Capability of Utilizing CYP3A5 Polymorphisms to Predict Therapeutic Dosage of Tacrolimus at Early Stage Post-Renal Transplantation
Abstract
:1. Introduction
2. Results
| Items | Value | p-Value |
|---|---|---|
| Sex (males:females) | 34:16 | - |
| CYP3A5 genotype groups | ||
| *1/*1:*1/*3:*3/*3 | 4:13:33 | - |
| Age (years, at transplantation) | 50.5 ± 12.0 (21–70) | - |
| Body weight (kg) | 57.9 ± 13.0 (34–91) | - |
| Biochemical Data | ||
| Hematocrit (%) at pre-transplantation | 30.3 (27.2–34.4) | - |
| on Day 7 | 28.8 (25.9–31.4) | <0.001 |
| on Day 14 | 30.2 (28.0–33.2) | |
| on Day 21 | 31.4 (29.4–34.0) | |
| on Day 28 | 33.1 (29.8–35.2) | |
| Serum albumin (g/dL) at pre-transplantation | 3.7 (3.5–4.0) | - |
| on Day 7 | 3.1 (2.8–3.4) | <0.001 |
| on Day 14 | 3.6 (3.3–3.9) | |
| on Day 21 | 3.7 (3.5–3.9) | |
| on Day 28 | 3.9 (3.5–4.0) | |
| Tacrolimus Data | ||
| Dose/day (mg) at pre-transplantation | 12.0 (10.0–14.0) | - |
| on Day 7 | 12.0 (10.0–13.0) | <0.001 |
| on Day 14 | 10.5 (9.0–13.0) | |
| on Day 21 | 9.0 (8.0–12.0) | |
| on Day 28 | 8.5 (6.0–11.0) | |
| C24h (ng/mL) at pre-transplantation | 10.6 (8.1–13.4) | - |
| C0h on Day 7 | 11.0 (8.5–13.2) | <0.001 |
| C0h on Day 14 | 9.5 (7.9–12.4) | |
| C0h on Day 21 | 8.2 (7.2–9.9) | |
| C0h on Day 28 | 7.3 (6.6–8.0) | |
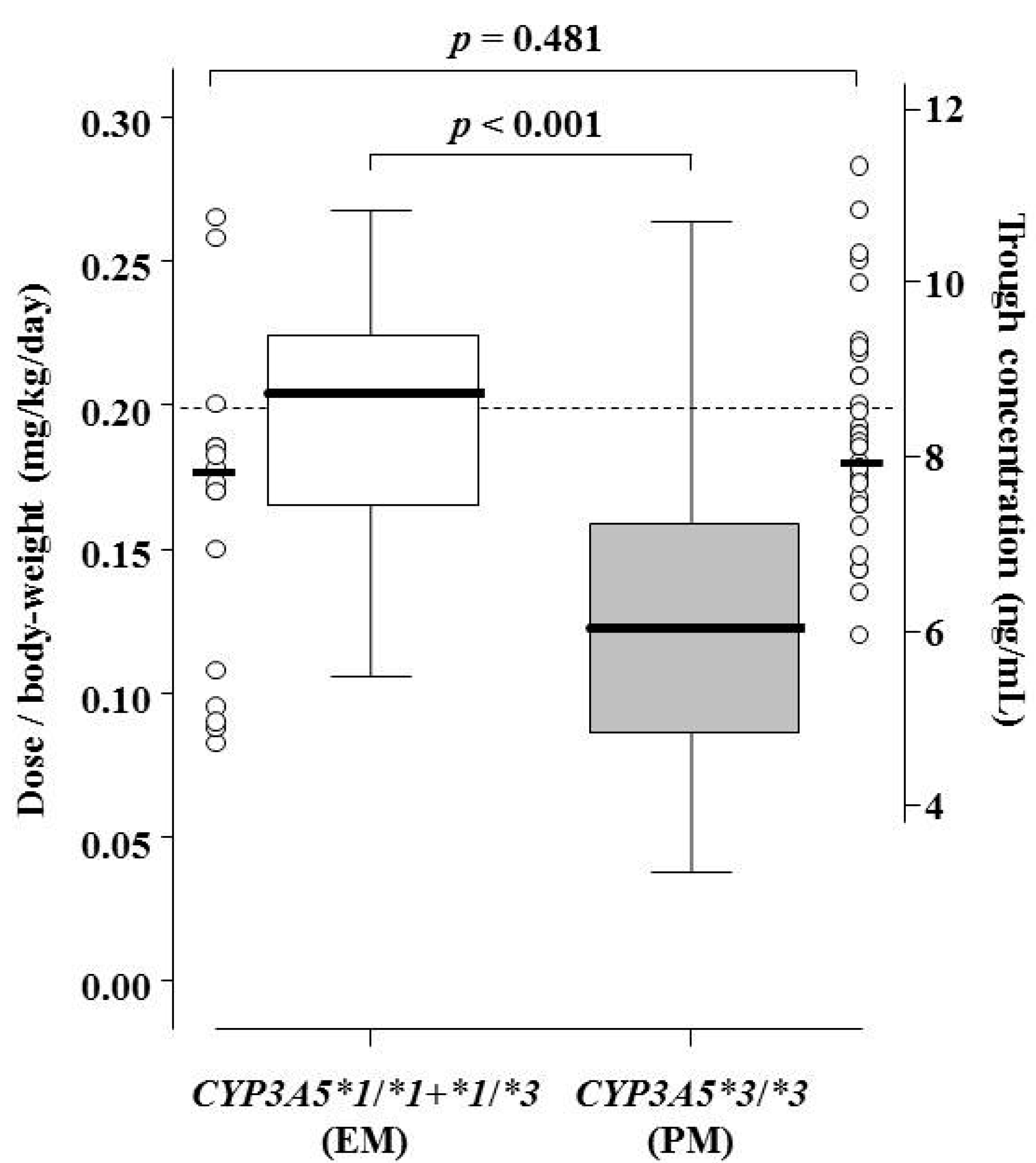
| Dose/body weight/day (mg/kg/day) | 0.20 (initial oral dose in all patients) | - |
| on Day 7 | 0.21 (0.15–0.23) | <0.001 |
| on Day 14 | 0.19 (0.12–0.23) | |
| on Day 21 | 0.16 (0.11–0.21) | |
| on Day 28 | 0.14 (0.10–0.21) | |
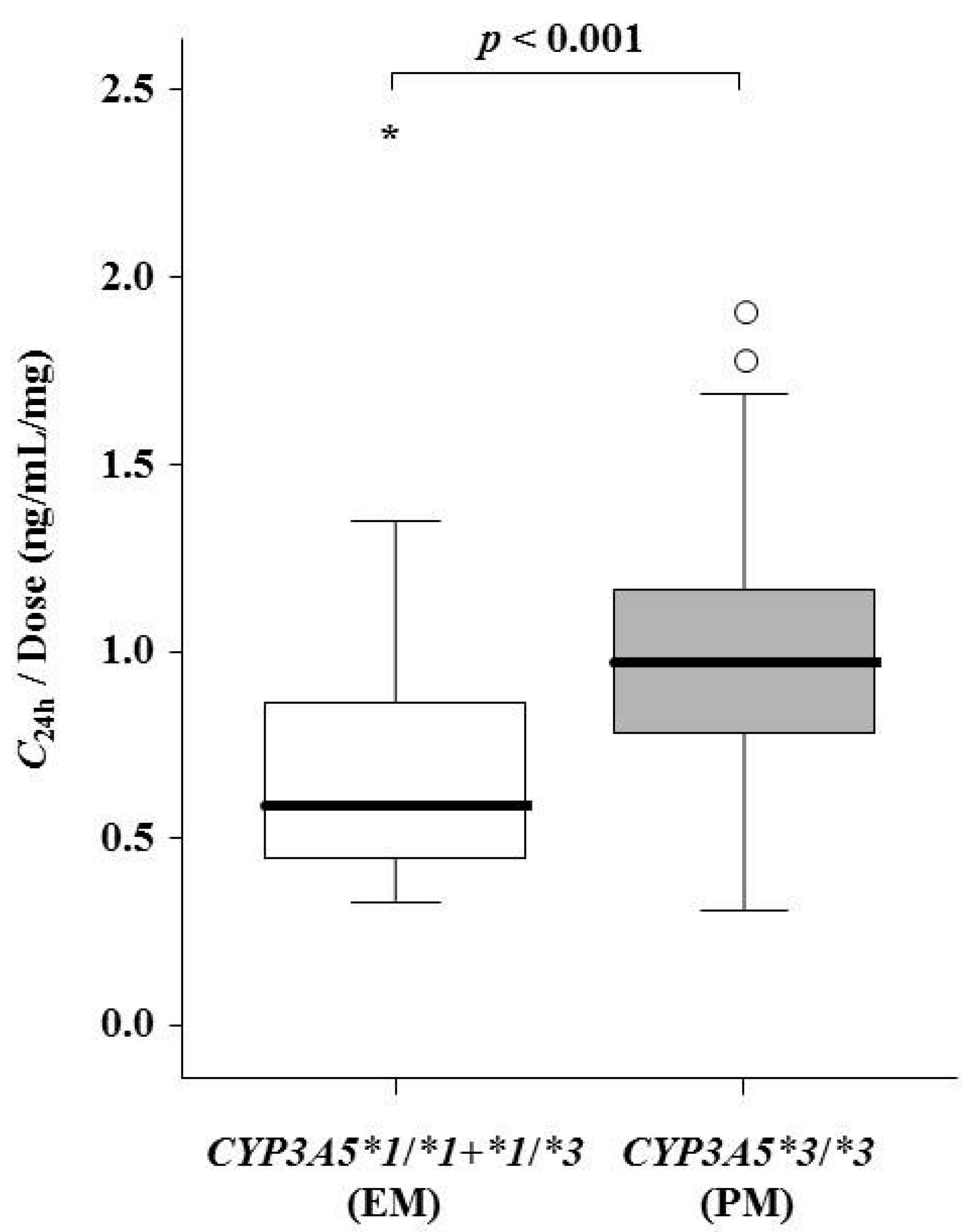
| Items | Day 7 | Day 14 | Day 21 | Day 28 | ||||
|---|---|---|---|---|---|---|---|---|
| Median (Quartiles 1–3) | p-Value | Median (Quartiles 1–3) | p-Value | Median (Quartiles 1–3) | p-Value | Median (Quartiles 1–3) | p-Value | |
| Sex | - | 0.001 | 0.096 | 0.660 | - | 0.409 | ||
| males | 12.5 (11.0–14.0) | - | 12.0 (10.0–13.0) | - | 9.0 (7.0–12.0) | - | 8.0 (6.0–12.0) | - |
| females | 10.0 (9.0–11.5) | - | 9.0 (9.0–10.5) | - | 9.0 (9.0–10.5) | - | 9.0 (8.0–10.5) | - |
| CYP3A5 genotype groups | 0.629 | 0.363 | 0.010 | 0.010 | ||||
| *1/*1 | 12.5 (10.5–14.0) | - | 12.5 (10.5–17.0) | - | 12.5 (10.5–14.0) | - | 11.5 (10.5–14.0) | - |
| *1/*3 | 12.0 (9.0–14.0) | - | 12.0 (9.0–12.0) | - | 10.0 (9.0–12.0) | - | 10.0 (9.0–12.0) | - |
| *3/*3 | 12.0 (11.0–13.0) | - | 10.0 (9.0–13.0) | - | 8.0 (6.0–10.0) | - | 7.0 (5.0–10.0) | - |
| Correlation Coefficient (r) | p-Value | Correlation Coefficient (r) | p-Value | Correlation Coefficient (r) | p-Value | Correlation Coefficient (r) | p-Value | |
| Age | −0.015 | 0.920 | −0.076 | 0.602 | −0.228 | 0.111 | −0.168 | 0.244 |
| Body-weight | 0.704 | <0.001 | 0.426 | 0.002 | 0.247 | 0.083 | 0.165 | 0.253 |
| C24h at pre-transplantation | 0.061 | 0.674 | −0.070 | 0.631 | −0.023 | 0.876 | −0.025 | 0.864 |
| CLt in CIV on day 3 | 0.382 | 0.006 | 0.549 | <0.001 | 0.384 | 0.006 | 0.297 | 0.036 |
| C0h on the day | −0.170 | 0.238 | −0.121 | 0.402 | −0.174 | 0.226 | −0.048 | 0.740 |
| Hematocrit on the day | −0.084 | 0.563 | −0.061 | 0.676 | −0.231 | 0.106 | −0.287 | 0.043 |
| Serum albumin on the day | −0.184 | 0.201 | −0.016 | 0.913 | −0.159 | 0.270 | −0.200 | 0.164 |
| Explanatory Variable | Slope | Bias * | SE * | p-Value * | R2 | |
|---|---|---|---|---|---|---|
| Day 7 | 0.389 | |||||
| Body-weight (kg) | 0.123 | 0.003 | 0.027 | 0.001 | ||
| Intercept = 4.901 | −0.128 | 1.500 | ||||
| Day 14 | 0.387 | |||||
| Body-weight (kg) | 0.066 | −0.003 | 0.031 | 0.033 | ||
| CLt in CIV on day 3 (L/h) | 1.174 | 0.063 | 0.404 | 0.010 | ||
| CYP3A5 *1/*1 (=1) | 3.461 | −0.019 | 1.494 | 0.011 | ||
| Intercept = 2.695 | 0.009 | 1.746 | ||||
| Day 21 | 0.495 | |||||
| Body-weight (kg) | 0.078 | 0.000 | 0.031 | 0.016 | ||
| CLt in CIV on day 3 (L/h) | 0.885 | 0.026 | 0.346 | 0.012 | ||
| CYP3A5 *3/*3 (=1) | −3.241 | 0.000 | 0.695 | 0.001 | ||
| Hematocrit on the day (%) | −0.194 | 0.005 | 0.084 | 0.023 | ||
| Intercept = 9.839 | −0.201 | 2.946 | ||||
| Day 28 | 0.501 | |||||
| Body-weight (kg) | 0.092 | −0.001 | 0.035 | 0.019 | ||
| CLt in CIV on day 3 (L/h) | 0.601 | 0.038 | 0.390 | 0.112 | ||
| CYP3A5 *3/*3 (=1) | −3.747 | 0.025 | 0.720 | 0.001 | ||
| Hematocrit on the day (%) | −0.284 | 0.011 | 0.071 | 0.002 | ||
| Intercept = 12.889 | −0.431 | 3.093 |

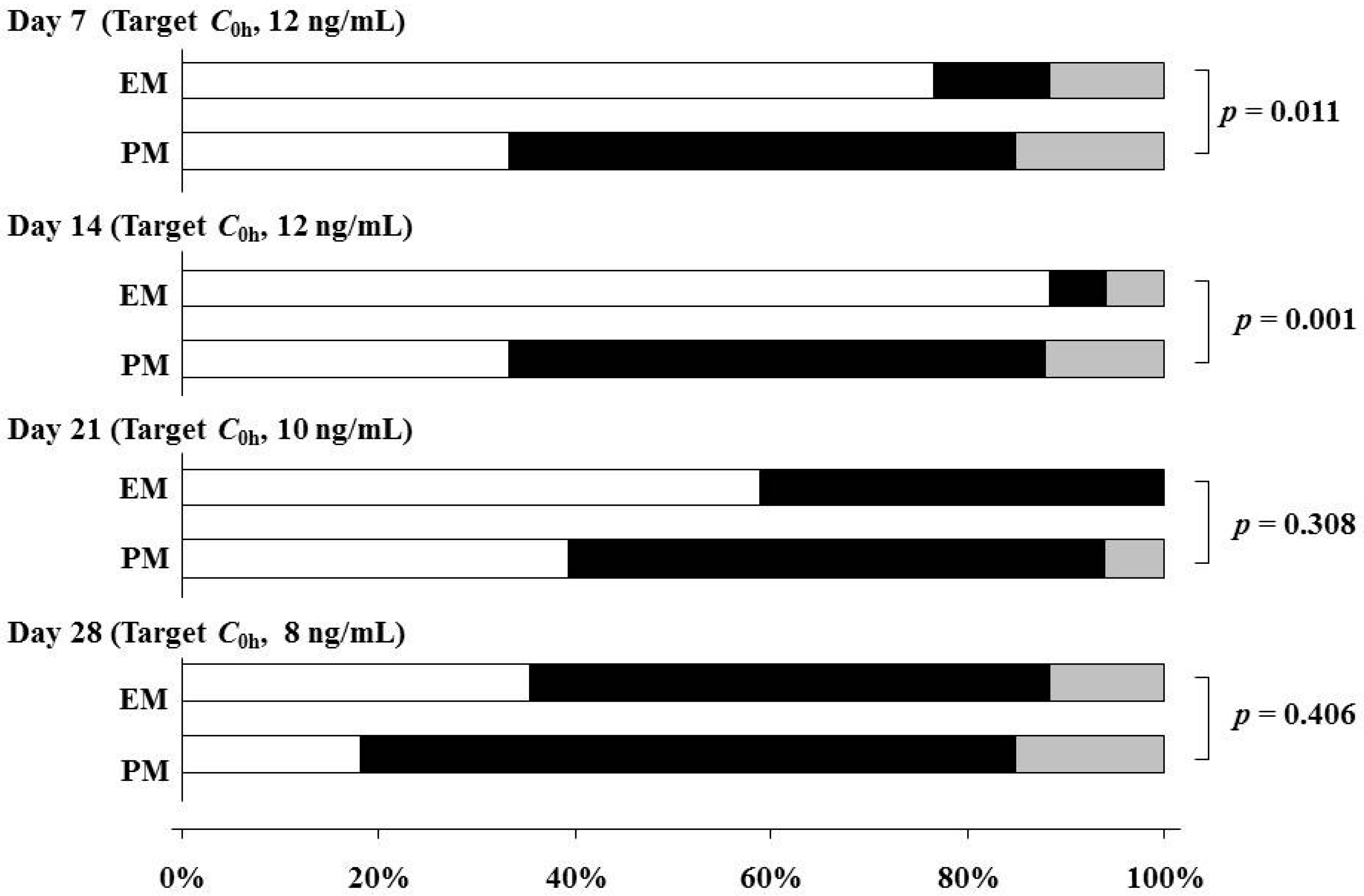
3. Discussion
4. Experimental Section
4.1. Patients and Protocols
4.2. Sample Collection and Analytical Methods
4.3. Genotyping
4.4. Pharmacokinetic Analysis
4.5. Statistical Procedures
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Scott, L.J.; McKeage, K.; Keam, S.J.; Plosker, G.L. Tacrolimus: A further update of its use in the management of organ transplantation. Drugs 2003, 63, 1247–1297. [Google Scholar] [CrossRef] [PubMed]
- Staatz, C.E.; Tett, S.E. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin. Pharmacokinet. 2004, 43, 623–653. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, P.A.; Oetting, W.S.; Brearley, A.M.; Leduc, R.; Guan, W.; Schladt, D.; Matas, A.J.; Lamba, V.; Julian, B.A.; Mannon, R.B.; et al. Novel polymorphisms associated with tacrolimus trough concentrations: Results from a multicenter kidney transplant consortium. Transplantation 2011, 91, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Ekberg, H.; Mamelok, R.D.; Pearson, T.C.; Vincenti, F.; Tedesco-Silva, H.; Daloze, P. The challenge of achieving target drug concentrations in clinical trials: Experience from the symphony study. Transplantation 2009, 87, 1360–1366. [Google Scholar] [CrossRef] [PubMed]
- Staatz, C.; Taylor, P.; Tett, S. Low tacrolimus concentrations and increased risk of early acute rejection in adult renal transplantation. Nephrol. Dial. Transplant. 2001, 16, 1905–1909. [Google Scholar] [CrossRef] [PubMed]
- Hesselink, D.A.; van Schaik, R.H.; van der Heiden, I.P.; van der Werf, M.; Gregoor, P.J.; Lindemans, J.; Weimar, W.; van Gelder, T. Genetic polymorphisms of the CYP3A4, CYP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clin. Pharmacol. Ther. 2003, 74, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, N.; Satoh, S.; Tada, H.; Li, Z.; Ohyama, C.; Sato, K.; Suzuki, T.; Habuchi, T.; Kato, T. Influence of CYP3A5 and MDR1 (ABCB1) polymorphisms on the pharmacokinetics of tacrolimus in renal transplant recipients. Transplantation 2004, 78, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Haufroid, V.; Wallemacq, P.; VanKerckhove, V.; Elens, L.; de Meyer, M.; Eddour, D.C.; Malaise, J.; Lison, D.; Mourad, M. CYP3A5 and ABCB1 polymorphisms and tacrolimus pharmacokinetics in renal transplant candidates: Guidelines from an experimental study. Am. J. Transplant. 2006, 6, 2706–2713. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, H.; de Loor, H.; Verbeke, K.; Vanrenterghem, Y.; Kuypers, D.R. Impact of CYP3A5 genotype on tacrolimus versus midazolam clearance in renal transplant recipients: New insights in CYP3A5-mediated drug metabolism. Pharmacogenomics 2013, 14, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Vannaprasaht, S.; Reungjui, S.; Supanya, D.; Sirivongs, D.; Pongskul, C.; Avihingsanon, Y.; Tassaneeyakul, W. Personalized tacrolimus doses determined by CYP3A5 genotype for induction and maintenance phases of kidney transplantation. Clin. Ther. 2013, 35, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Åsberg, A.; Midtvedt, K.; van Guilder, M.; Størset, E.; Bremer, S.; Bergan, S.; Jelliffe, R.; Hartmann, A.; Neely, M.N. Inclusion of CYP3A5 genotyping in a nonparametric population model improves dosing of tacrolimus early after transplantation. Transpl. Int. 2013, 26, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Hebert, M.F.; Isoherranen, N.; Davis, C.L.; Marsh, C.; Shen, D.D.; Thummel, K.E. Effect of CYP3A5 polymorphism on tacrolimus metabolic clearance in vitro. Drug Metab. Dispos. 2006, 34, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Barraclough, K.A.; Isbel, N.M.; Johnson, D.W.; Campbell, S.B.; Staatz, C.E. Once- versus twice-daily tacrolimus: Are the formulations truly equivalent? Drugs 2011, 71, 1561–1577. [Google Scholar] [CrossRef] [PubMed]
- Hougardy, J.M.; de Jonge, H.; Kuypers, D.; Abramowicz, D. The once-daily formulation of tacrolimus: A step forward in kidney transplantation? Transplantation 2012, 93, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Niioka, T.; Satoh, S.; Kagaya, H.; Numakura, K.; Inoue, T.; Saito, M.; Narita, S.; Tsuchiya, N.; Habuchi, T.; Miura, M. Comparison of pharmacokinetics and pharmacogenetics of once- and twice-daily tacrolimus in the early stage after renal transplantation. Transplantation 2012, 94, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Niioka, T.; Kagaya, H.; Miura, M.; Numakura, K.; Saito, M.; Inoue, T.; Habuchi, T.; Satoh, S. Pharmaceutical and genetic determinants for interindividual differences of tacrolimus bioavailability in renal transplant recipients. Eur. J. Clin. Pharmacol. 2013, 69, 1659–1665. [Google Scholar] [CrossRef] [PubMed]
- Wallemacq, P.; Armstrong, V.W.; Brunet, M.; Haufroid, V.; Holt, D.W.; Johnston, A.; Kuypers, D.; Meur, Y.L.; Marquet, P.; Oellerich, M.; et al. Opportunities to optimize tacrolimus therapy in solid organ transplantation: Report of the European consensus conference. Ther. Drug Monit. 2009, 31, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Borobia, A.M.; Romero, I.; Jimenez, C.; Gil, F.; Ramirez, E.; de Gracia, R.; Escuin, F.; Gonzalez, E.; Sansuán, A.J. Trough tacrolimus concentrations in the first week after kidney transplantation are related to acute rejection. Ther. Drug Monit. 2009, 31, 436–442. [Google Scholar] [CrossRef] [PubMed]
- O’Seaghdha, C.M.; McQuillan, R.; Moran, A.M.; Lavin, P.; Dorman, A.; O’Kelly, P.; Mohan, D.M.; Little, P.; Hickey, D.P.; Conlon, P.J. Higher tacrolimus trough levels on days 2–5 post-renal transplant are associated with reduced rates of acute rejection. Clin. Transplant. 2009, 23, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Thervet, E.; Loriot, M.A.; Barbier, S.; Buchler, M.; Ficheux, M.; Choukroun, G.; Toupance, O.; Touchard, G.; Alberti, C.; le Pogamp, P.; et al. Optimization of initial tacrolimus dose using pharmacogenetic testing. Clin. Pharmacol. Ther. 2010, 87, 721–726. [Google Scholar] [PubMed]
- Zhao, W.; Elie, V.; Roussey, G.; Brochard, K.; Niaudet, P.; Leroy, V.; Loirat, C.; Cochat, P.; Cloarec, S.; André, J.L.; et al. Population pharmacokinetics and pharmacogenetics of tacrolimus in de novo pediatric kidney transplant recipients. Clin. Pharmacol. Ther. 2009, 86, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Benkali, K.; Prémaud, A.; Picard, N.; Rérolle, J.P.; Toupance, O.; Hoizey, G.; Turcant, A.; Villemain, F.; Meur, Y.L.; Marquet, P.; et al. Tacrolimus population pharmacokinetic-pharmacogenetic analysis and Bayesian estimation in renal transplant recipients. Clin. Pharmacokinet. 2009, 48, 805–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woillard, J.B.; de Winter, B.C.; Kamar, N.; Marquet, P.; Rostaing, L.; Rousseau, A. Population pharmacokinetic model and Bayesian estimator for two tacrolimus formulations—Twice daily Prograf® and once daily Advagraf®. Br. J. Clin. Pharmacol. 2011, 71, 391–402. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, H.; de Loor, H.; Verbeke, K.; Vanrenterghem, Y.; Kuypers, D.R. In vivo CYP3A4 activity, CYP3A5 genotype, and hematocrit predict tacrolimus dose requirements and clearance in renal transplant patients. Clin. Pharmacol. Ther. 2012, 92, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Yun, H.Y.; Hong, J.Y.; Kim, I.W.; Ji, E.; Hong, S.H.; Kim, Y.S.; Ha, J.; Shin, W.G.; Oh, J.M. Prediction of the tacrolimus population pharmacokinetic parameters according to CYP3A5 genotype and clinical factors using NONMEM in adult kidney transplant recipients. Eur. J. Clin. Pharmacol. 2013, 69, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, T.K.; Hennig, S.; Barraclough, K.A.; Isbel, N.M.; Staatz, C.E. Population pharmacokinetics of tacrolimus in adult kidney transplant patients: Impact of CYP3A5 genotype on starting dose. Ther. Drug Monit. 2014, 36, 62–70. [Google Scholar] [PubMed]
- Wallemacq, P.; Goffinet, J.S.; O’Morchoe, S.; Rosiere, T.; Maine, G.T.; Labalette, M.; Aimo, G.; Dickson, D.; Schmidt, E.; Schwinzer, R.; et al. Multi-site analytical evaluation of the Abbott ARCHITECT tacrolimus assay. Ther. Drug Monit. 2009, 31, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Doki, K.; Homma, M.; Hori, T.; Tomita, T.; Hasegawa, Y.; Ito, S.; Fukunaga, K.; Kaneko, M.; Chiba, S.; Sumida, T.; et al. Difference in blood tacrolimus concentration between ACMIA and MEIA in samples with low haematocrit values. J. Pharm. Pharmacol. 2010, 62, 1185–1188. [Google Scholar] [CrossRef] [PubMed]
- Bouzas, L.; Ortega, F.J.; Casado, P.; Arranz, M.I.; Carcas, A.; Tutor, J.C. Effect of the hematocrit and its correction on the relationship between blood tacrolimus concentrations obtained using the microparticle enzyme immunoassay (MEIA) and enzyme multiplied immunoassay technique (EMIT). Clin. Lab. 2007, 53, 591–596. [Google Scholar] [PubMed]
- Racusen, L.C.; Solez, K.; Colvin, R.B.; Bonsib, S.M.; Castro, M.C.; Cavallo, T.; Croker, B.P.; Demetris, A.J.; Drachenberg, C.B.; Fogo, A.B.; et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999, 55, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Solez, K.; Colvin, R.B.; Racusen, L.C.; Sis, B.; Halloran, P.F.; Birk, P.E.; Campbell, P.M.; Cascalho, M.; Collins, A.B.; Demetris, A.J.; et al. Banff 05 Meeting Report: Differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (“CAN”). Am. J. Transplant. 2007, 7, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Solez, K.; Colvin, R.B.; Racusen, L.C.; Haas, M.; Sis, B.; Mengel, M.; Halloran, P.F.; Baldwin, W.; Banfi, G.; Collins, A.B.; et al. Banff 07 classification of renal allograft pathology: Updates and future directions. Am. J. Transplant. 2008, 8, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Fukuen, S.; Fukuda, T.; Maune, H.; Ikenaga, Y.; Yamamoto, I.; Inaba, T.; Azuma, J. Novel detection assay by PCR-RFLP and frequency of the CYP3A5 SNPs, CYP3A5*3 and *6, in a Japanese population. Pharmacogenetics 2002, 12, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman & Hall: New York, NY, USA, 1993. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niioka, T.; Kagaya, H.; Saito, M.; Inoue, T.; Numakura, K.; Habuchi, T.; Satoh, S.; Miura, M. Capability of Utilizing CYP3A5 Polymorphisms to Predict Therapeutic Dosage of Tacrolimus at Early Stage Post-Renal Transplantation. Int. J. Mol. Sci. 2015, 16, 1840-1854. https://doi.org/10.3390/ijms16011840
Niioka T, Kagaya H, Saito M, Inoue T, Numakura K, Habuchi T, Satoh S, Miura M. Capability of Utilizing CYP3A5 Polymorphisms to Predict Therapeutic Dosage of Tacrolimus at Early Stage Post-Renal Transplantation. International Journal of Molecular Sciences. 2015; 16(1):1840-1854. https://doi.org/10.3390/ijms16011840
Chicago/Turabian StyleNiioka, Takenori, Hideaki Kagaya, Mitsuru Saito, Takamitsu Inoue, Kazuyuki Numakura, Tomonori Habuchi, Shigeru Satoh, and Masatomo Miura. 2015. "Capability of Utilizing CYP3A5 Polymorphisms to Predict Therapeutic Dosage of Tacrolimus at Early Stage Post-Renal Transplantation" International Journal of Molecular Sciences 16, no. 1: 1840-1854. https://doi.org/10.3390/ijms16011840





