Synergistic Effect of Bolus Exposure to Zinc Oxide Nanoparticles on Bleomycin-Induced Secretion of Pro-Fibrotic Cytokines without Lasting Fibrotic Changes in Murine Lungs
Abstract
:1. Introduction
2. Results
2.1. Characterization of ZnO Nanoparticles
2.2. Effects of BLM and ZnO Nanoparticles on Body and Lung Weights
| Subcutaneous Infusion | SALINE | BLM | ||||
|---|---|---|---|---|---|---|
| Groups | No ZnO | 10 µg ZnO | 30 µg ZnO | No ZnO | 10 µg ZnO | 30 µg ZnO |
| Number of mice | 6 | 7 | 6 | 6 | 6 | 7 |
| Body weight (g) | 22.65 ± 0.53 | 22.36 ± 0.81 | 21.03 ± 1.36 * | 19.17 ± 0.49 | 17.99 ± 0.38 * | 15.63 ± 0.74 ** |
| Relative lung weight a | 9.90 ± 1.26 | 11.52 ± 1.39 | 18.04 ± 1.73 ** | 14.33 ± 1.95 | 15.90 ± 0.91 | 18.91 ± 0.64 ** |
2.3. Effects of ZnO Nanoparticles on Lung Histopathology
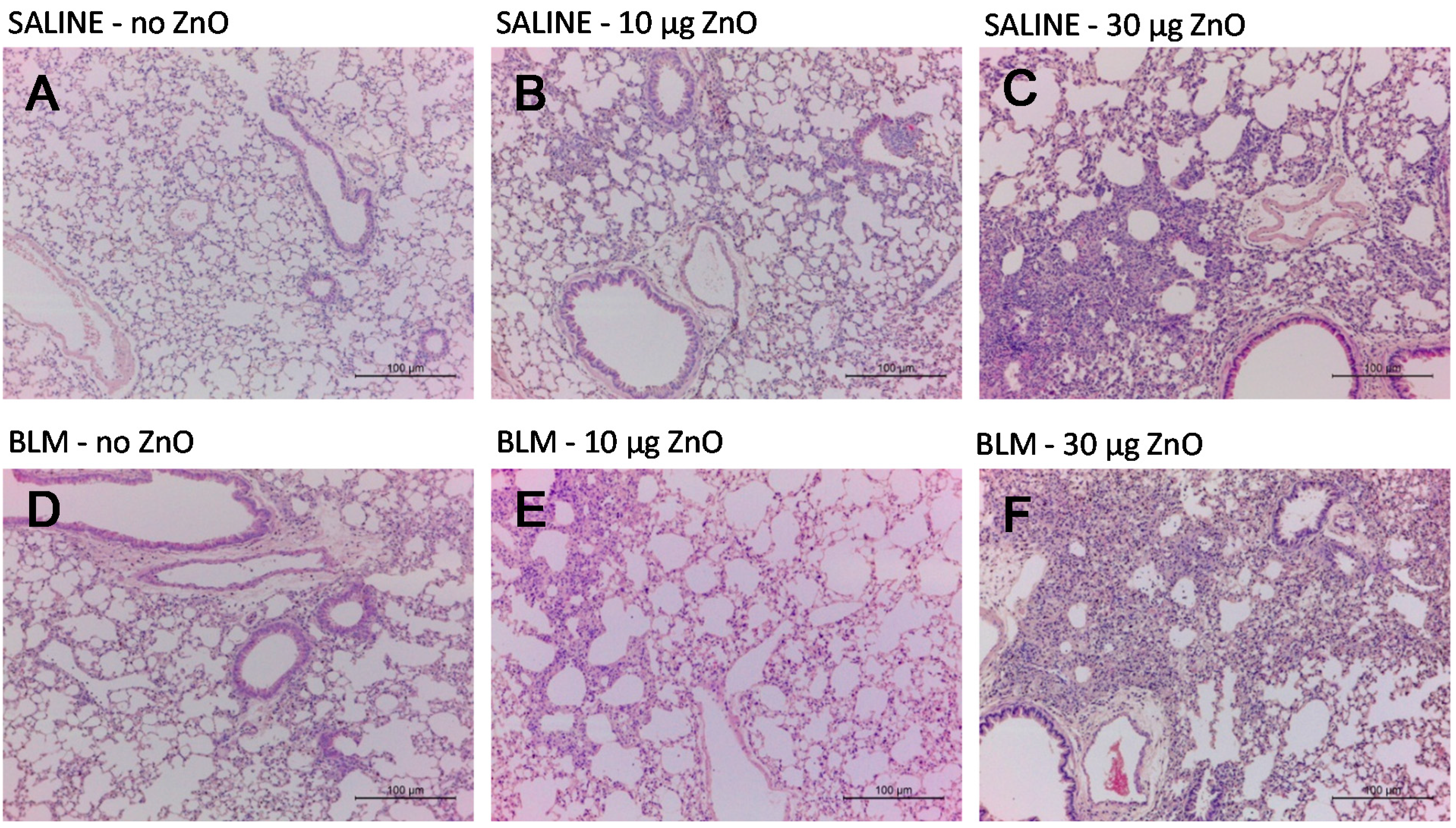

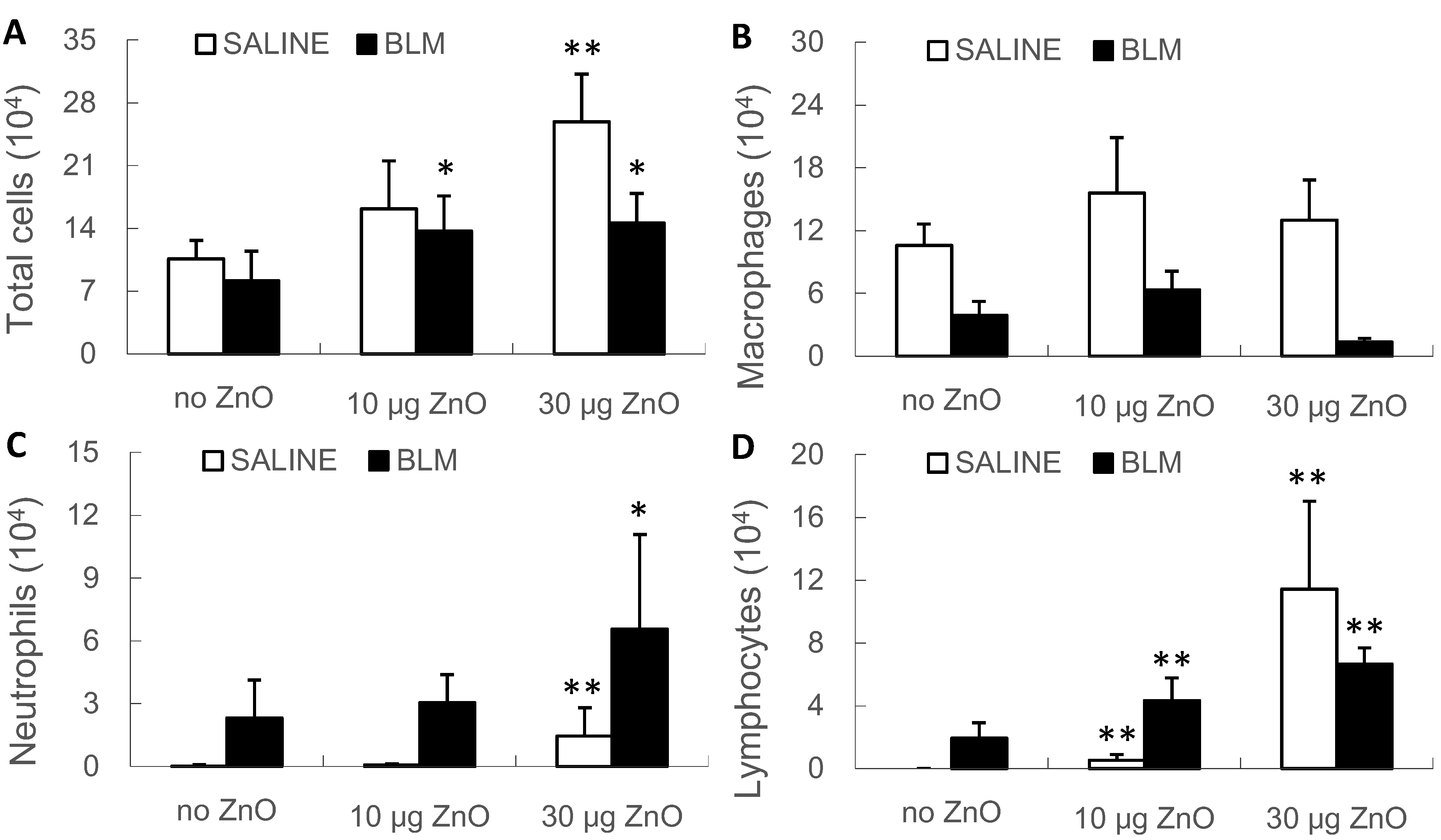
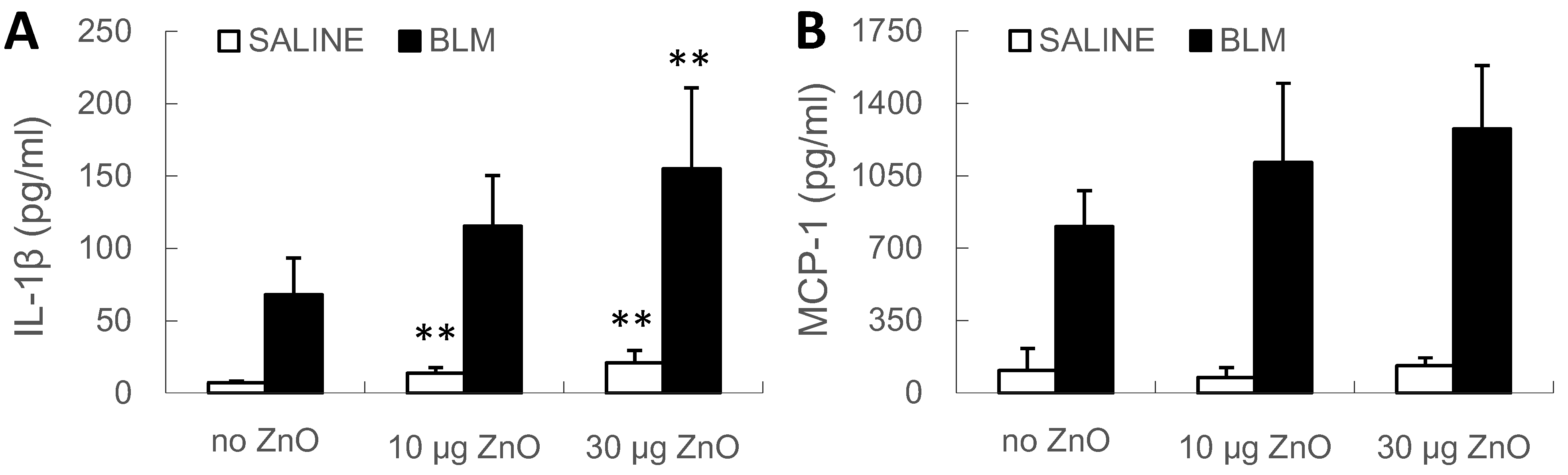
2.4. Effects of ZnO Nanoparticles on BALF Cytology and Cytokine Concentrations
2.5. Interaction between the BLM Treatment and ZnO Exposure
| Parameters | SALINE | BLM | ||
|---|---|---|---|---|
| Coefficient ± SEM | p-Value | Coefficient ± SEM | p-Value | |
| Body weight (g) | ||||
| ZnO exposure level (×10−1/µg) | −0.56 ± 0.18 | 0.007 | −1.18 ± 0.11 | <0.001 |
| IL-1β (µg/mL) | ||||
| ZnO exposure level (per µg) | 0.44 ± 0.11 | 0.725 | 2.90 ± 0.85 | 0.005 |
| MCP-1 (µg/mL) | ||||
| ZnO exposure level (per µg) | 1.11 ± 1.44 | 0.453 | 15.76 ± 7.02 | 0.043 |
2.6. Status at Day 14 after Administration


3. Discussion
4. Experimental Section
4.1. ZnO Nanoparticles
4.2. Animals
4.3. Histopathological Examination
4.4. Total and Differential Cell Count in BALF
4.5. BALF Biochemical Analysis
4.6. Real-Time Quantitative Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
4.7. Hydroxyproline Content
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Donaldson, K.; Stone, V.; Tran, C.L.; Kreyling, W.; Borm, P.J. Nanotoxicology. Occup. Environ. Med. 2004, 61, 727–728. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Becheri, A.; Dürr, M.; Nostro, P.L.; Baglioni, P. Synthesis and characterization of zinc oxide nanoparticles: Application to textiles as UV-absorbers. J. Nanopart. Res. 2008, 10, 679–689. [Google Scholar] [CrossRef]
- Rekha, K.; Nirmala, M.; Nair, M.G.; Anukaliani, A. Structural, optical, photocatalytic and antibacterial activity of zinc oxide and manganese doped zinc oxide nanoparticles. Phys. B 2010, 405, 3180–3185. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Fazlollahi, F.; Kennedy, I.M.; Yacobi, N.R.; Hamm-Alvarez, S.F.; Borok, Z.; Kim, K.J.; Crandall, E.D. Alveolar epithelial cell injury due to zinc oxide nanoparticle exposure. Am. J. Respir. Crit. Care Med. 2010, 182, 1398–1409. [Google Scholar] [CrossRef] [PubMed]
- Krug, H.F.; Wick, P. Nanotoxicology: An interdisciplinary challenge. Angew. Chem. Int. Ed. Engl. 2011, 50, 1260–1278. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.J.; Ahamed, M.; Kumar, S.; Khan, M.M.; Ahmad, J.; Alrokayan, S.A. Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. Int. J. Nanomed. 2012, 7, 845–857. [Google Scholar]
- Kang, T.; Guan, R.; Chen, X.; Song, Y.; Jiang, H.; Zhao, J. In vitro toxicity of different-sized ZnO nanoparticles in Caco-2 cells. Nanoscale Res. Lett. 2013, 8, 496. [Google Scholar] [CrossRef] [PubMed]
- Sahu, D.; Kannan, G.M.; Vijayaraghavan, R.; Anand, T.; Khanum, F. Nanosized zinc oxide induces toxicity in human lung cells. ISRN Toxicol. 2013, 2013, 316075. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmi, V.; Fischer, U.; Weighardt, H.; Schulze-Osthoff, K.; Nickel, C.; Stahlmecke, B.; Kuhlbusch, T.A.; Scherbart, A.M.; Esser, C.; Schins, R.P.; et al. Zinc oxide nanoparticles induce necrosis and apoptosis in macrophages in a p47phox- and Nrf2-independent manner. PLoS One 2013, 8, e65704. [Google Scholar]
- Warheit, D.B.; Sayes, C.M.; Reed, K.L. Nanoscale and fine zinc oxide particles: Can in vitro assays accurately forecast lung hazards following inhalation exposures? Environ. Sci. Technol. 2009, 43, 7939–7945. [Google Scholar] [CrossRef] [PubMed]
- Fukui, H.; Horie, M.; Endoh, S.; Kato, H.; Fujita, K.; Nishio, K.; Komaba, L.K.; Maru, J.; Miyauhi, A.; Nakamura, A.; et al. Association of zinc ion release and oxidative stress induced by intratracheal instillation of ZnO nanoparticles to rat lung. Chem. Biol. Interact. 2012, 198, 29–37. [Google Scholar]
- Cho, W.S.; Duffin, R.; Poland, C.A.; Howie, S.E.; MacNee, W.; Bradley, M.; Megson, I.L.; Donaldson, K. Metal oxide nanoparticles induce unique inflammatory footprints in the lung: Important implications for nanoparticle testing. Environ. Health Perspect. 2010, 118, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.S.; Duffin, R.; Howie, S.E.; Scotton, C.J.; Wallace, W.A.; Macnee, W.; Bradley, M.; Megson, I.L.; Donaldson, K. Progressive severe lung injury by zinc oxide nanoparticles; the role of Zn2+ dissolution inside lysosomes. Part. Fibre Toxicol. 2011, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Moeller, A.; Ask, K.; Warburton, D.; Gauldie, J.; Kolb, M. The bleomycin animal model: A useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int. J. Biochem. Cell Biol. 2008, 40, 362–382. [Google Scholar] [CrossRef] [PubMed]
- Manali, E.D.; Moschos, C.; Triantafillidou, C.; Kotanidou, A.; Psallidas, I.; Karabela., S.P.; Roussos, C.; Papiris, S.; Armaganidis, A.; Stathopoulos, GT.; et al. Static and dynamic mechanics of the murine lung after intratracheal bleomycin. BMC Pulm. Med. 2011, 11, 33. [Google Scholar]
- Tsai, K.D.; Yang, S.M.; Lee, J.C.; Wong, H.Y.; Shih, C.M.; Lin, T.H.; Tseng, M.J.; Chen, W. Panax notoginseng attenuates bleomycin-induced pulmonary fibrosis in mice. Evid. Based Complement. Alternat. Med. 2011, 2011, 404761. [Google Scholar]
- Bogatkevich, G.S.; Ludwicka-Bradley, A.; Nietert, P.J.; Akter, T.; van Ryn, J.; Silver, R.M. Antiinflammatory and antifibrotic effects of the oral direct thrombin inhibitor dabigatran etexilate in a murine model of interstitial lung disease. Arthritis Rheumatol. 2011, 63, 1416–1425. [Google Scholar] [CrossRef]
- Harrison, J.H., Jr.; Lazo, J.S. High dose continuous infusion of bleomycin in mice: A new model for drug-induced pulmonary fibrosis. J. Pharmacol. Exp. Ther. 1987, 243, 1185–1194. [Google Scholar] [PubMed]
- Yasui, H.; Gabazza, E.C.; Tamaki, S.; Kobayashi, T.; Hataji, O.; Yuda, H.; Shimizu, S.; Suzuki, K.; Adachi, Y.; Taguchi, O. Intratracheal administration of activated protein C inhibits bleomycin-induced lung fibrosis in the mouse. Am. J. Respir. Crit. Care Med. 2001, 163, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Aono, Y.; Nishioka, Y.; Inayama, M.; Ugai, M.; Kishi, J.; Uehara, H.; Izumi, K.; Sone, S. Imatinib as a novel antifibrotic agent in bleomycin-induced pulmonary fibrosis in mice. Am. J. Respir. Crit. Care Med. 2005, 171, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Gharaee-Kermani, M.; McCullumsmith, R.E.; Charo, I.F.; Kunkel, S.L.; Phan, S.H. CC-chemokine receptor 2 required for bleomycin-induced pulmonary fibrosis. Cytokine 2003, 24, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Olman, M.A.; White, K.E.; Ware, L.B.; Simmons, W.L.; Benveniste, E.N.; Zhu, S.; Pugin, J.; Matthay, M.A. Pulmonary edema fluid from patients with early lung injury stimulates fibroblast proliferation through IL-1 beta-induced IL-6 expression. J. Immunol. 2004, 172, 2668–2677. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, G.; Kutuzov, M.A.; Ridge, K.M. The inflammasome in lung diseases. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 303, L627–L633. [Google Scholar] [CrossRef] [PubMed]
- El-Zein, M.; Malo, J.L.; Infante-Rivard, C.; Gautrin, D. Prevalence and association of welding related systemic and respiratory symptoms in welders. Occup. Environ. Med. 2003, 60 (Suppl. 4), 655–661. [Google Scholar] [CrossRef]
- Kelleher, P.; Pacheco, K.; Newman, L.S. Inorganic dust pneumonias: The metal-related parenchymal disorders. Environ. Health Perspect. 2000, 108, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Lagente, V.; Manoury, B.; Nénan, S.; Le Quément, C.; Martin-Chouly, C.; Boichot, E. Role of matrix metalloproteinases in the development of airway inflammation and remodeling. Braz. J. Med. Biol. Res. 2005, 38, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Ho, C.C.; Yang, C.S.; Chang, W.H.; Tsai, M.H.; Tsai, H.T.; Lin, P. Involvement of MyD88 in zinc oxide nanoparticle-induced lung inflammation. Exp. Toxicol. Pathol. 2013, 65, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Adamcakova-Dodd, A.; Stebounova, L.V.; Kim, J.S.; Vorrink, S.U.; Ault, A.P.; O’Shaughnessy, P.T.; Grassian, V.H.; Thorne, P.S. Toxicity assessment of zinc oxide nanoparticles using sub-acute and sub-chronic murine inhalation models. Part. Fibre Toxicol. 2014, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, E.; Mangum, J.B.; Wong, B.A.; Asgharian, B.; Hext, P.M.; Warheit, D.B.; Everitt, J.I. Pulmonary responses of mice, rats, and hamsters to subchronic inhalation of ultrafine titanium dioxide particles. Toxicol. Sci. 2004, 77, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Voznesenskiĭ, N.K. Exogenous fibrosing alveolitis due to the condensation aerosol (smoke) of zinc oxide (abstract). Vestn. Ross. Akad. Med. Nauk 2004, 3, 18–25. [Google Scholar] [PubMed]
- Osier, M.; Baggs, R.B.; Oberdörster, G. Intratracheal instillation versus intratracheal inhalation: Influence of cytokines on inflammatory response. Environ. Health Perspect. 1997, 105 (Suppl. 5), 1265–1271. [Google Scholar] [CrossRef]
- Xu, M.; Li, J.; Hanagata, N.; Su, H.; Chen, H.; Fujita, D. Challenge to assess the toxic contribution of metal cation released from nanomaterials for nanotoxicology—The case of ZnO nanoparticles. Nanoscale 2013, 5, 4763–4769. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.S.; Duffin, R.; Poland, C.A.; Duschl, A.; Oostingh, G.J.; Macnee, W.; Bradley, M.; Megson, I.L.; Donaldson, K. Differential pro-inflammatory effects of metal oxide nanoparticles and their soluble ions in vitro and in vivo; zinc and copper nanoparticles, but not their ions, recruit eosinophils to the lungs. Nanotoxicology 2012, 6, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.; Sriram, K.; Wolfarth, M.; Jefferson, A.; Schwegler-Berry, D.; Andrew, M.; Castranova, V. A biocompatible medium for nanoparticle dispersion. Nanotoxicology 2008, 2, 144–154. [Google Scholar] [CrossRef]
- Wu, W.; Ichihara, G.; Suzuki, Y.; Izuoka, K.; Oikawa-Tada, S.; Chang, J.; Sakai, K.; Miyazawa, K.; Porter, D.; Castranova, V.; et al. Dispersion method for safety research on manufactured nanomaterials. Ind. Health 2014, 52, 54–65. [Google Scholar]
- Gabazza, E.C.; Kasper, M.; Ohta, K.; Keane, M.; D’Alessandro-Gabazza, C.; Fujimoto, H.; Nishii, Y.; Nakahara, H.; Takagi, T.; Menon, A.G.; et al. Decreased expression of aquaporin-5 in bleomycin-induced lung fibrosis in the mouse. Pathol. Int. 2004, 54, 774–780. [Google Scholar]
- Scanlan, C.L.; Wilkins, R.; Stoller, J.K. Egan’s Fundamentals of Respiratory Care, 7th ed.; Mosby: St. Louis, MO, USA, 1998. [Google Scholar]
- Porter, D.W.; Hubbs, A.F.; Mercer, R.R.; Wu, N.; Wolfarth, M.G.; Sriram, K.; Leonard, S.; Battelli, L.; Schwegler-Berry, D.; Friend, S.; et al. Mouse pulmonary dose- and time course-responses induced by exposure to multi-walled carbon nanotubes. Toxicology 2010, 269, 136–147. [Google Scholar]
- Braber, S.; Henricks, P.A.; Nijkamp, F.P.; Kraneveld, A.D.; Folkerts, G. Inflammatory changes in the airways of mice caused by cigarette smoke exposure are only partially reversed after smoking cessation. Respir. Res. 2010, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Boveda-Ruiz, D.; D’Alessandro-Gabazza, C.N.; Toda, M.; Takagi, T.; Naito, M.; Matsushima, Y.; Matsumoto, T.; Kobayashi, T.; Gil-Bernabe, P.; Chelakkot-Govindalayathil, A.L.; et al. Differential role of regulatory T cells in early and late stages of pulmonary fibrosis. Immunobiology 2013, 218, 245–254. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Ichihara, G.; Hashimoto, N.; Hasegawa, Y.; Hayashi, Y.; Tada-Oikawa, S.; Suzuki, Y.; Chang, J.; Kato, M.; D'Alessandro-Gabazza, C.N.; et al. Synergistic Effect of Bolus Exposure to Zinc Oxide Nanoparticles on Bleomycin-Induced Secretion of Pro-Fibrotic Cytokines without Lasting Fibrotic Changes in Murine Lungs. Int. J. Mol. Sci. 2015, 16, 660-676. https://doi.org/10.3390/ijms16010660
Wu W, Ichihara G, Hashimoto N, Hasegawa Y, Hayashi Y, Tada-Oikawa S, Suzuki Y, Chang J, Kato M, D'Alessandro-Gabazza CN, et al. Synergistic Effect of Bolus Exposure to Zinc Oxide Nanoparticles on Bleomycin-Induced Secretion of Pro-Fibrotic Cytokines without Lasting Fibrotic Changes in Murine Lungs. International Journal of Molecular Sciences. 2015; 16(1):660-676. https://doi.org/10.3390/ijms16010660
Chicago/Turabian StyleWu, Wenting, Gaku Ichihara, Naozumi Hashimoto, Yoshinori Hasegawa, Yasuhiko Hayashi, Saeko Tada-Oikawa, Yuka Suzuki, Jie Chang, Masashi Kato, Corina N. D'Alessandro-Gabazza, and et al. 2015. "Synergistic Effect of Bolus Exposure to Zinc Oxide Nanoparticles on Bleomycin-Induced Secretion of Pro-Fibrotic Cytokines without Lasting Fibrotic Changes in Murine Lungs" International Journal of Molecular Sciences 16, no. 1: 660-676. https://doi.org/10.3390/ijms16010660






