Protection of Retina by Mini-αA in NaIO3-Induced Retinal Pigment Epithelium Degeneration Mice
Abstract
:1. Introduction
2. Results
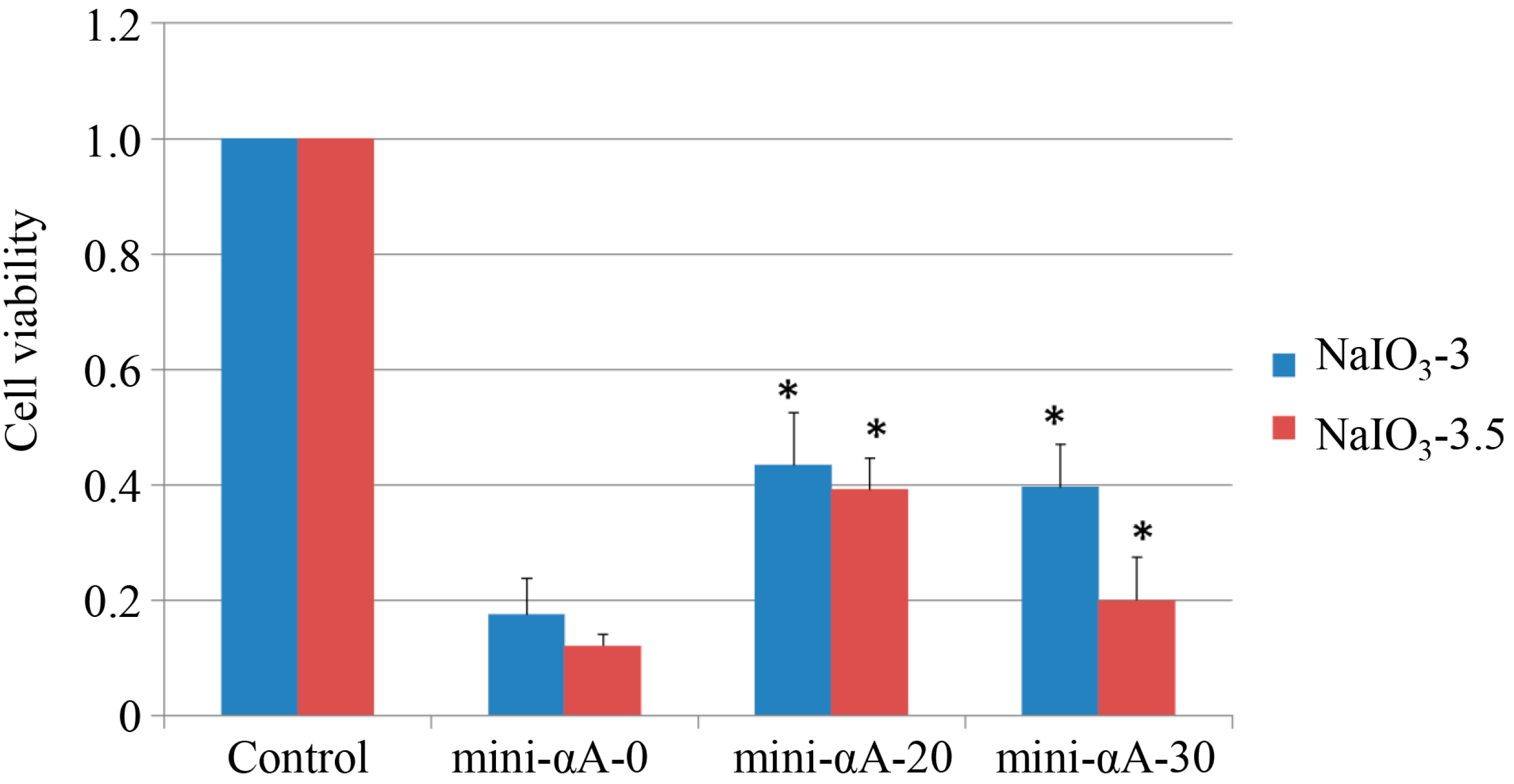
2.1. Mini-αA Protected ARPE-19 from NaIO3-Induced Apoptosis
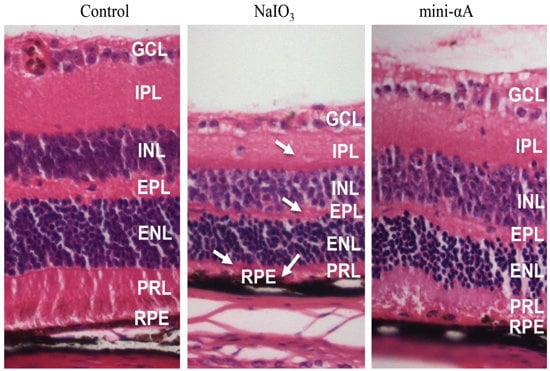
2.2. Mini-αA Reduced Retinal Damage in Mice with NaIO3-Induced Retinal Degeneration
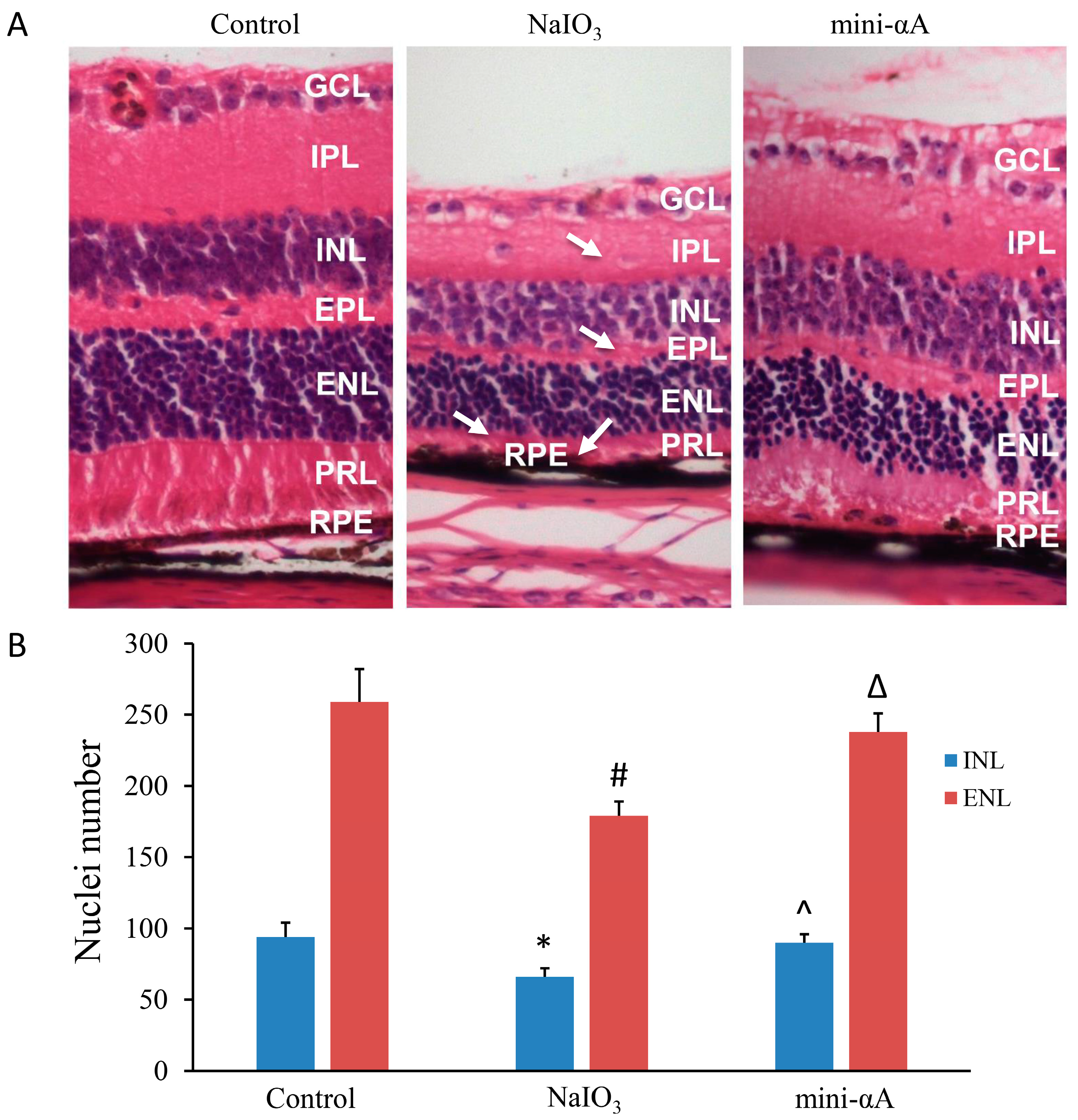
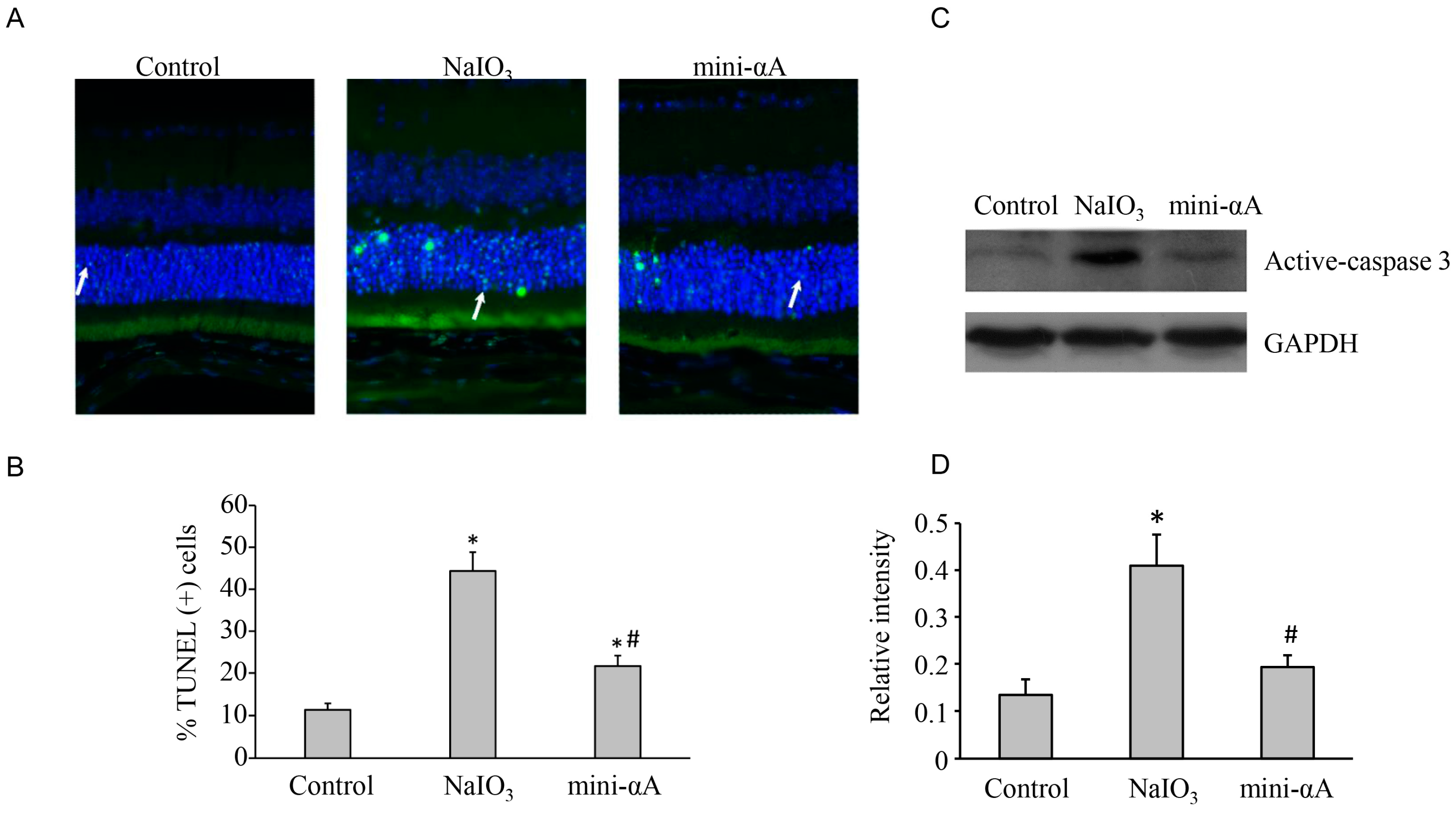
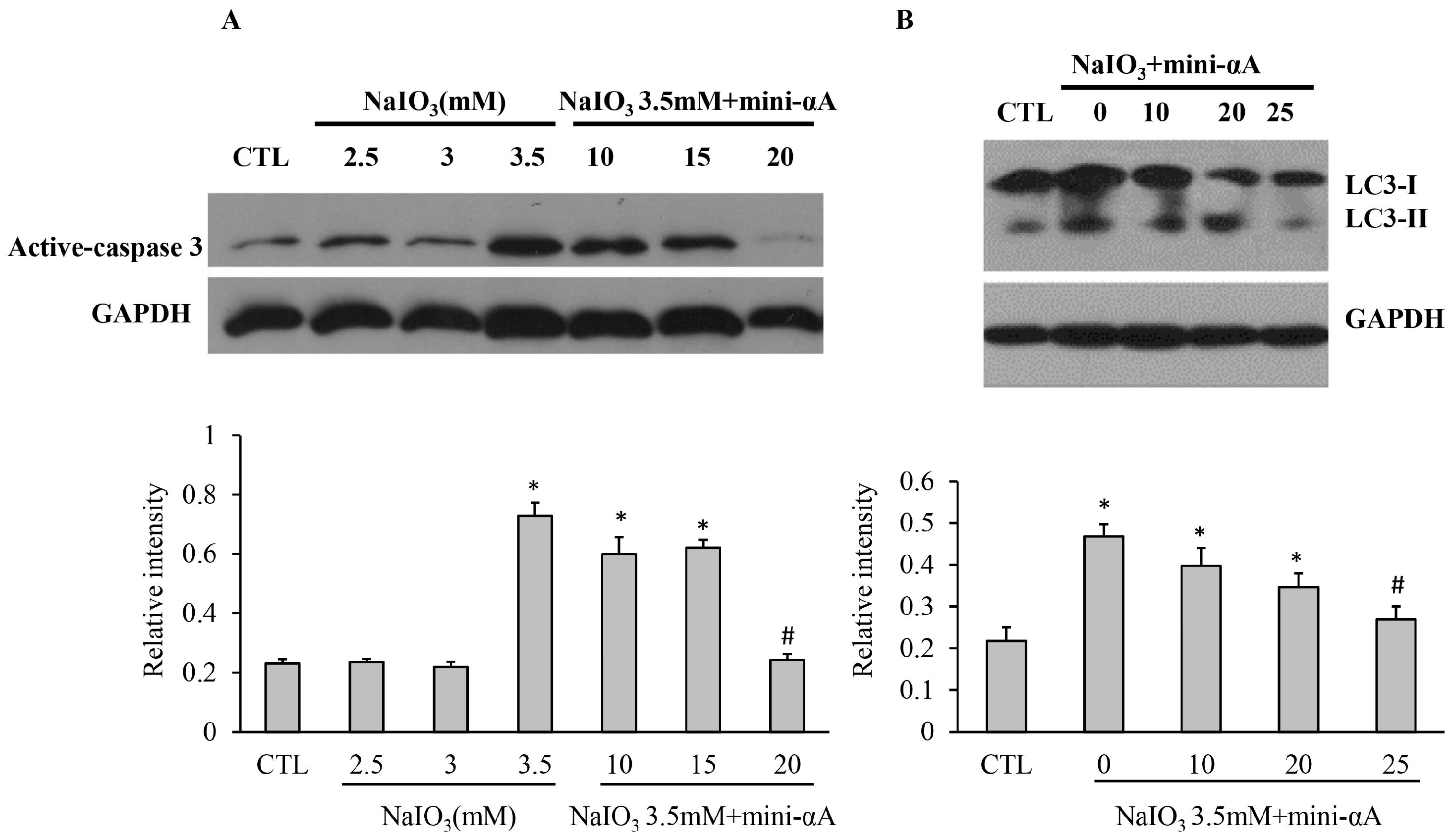
2.3. Mini-αA Reduced NaIO3-Induced Apoptosis and Autophagy Level
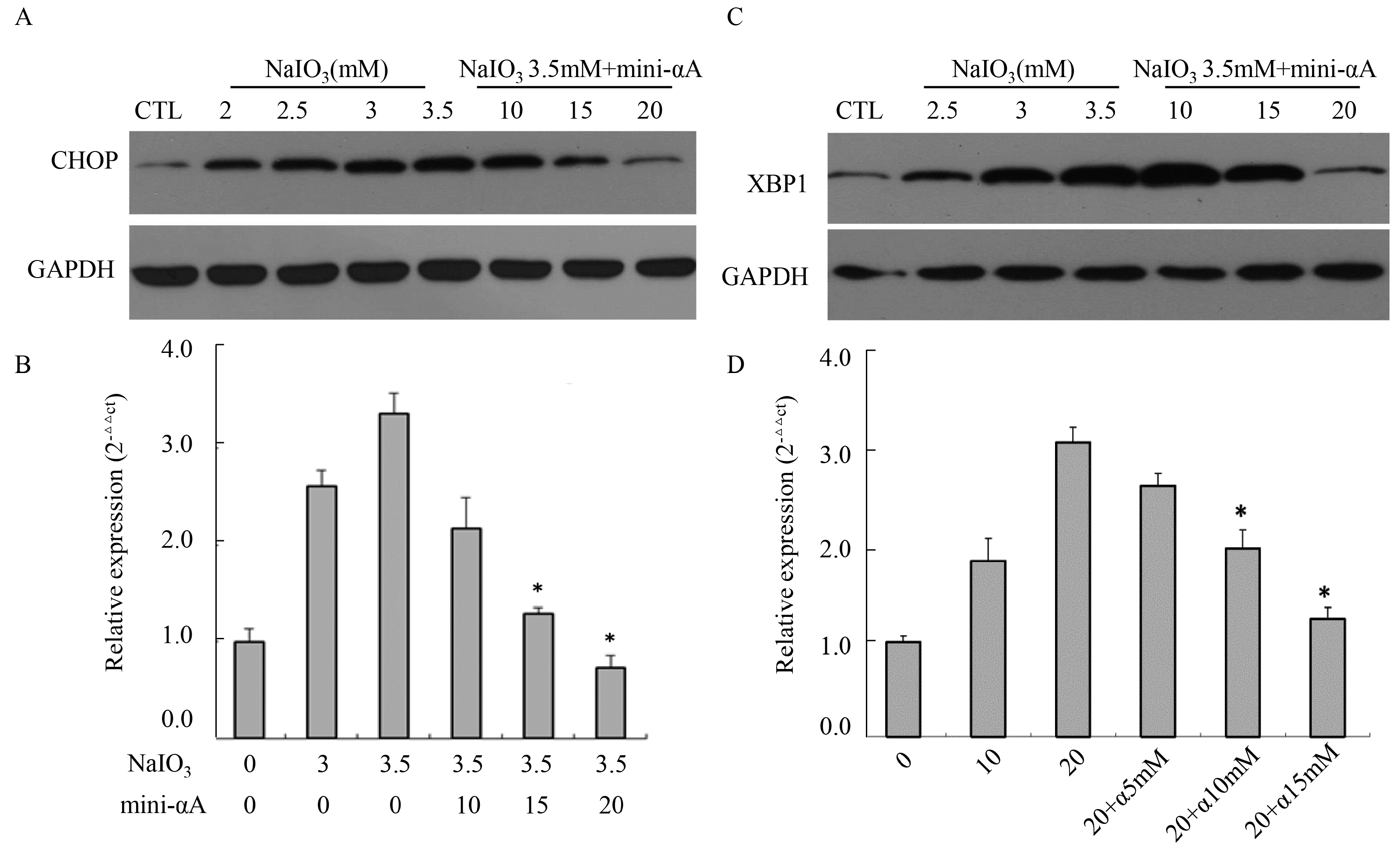
2.4. Mini-αA’s Protective Function against NaIO3-Induced RPE Cell Apoptosis Is Related with UPR
3. Discussion
4. Material and Methods
4.1. Cell Culture
4.2. Animals
4.3. Tissue Processing
4.4. TUNEL Assay
4.5. MTT Assay
4.6. RT-qPCR
| Primer | Sequence (5'–3') |
|---|---|
| CHOP-F | GGGAGCTGGAAGCCTGGTATG |
| CHOP-R | GACCTCTGCTGGTTCTGGCTC |
| XBP1-F | GACACGCTTGGGGATGAATGC |
| XBP1-R | TGTTCTGGGGAGGTGACAAC |
| GAPDH-F | AACGGATTTGGTCGTATTGG |
| GAPDH-R | TGGAAGATGGTGATGGGATT |
4.7. Western Blot
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ingolia, T.D.; Craig, E.A. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc. Natl. Acad. Sci. USA 1982, 79, 2360–2364. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Shinohara, H.; Kurobe, N.; Goto, S.; inaguma, Y.; Ohshima, K. Immunoreactive alpha A crystallin in rat non-lenticular tissues detected with a sensitive immunoassay method. Biochim. Biophys. Acta 1991, 1080, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, H.; Miyagi, M.; Darrow, R.M.; Crabb, J.S.; Hollyfield, J.G.; Organisciak, D.T.; Crabb, J.W. Intense light exposure changes the crystallin content in retina. Exp. Eye Res. 2003, 76, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Chona, F.; Song, B.K.; Geisert, E.J. Temporal changes in gene expression after injury in the rat retina. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2737–2746. [Google Scholar] [CrossRef]
- Steele, M.R.; Inman, D.M.; Calkins, D.J.; Horner, P.J.; Vetter, M.L. Microarray analysis of retinal gene expression in the DBA/2J model of glaucoma. Investig. Ophthalmol. Vis. Sci. 2006, 47, 977–985. [Google Scholar] [CrossRef]
- Zhou, P.; Ye, H.-F.; Jiang, Y.-X.; Yang, J.; Zhu, X.-J.; Sun, X.-H.; Luo, Y.; Dou, G.-R.; Wang, Y.-S.; Lu, Y. αA crystallin may protect against geographic atrophy-meta-analysis of cataract vs. cataract surgery for geographic atrophy and experimental studies. PLoS One 2012, 7, e43173. [Google Scholar]
- Pasupuleti, N.; Matsuyama, S.; Voss, O.; Doseff, A.I.; Song, K.; Danielpour, D.; Nagaraj, R.H. The anti-apoptotic function of human alphaA-crystallin is directly related to its chaperone activity. Cell Death Dis. 2010, 1, e31. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, J.; Sharma, K.K. Conformational specificity of mini-alphaA-crystallin as a molecular chaperone. J. Pept. Res. 2001, 57, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, P.G.; Chothe, P.; Sharma, K.K.; Baid, R.; Kompella, U.; Spee, C.; Kannan, N.; Manh, C.; Ryan, S.J.; Ganapathy, V.; et al. Antiapoptotic properties of alpha-crystallin-derived peptide chaperones and characterization of their uptake transporters in human RPE cells. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2787–2798. [Google Scholar] [CrossRef]
- Chen, C.; Cano, M.; Wang, J.J.; Li, J.; Huang, C.; Yu, Q.; Herbert, T.P.; Handa, J.T.; Zhang, S.X. Role of unfolded protein response dysregulation in oxidative injury of retinal pigment epithelial cells. Antioxid. Redox Signal. 2014, 20, 2091–2106. [Google Scholar] [CrossRef] [PubMed]
- Kiuchi, K.; Yoshizawa, K.; Shikata, N.; Moriguchi, K.; Tsubura, A. Morphologic characteristics of retinal degeneration induced by sodium iodate in mice. Curr. Eye Res. 2002, 25, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Machalinska, A.; Lubinski, W.; Klos, P.; Kawa, M.; Baumert, B.; Penkala, K.; Grzegrzolka, R.; Karczewicz, D.; Wiszniewska, B.; Machalinski, B. Sodium iodate selectively injuries the posterior pole of the retina in a dose-dependent manner: Morphological and electrophysiological study. Neurochem. Res. 2010, 35, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Mizota, A.; Adachi-Usami, E. Functional recovery of retina after sodium iodate injection in mice. Vis. Res. 1997, 37, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Machalinska, A.; Klos, P.; Baumert, B.; Baskiewicz, M.; Kawa, M.; Rudnicki, M.; Lubinski, W.; Wiszniewska, B.; Karczewicz, D.; Machalinski, B. Stem Cells are mobilized from the bone marrow into the peripheral circulation in response to retinal pigment epithelium damage—A pathophysiological attempt to induce endogenous regeneration. Curr. Eye Res. 2011, 36, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Redfern, W.S.; Storey, S.; Tse, K.; Hussain, Q.; Maung, K.P.; Valentin, J.-P.; Ahmed, G.; Bigley, A.; Heathcote, D.; McKay, J.S. Evaluation of a convenient method of assessing rodent visual function in safety pharmacology studies: Effects of sodium iodate on visual acuity and retinal morphology in albino and pigmented rats and mice. J. Pharmacol. Toxicol. Methods 2011, 63, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Enzmann, V.; Row, B.W.; Yamauchi, Y.; Kheirandish, L.; Gozal, D.; Kaplan, H.J.; McCall, M.A. Behavioral and anatomical abnormalities in a sodium iodate-induced model of retinal pigment epithelium degeneration. Exp. Eye Res. 2006, 82, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Machalinska, A.; Kawa, M.P.; Pius-Sadowska, E.; Roginska, D.; Klos, P.; Baumert, B.; Wiszniewska, B.; Machalinski, B. Endogenous regeneration of damaged retinal pigment epithelium following low dose sodium iodate administration: An insight into the role of glial cells in retinal repair. Exp. Eye Res. 2013, 112, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Hariri, S.; Tam, M.C.; Lee, D.; Hileeto, D.; Moayed, A.A.; Bizheva, K. Noninvasive imaging of the early effect of sodium iodate toxicity in a rat model of outer retina degeneration with spectral domain optical coherence tomography. J. Biomed. Opt. 2013, 18, 26017. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-T.; Deng, X.-G.; Gao, Y.; He, M.-F.; Li, N. Pathological and SOD, CAT changes in rats with retinal injuries induced by Sodium iodate. Chin. J. Pathophysiol. 2010, 9, 048. (In Chinese) [Google Scholar]
- Zhang, T.; Zhang, N.; Baehr, W.; Fu, Y. Cone opsin determines the time course of cone photoreceptor degeneration in Leber congenital amaurosis. Proc. Natl. Acad. Sci. USA 2011, 108, 8879–8884. [Google Scholar] [CrossRef] [PubMed]
- Mendes, C.S.; Levet, C.; Chatelain, G.; Dourlen, P.; Fouillet, A.; Dichtel-Danjoy, M.-L.; Gambis, A.; Ryoo, H.D.; Steller, H.; Mollereau, B. ER stress protects from retinal degeneration. EMBO J. 2009, 28, 1296–1307. [Google Scholar] [CrossRef] [PubMed]
- Farrar, G.J.; Palfi, A.; O’Reilly, M. Gene therapeutic approaches for dominant retinopathies. Curr. Gene. Ther. 2010, 10, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kume, S.; Kitada, M.; Kanasaki, K.; Uzu, T.; Maegawa, H.; Koya, D. Autophagy as a therapeutic target in diabetic nephropathy. Exp. Diabetes Res. 2012, 2012, 628978. [Google Scholar] [CrossRef] [PubMed]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Hummasti, S.; Hotamisligil, G.S. Endoplasmic reticulum stress and inflammation in obesity and diabetes. Circ. Res. 2010, 107, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, D.T.; Kaufman, R.J. That which does not kill me makes me stronger: Adapting to chronic ER stress. Trends Biochem. Sci. 2007, 32, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Marino, G.; Levine, B. Autophagy and the integrated stress response. Mol. Cell 2010, 40, 280–293. [Google Scholar]
- Rao, N.A.; Saraswathy, S.; Wu, G.S; Katselis, G.S.; Wawrousek, E.F.; Bhat, S. Elevated retina-specific expression of the small heat shock protein, alphaA-crystallin, is associated with photoreceptor protection in experimental uveitis. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1161–1171. [Google Scholar]
- Bahk, S.C.; Jang, J.U.; Choi, C.U.; Lee, S.-H.; Park, Z.-Y.; Yang, J.-Y.; Kim, J.-D.; Yang, Y.-S.; Chung, H.-T. Post-translational modification of crystallins in vitreous body from experimental autoimmune uveitis of rats. J. Proteome Res. 2007, 6, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.; Sreekumar, P.G.; Hinton, D.R. Novel roles for alpha-crystallins in retinal function and disease. Prog. Retin. Eye Res. 2012, 31, 576–604. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.Z.; Fu, X.B.; Yao, Y.M.; Yang, Y.H.; Chen, W.; Zhao, Z.L.; Sun, X.Q. An ideal way of intravenous medication administration in male rats. Shi Yan Dong Wu Ke Xue Yu Guan Li 2002, 19, 46. (In Chinese) [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhao, X.; Cai, Y.; Li, Y.; Yu, X.; Lu, L. Protection of Retina by Mini-αA in NaIO3-Induced Retinal Pigment Epithelium Degeneration Mice. Int. J. Mol. Sci. 2015, 16, 1644-1656. https://doi.org/10.3390/ijms16011644
Zhang J, Zhao X, Cai Y, Li Y, Yu X, Lu L. Protection of Retina by Mini-αA in NaIO3-Induced Retinal Pigment Epithelium Degeneration Mice. International Journal of Molecular Sciences. 2015; 16(1):1644-1656. https://doi.org/10.3390/ijms16011644
Chicago/Turabian StyleZhang, Jinglin, Xiujuan Zhao, Yu Cai, Yonghao Li, Xiling Yu, and Lin Lu. 2015. "Protection of Retina by Mini-αA in NaIO3-Induced Retinal Pigment Epithelium Degeneration Mice" International Journal of Molecular Sciences 16, no. 1: 1644-1656. https://doi.org/10.3390/ijms16011644