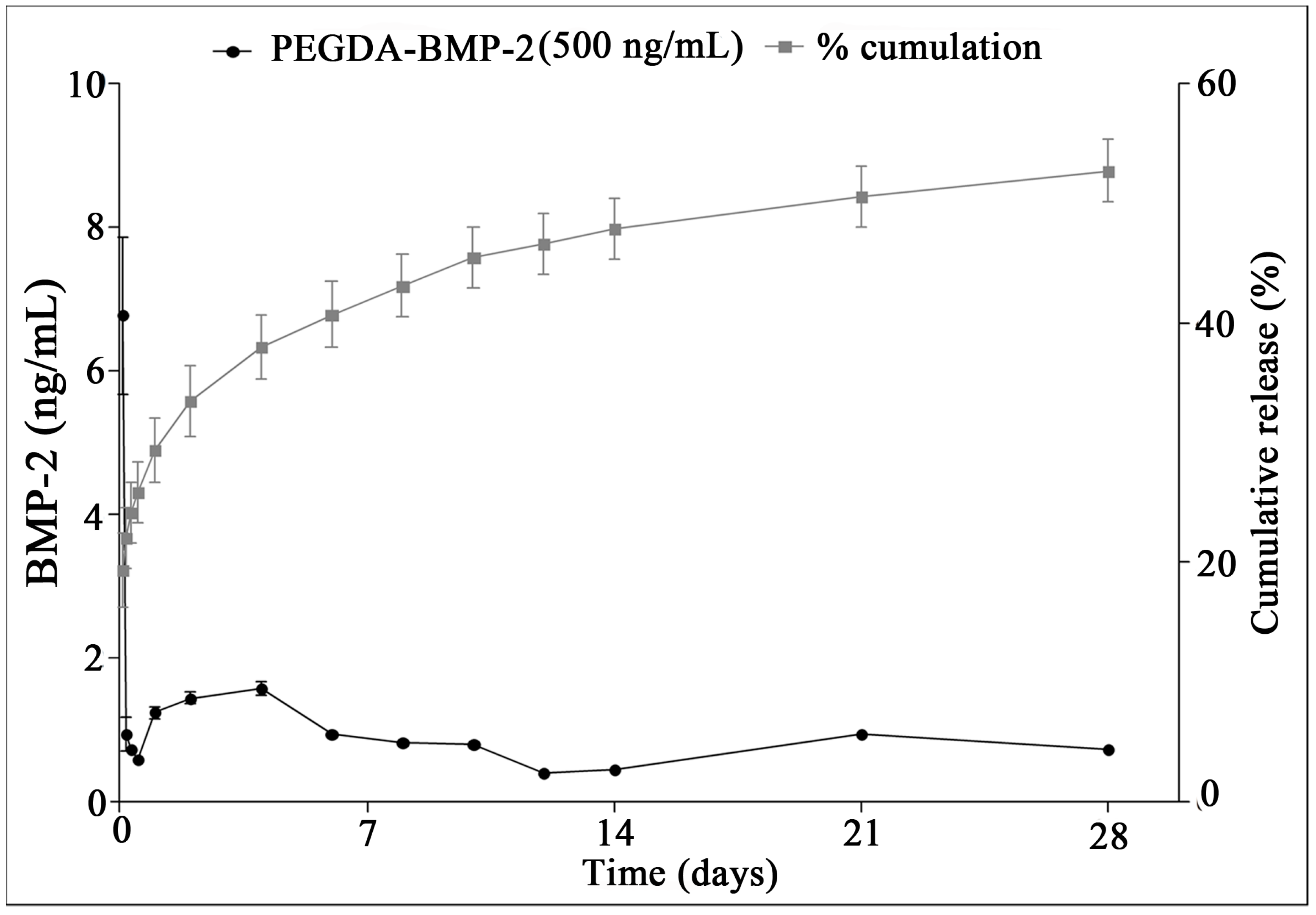
4.5. In Vitro Release Kinetics of BMP-2
4.6. Bioreactor Cultivation in Vitro
4.7. In Vitro Release Profile of BMP-2
4.8. In Vivo Implantation: Nude Mice Model
4.9. Radiographic Assessment
4.10. Cell Viability
4.11. Alkaline Phosphatase Activity and Cell Proliferation
4.12. Quantification of Calcium Content
4.13. Real-Time PCR Analysis
| Gene Symbol | Primer Sequence (5ʹ→3ʹ) | Tm (°C) | |
|---|---|---|---|
| GAPDH | F: | 5′-GAGCTGAACGGGAAACTCAC-3ʹ | 67.8 |
| R: | 5′-GGTCTGGGATGGAAACTGTG-3ʹ | ||
| Collagen I | F: | 5′-GATGGTCAGCCTGGACACA-3ʹ | 67.8 |
| R: | 5′-CGAAGGCCAGCAGGTCCAA-3ʹ | ||
| Osteopontin | F: | 5′-CAGTGGCTCAGCACCTGAA-3ʹ | 67.8 |
| R: | 5′-CGGCTCGATGGCTAGCTT-3ʹ | ||
4.14. Histology Staining
4.15. Immunofluorescence Staining
4.16. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Specchia, N.; Pagnotta, A.; Gigante, A.; Logroscino, G.; Toesca, A. Characterization of cultured human ligamentum flavum cells in lumbar spine stenosis. J. Orthop. Res. 2001, 19, 294–300. [Google Scholar] [CrossRef]
- Moon, S.H.; Park, S.R.; Kim, H.; Kwon, U.H.; Kim, K.H.; Kim, H.S.; Lee, H.M. Biologic modification of ligamentum flavum cells by marker gene transfer and recombinant human bone morphogenetic protein-2. Spine 2004, 29, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Mimatsu, K.; Sato, K.; Hashizume, Y. Histopathologic and morphometric study of spinal cord lesion in a chronic cord compression model using bone morphogenetic protein in rabbits. Spine 1992, 17, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Nagai, E.; Murata, H.; Tsubone, T.; Shirakura, Y.; Sugiyama, T.; Taguchi, T.; Kawai, S. Involvement of bone morphogenic protein-2 in the pathological ossification process of the spinal ligament. Rheumatology 2001, 40, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- De Jong, D.S.; Steegenga, W.T.; Hendriks, J.M.; van Zoelen, E.J.; Olijve, W.; Dechering, K.J. Regulation of notch signaling genes during BMP2-induced differentiation of osteoblast precursor cells. Biochem. Biophys. Res. Commun. 2004, 320, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.D.; Valentini, R.F. Retention and activity of BMP-2 in hyaluronic acid-based scaffolds in vitro. J. Biomed. Mater. Res. 2002, 59, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Ishidou, Y.; Yonemori, K.; Nagamine, T.; Origuchi, N.; Maeda, S.; Imamura, T.; Kato, M.; Yoshida, H.; Sampath, T.K.; et al. Expression and localization of bone morphogenetic proteins (BMPs) and BMP receptors in ossification of the ligamentum flavum. Bone 1997, 21, 23–30. [Google Scholar] [CrossRef]
- Kim, H.N.; Min, W.K.; Jeong, J.H.; Kim, S.G.; Kim, J.R.; Kim, S.Y.; Choi, J.Y.; Park, B.C. Combination of Runx2 and BMP2 increases conversion of human ligamentum flavum cells into osteoblastic cells. BMB Rep. 2011, 44, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.H.; Kim, H.; Kwon, U.H. De novo osteogenesis from human ligamentum flavum by adenovirus-mediated bone morphogenetic protein-2 gene transfer. Spine 2005, 24, 2749–2754. [Google Scholar] [CrossRef]
- Musumeci, G.; Loreto, C.; Castorina, S.; Imbesi, R.; Leonardi, R.; Castrogiovanni, P. New perspectives in the treatment of cartilage damage. Poly(ethylene glycol) diacrylate (PEGDA) scaffold. A review. Ital. J. Anat. Embryol. 2013, 118, 204–210. [Google Scholar] [PubMed]
- Ingavle, G.C.; Dormer, N.H.; Gehrke, S.H.; Detamore, M.S. Using chondroitin sulfate to improve the viability and biosynthesis of chondrocytes encapsulated in interpenetrating network (IPN) hydrogels of agarose and poly(ethylene glycol) diacrylate. J. Mater. Sci. 2012, 23, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Ramirez, W.F.; Anseth, K.S. Photopolymerizaed, multilaminated matrix devices with optimized nonuniform initial concentration profiles to control drug release. J. Pharm. Sci. 2000, 89, 45–51. [Google Scholar] [CrossRef]
- Fukuyama, S.; Nakamura, T.; Ikeda, T.; Takagi, K. The effect of mechanical stress on hypertrophy of the lumbar ligamentum flavum. J. Spinal Disord. 1995, 8, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Yonenobu, K.; Miyamoto, S.; Okada, K. Pathology of ossification of the posterior longitudinal ligament and ligamentum flavum. Clin. Orthop. Relat. Res. 1999, 359, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Aspenberg, P.; Jeppsson, C.; Economides, A.N. The bone morphogenetic proteins antagonist Noggin inhibits membranous ossification. J. Bone Miner. Res. 2001, 16, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.M.; Suk, K.S.; Lee, B.H.; Kim, H.S.; Lee, K.I.; Park, S.Y.; Lee, H.M.; Moon, S.H. Herniated intervertebral disk induces hypertrophy and ossification of ligamentum flavum. J. Spinal Disord. Tech. 2014, 27, 382–389. [Google Scholar] [CrossRef]
- Zhong, Z.M.; Chen, J.T. Phenotypic characterization of ligamentum flavum cells from patients with ossification of ligamentum flavum. Yonsei Med. J. 2009, 50, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Wei, Y.H.; Chu, I.M.; Yao, C.L. Effect of chondroitin sulphate C on the in vitro and in vivo chondrogenesis of mesenchymal stem cells in crosslinked type II collagen scaffolds. J. Tissue Eng. Regen. Med. 2013, 7, 665–672. [Google Scholar] [CrossRef]
- Hassan, W.; Dong, Y.; Wang, W. Encapsulation and 3D culture of human adipose-derived stem cells in an in-situ crosslinked hybrid hydrogel composed of PEG-based hyperbranched copolymer and hyaluronic acid. Stem Cell Res. Ther. 2013, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Fermanian, S.; Gibson, M.; Unterman, S.; Herzka, D.A.; Cascio, B.; Coburn, J.; Hui, A.Y.; Marcus, N.; Gold, G.E.; et al. Human cartilage repair with a photoreactive adhesive-hydrogel composite. Sci. Trans. Med. 2013, 5, 167ra6. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Chang, C.H.; Wang, K.C.; Su, C.I.; Liu, H.T.; Yu, C.M.; Wong, C.B.; Wang, I.C.; Whu, S.W.; Liu, H.W. Enhancement of rotator cuff tendon-bone healing with injectable periosteum progenitor cells-BMP-2 hydrogel in vivo. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Olabisi, R.M.; Lazard, Z.W.; Franco, C.L.; Hall, M.A.; Kwon, S.K.; Sevick-Muraca, E.M.; Hipp, J.A.; Davis, A.R.; Olmsted-Davis, E.A.; West, J.L. Hydrogel microsphere encapsulation of a cell-based gene therapy system increases cell survival of injected cells, transgene expression, and bone volume in a model of heterotopic ossification. Tissue Eng. Part A 2010, 16, 3727–3736. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.F.; Fan, D.W.; Sun, C.G. Recombinant human bone morphogenetic protein-2-induced ossification of the ligamentum flavum in rats and the associated global modification of histone H3. J. Neurosurg. 2014, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rauch, F.; Lauzier, D.; Croteau, S.; Travers, R.; Glorieux, F.H.; Hamdy, R. Temporal and spatial expression of bone morphogenetic protein-2, -4, and -7 during distraction osteogenesis in rabbits. Bone 2000, 27, 453–459. [Google Scholar] [CrossRef]
- Canalis, E.; Economides, A.N.; Gazzerro, E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr. Rev. 2003, 24, 218–235. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Cao, X. BMP signaling in skeletal development. Biochem. Biophys. Res. Commun. 2005, 328, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Yamamoto, M.; Tabata, Y. Enhanced osteoinduction by controlled release of bone morphogenetic protein-2 from biodegradable sponge composed of gelatin and β-tricalcium phosphate. Biomaterials 2005, 26, 4856–4865. [Google Scholar] [CrossRef] [PubMed]
- Murakami, N.; Saito, N.; Takahashi, J.; Ota, H.; Horiuchi, H.; Nawatam, M.; Okada, T.; Nozaki, K.; Takaoka, K. Repair of a proximal femoral bone defect in dogs using a porous surfaced prosthesis in combination with recombinant BMP-2 and a synthetic polymer carrier. Biomaterials 2003, 24, 2153–2159. [Google Scholar] [CrossRef]
- Chen, D.; Harris, M.A.; Rossini, G.; Dunstan, C.R.; Dallas, S.L.; Feng, J.Q.; Mundy, G.R.; Harris, S.E. Bone morphogenetic protein 2 (BMP-2) enhances BMP-3, BMP-4, and bone cell differentiation marker gene expression during the induction of mineralized bone matrix formation in cultures of fetal rat calvarial osteoblasts. Calcif. Tissue Int. 1997, 60, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Luppen, C.A.; Smith, E.; Spevak, L.; Boskey, A.L.; Frenkel, B. Bone morphogenetic protein-2 restores mineralization in glucocorticoid-inhibited MC3T3-E1 osteoblast cultures. J. Bone Miner.Res. 2003, 18, 1186–1197. [Google Scholar] [CrossRef] [PubMed]
- Luppen, C.A.; Leclerc, N.; Noh, T.; Barski, A.; Khokhar, A.; Boskey, A.L.; Smith, E.; Frenkel, B. Brief bone morphogenetic protein 2 treatment of glucocorticoid-inhibited MC3T3-E1 osteoblasts rescues commitment-associated cell cycle and mineralization without alteration of Runx2. J. Biol. Chem. 2003, 278, 44995–45003. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Katagiri, T.; Toyoda, H.; Takada, T.; Yanai, T.; Fukuda, T.; Chung, U.I.; Koike, T.; Takaoka, K.; Kamijo, R. Heparin potentiates the in vivo ectopic bone formation induced by bone morphogenetic protein-2. J. Biol. Chem. 2006, 281, 23246–23253. [Google Scholar] [CrossRef] [PubMed]
- Na, K.; Kim, S.W.; Sun, B.K.; Woo, D.G.; Yang, H.N.; Chung, H.M.; Park, K.H. Osteogenic differentiation of rabbit mesenchymal stem cells in thermo-reversible hydrogel constructs containing hydroxyapatite. Biomaterials 2007, 28, 2631–2637. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, I.S.; Cho, T.H.; Lee, K.B.; Hwang, S.J.; Tae, G.; Noh, I.; Lee, S.H.; Park, Y.; Sun, K. Bone regeneration using hyaluronic acid-based hydrogel with bone morphogenic protein-2 and human mesenchymal stem cells. Biomaterials 2007, 28, 1830–1837. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.S.; Garland, J.; Infante, A.; Sanders, R.W.; Sagi, H.C. Wound complications associated with bone morphogenetic protein-2 in orthopaedic trauma surgery. J. Orthop. Trauma 2014, 28, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Govender, S.; Csimma, C.; Genant, H.K.; Valentin-Opran, A.; Amit, Y.; Arbel, R.; Aro, H.; Atar, D.; Bishay, M.; Börner, M.G.; et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: A prospective, controlled, randomized study of four hundred and fifty patients. J. Bone Jt. Surg. Am. 2002, 84, 2123–2134. [Google Scholar] [CrossRef]
- Lykissas, M.G.; Aichmair, A.; Sama, A.A.; Hughes, A.P.; Lebl, D.R.; Cammisa, F.P.; Girardi, F.P. Nerve injury and recovery after lateral lumbar interbody fusion with and without bone morphogenetic protein-2 augmentation: A cohort-controlled study. Spine J. 2014, 14, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.A.; Gruber, H.E.; Howard, B.A.; Tabor, O.B.; Murakami, T.; Kwiatkowski, T.C.; Wozney, J.M.; Hanley, E.N. Safety of recombinant human bone morphogenetic protein-2 after spinal laminectomy in the dog. Spine 1999, 24, 747–754. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, C.-W.; Chen, W.-C.; Liu, H.-W.; Wang, I.-C.; Chen, C.-H. Evaluating Osteogenic Potential of Ligamentum Flavum Cells Cultivated in Photoresponsive Hydrogel that Incorporates Bone Morphogenetic Protein-2 for Spinal Fusion. Int. J. Mol. Sci. 2015, 16, 23318-23336. https://doi.org/10.3390/ijms161023318
Chiang C-W, Chen W-C, Liu H-W, Wang I-C, Chen C-H. Evaluating Osteogenic Potential of Ligamentum Flavum Cells Cultivated in Photoresponsive Hydrogel that Incorporates Bone Morphogenetic Protein-2 for Spinal Fusion. International Journal of Molecular Sciences. 2015; 16(10):23318-23336. https://doi.org/10.3390/ijms161023318
Chicago/Turabian StyleChiang, Chih-Wei, Wei-Chuan Chen, Hsia-Wei Liu, I-Chun Wang, and Chih-Hwa Chen. 2015. "Evaluating Osteogenic Potential of Ligamentum Flavum Cells Cultivated in Photoresponsive Hydrogel that Incorporates Bone Morphogenetic Protein-2 for Spinal Fusion" International Journal of Molecular Sciences 16, no. 10: 23318-23336. https://doi.org/10.3390/ijms161023318