Systemic Delivery of Protein Nanocages Bearing CTT Peptides for Enhanced Imaging of MMP-2 Expression in Metastatic Tumor Models
Abstract
:1. Introduction
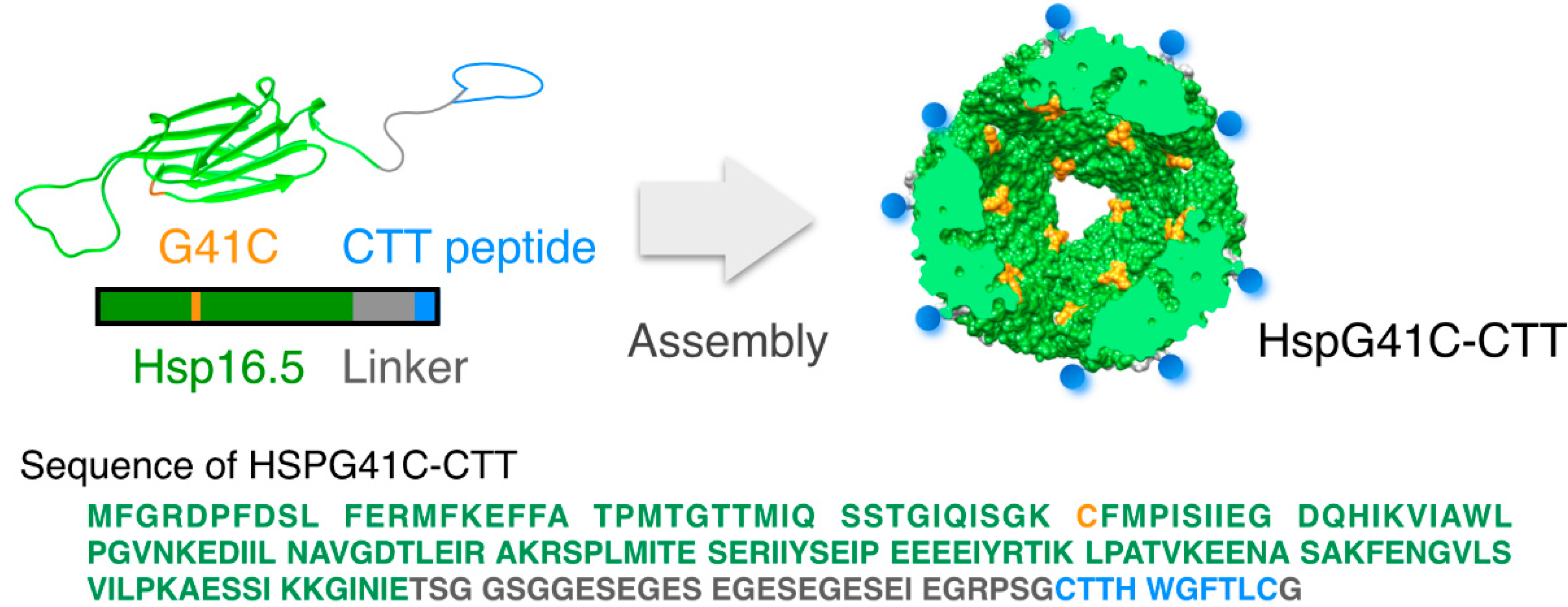
2. Results and Discussion
2.1. Characterization of the Protein Nanocages Modified with CTT Peptides


2.2. Cellular Uptake of the Protein Nanocages in Vitro

2.3. Biodistribution of the Protein Nanocages in Vivo
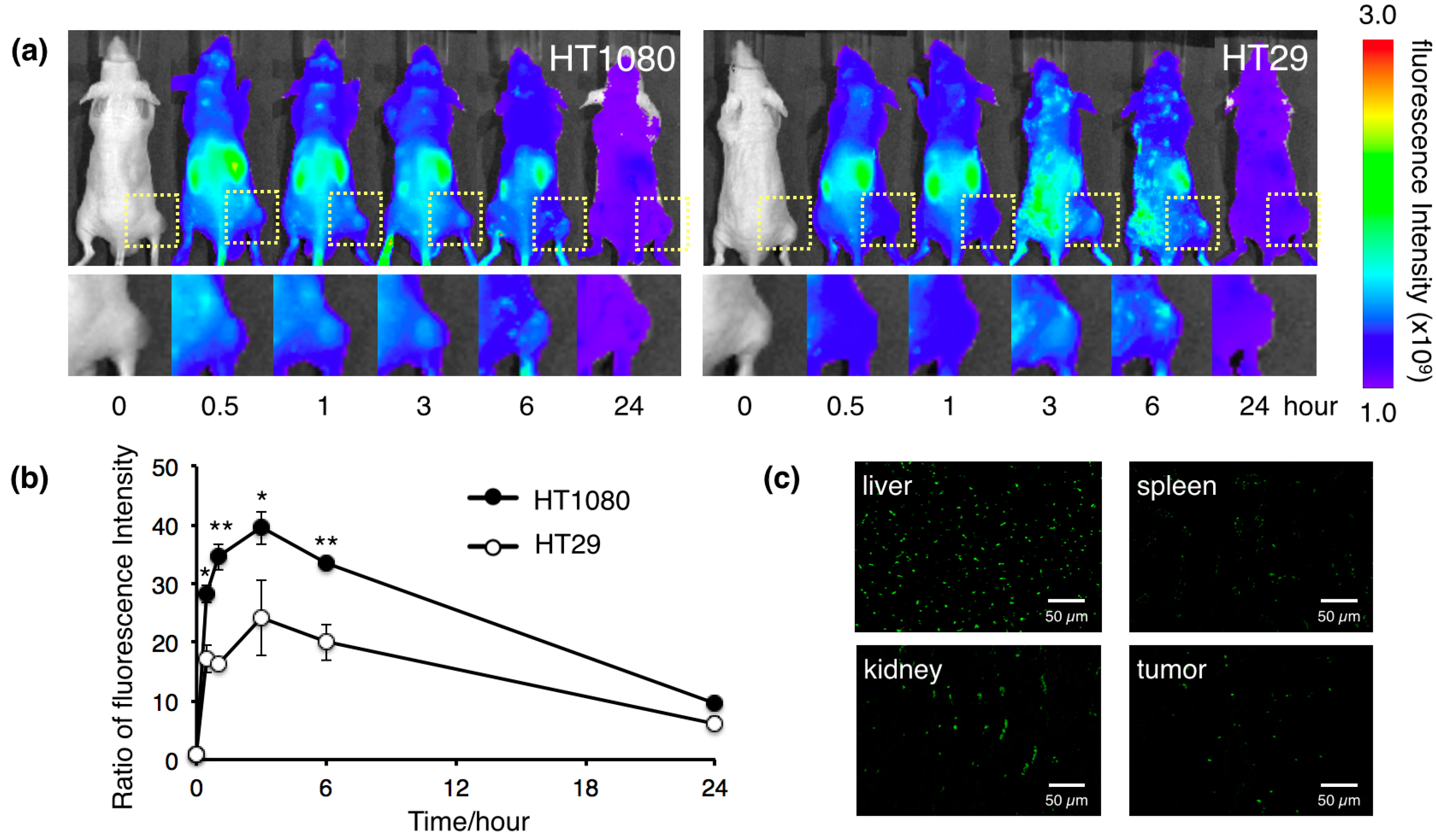
3. Experimental Section
3.1. Protein Expression
3.2. Protein Purification
3.3. Size Measurements
3.4. MMP-Binding Assay
3.5. Conjugation of Fluorophores to Protein Nanocages
3.6. Cell Study
3.7. Biodistribution in Vivo
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mignatti, P.; Rifkin, D.B. Biology and biochemistry of proteinases in tumor invasion. Physiol. Rev. 1993, 73, 161–195. [Google Scholar] [PubMed]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 163–176. [Google Scholar] [CrossRef]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, M.; Floyd, C.D.; Brown, P.; Gearing, A.J. Design and therapeutic application of matrix metalloproteinase inhibitors. Chem. Rev. 1999, 99, 2735–2776. [Google Scholar] [CrossRef] [PubMed]
- Woessner, J.F. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1990, 5, 2145–2154. [Google Scholar]
- Hrabec, E.; Strek, M.; Nowak, D.; Greger, J.; Suwalski, M.; Hrabec, Z. Activity of type IV collagenases (MMP-2 and MMP-9) in primary pulmonary carcinomas: A quantitative analysis. J. Cancer Res. Clin. Oncol. 2002, 128, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Vilinen, P.; Kahari, V.-M. Matrix metalloproteinases in cancer: Prognostic markers and therapeutic targets. Int. J. Cancer 2002, 99, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Koivunen, E.; Arap, W.; Valtanen, H.; Rainisalo, A.; Medina, O.P.; Heikkilä, P.; Kantor, C.; Gahmberg, C.G.; Salo, T.; Konttinen, Y.T.; et al. Tumor targeting with a selective gelatinase inhibitor. Nat. Biotechnol. 1999, 17, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Angiogenic zip code. Nat. Biotechol. 1999, 17. [Google Scholar] [CrossRef]
- Kuhnast, B.; Bodenstein, C.; Haubner, R.; Wester, H.J.; Senekowitsch-Schmidtke, R.; Schwaiger, M.; Weber, W.A. Targeting of gelatinase activity with a radiolabeled cyclic HWGF peptide. Nucl. Med. Biol. 2004, 31, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, H.; Mukai, T.; Habashita, S.; Asano, D.; Ogawa, K.; Kuroda, Y.; Akizawa, H.; Iida, Y.; Endo, K.; Saga, T.; et al. Chemical design of a radiolabeled gelatinase inhibitor peptide for the imaging of gelatinase activity in tumors. Nucl. Med. Biol. 2007, 34, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Sprague, J.E.; Li, W.P.; Liang, K.; Achilefu, S.; Anderson, C.J. In vitro and in vivo investigation of matrix metalloproteinase expression in metastatic tumor models. Nucl. Med. Biol. 2006, 33, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Adair, J.H.; Parette, M.P.; Altınoglu, E.I.; Kester, M. Nanoparticulate alternatives for drug delivery. ACS Nano 2010, 4, 4967–4970. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Brannon-Peppas, L.; Blanchette, J.O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Del. Rev. 2012, 64, 206–212. [Google Scholar] [CrossRef]
- Duncan, R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003, 2, 347–360. [Google Scholar] [PubMed]
- Kim, K.K.; Kim, R.; Kim, S.H. Crystal structure of a small heat-shock protein. Nature 1998, 394, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Lai, L.; Lee, H.-H.; Cheong, G.-W.; Kim, K.K.; Wu, Z.; Yokota, H.; Marqusee, S.; Kim, S.-H. On the mechanism of chaperone activity of the small heat-shock protein of Methanococcus jannaschii. Proc. Natl. Acad. Sci. USA 2003, 100, 8151–8155. [Google Scholar] [CrossRef] [PubMed]
- Koteiche, H.A.; Chiu, S.; Majdoch, R.L.; Stewart, P.L.; McHaourab, H.S. Atomic models by cryo-EM and site-directed spin labeling: Application to the N-Terminal region of Hsp16.5. Structure 2005, 13, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Sao, K.; Murata, M.; Umezaki, K.; Fujisaki, Y.; Mori, T.; Niidome, T.; Katayama, Y.; Hashizume, M. Molecular design of protein-based nanocapsules for stimulus-responsive characteristics. Bioorg. Med. Chem. 2009, 17, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Toita, R.; Murata, M.; Tabata, S.; Abe, K.; Narahara, S.; Piao, J.S.; Kang, J.-H.; Hashizume, M. Development of human hepatocellular carcinoma cell-targeted protein cages. Bioconjugate Chem. 2012, 23, 1494–1501. [Google Scholar] [CrossRef]
- Toita, R.; Murata, M.; Abe, K.; Narahara, S.; Piao, J.S.; Kang, J.-H.; Ohuchida, K.; Hashizume, M. Biological evaluation of protein nanocapsules containing doxorubicin. Int. J. Nanomed. 2013, 8, 1989–1999. [Google Scholar]
- Toita, R.; Murata, M.; Abe, K.; Narahara, S.; Piao, J.S.; Kang, J.-H.; Hashizume, M. A nanocarrier based on a genetically engineered protein cage to deliver doxorubicin to human hepatocellular carcinoma cells. Chem. Commun. 2013, 49, 7442–7444. [Google Scholar] [CrossRef]
- Puyraimond, A.; Fridman, R.; Lemesle, M.; Arbeille, B.; Menashi, S. MMP-2 colocalizes with caveolae on the surface of endothelial cells. Exp. Cell Res. 2001, 262, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, J.; Shekarriz, B.; Nemeth, J.A.; Dong, Z.; Cummings, G.D.; Fridman, R.; Sakr, W.; Grignon, D.J.; Cher, M.L. Membrane type 1-matrix metalloproteinase (MT1-MMP) and MMP-2 immunolocalization in human prostate: Change in cellular localization associated with high-grade prostatic intraepithelial neoplasia. Clin. Cancer Res. 1999, 5, 4105–4110. [Google Scholar] [PubMed]
- Wang, W.; Shao, R.; Wu, Q.; Ke, S.; McMurray, J.; Lang, F.F., Jr.; Charnsangavej, C.; Gelovani, J.G.; Li, C. Targeting gelatinases with a near-infrared fluorescent cyclic His-Try-Gly-Phe peptide. Mol. Imaging Biol. 2009, 11, 424–433. [Google Scholar] [CrossRef]
- Medina, O.P.; Söderlund, T.; Laakkonen, L.J.; Tuominen, E.K.J.; Koivunen, E.; Kinnunen, P.K.J. Binding of novel peptide inhibitors of type IV collagenases to phospholipid membranes and use in liposome targeting to tumor cells in vitro. Cancer Res. 2001, 61, 3948–3985. [Google Scholar]
- Kaiser, C.R.; Flenniken, M.L.; Gillitzer, E.; Harmsen, A.L.; Harmsen, A.G.; Jutila, M.A.; Douglas, T.; Young, M.J. Biodistribution studies of protein cage nanoparticles demonstrate broad tissue distribution and rapid clearance in vivo. Int. J. Nanomed. 2007, 2, 715–733. [Google Scholar]
- Haubner, R.; Wester, H.J.; Burkhart, F.; Senekowitsch-Schmidtke, R.; Weber, W.; Goodman, S.L.; Kessler, H.; Schwaiger, M. Glycosylated RGD-containing peptides: Tracer for tumor targeting and angiogenesis imaging with improved biokinetics. J. Nucl. Med. 2001, 42, 326–336. [Google Scholar] [PubMed]
- Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M.R.; Miyazono, K.; Uesaka, M.; et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011, 6, 815–823. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawano, T.; Murata, M.; Piao, J.S.; Narahara, S.; Hamano, N.; Kang, J.-H.; Hashizume, M. Systemic Delivery of Protein Nanocages Bearing CTT Peptides for Enhanced Imaging of MMP-2 Expression in Metastatic Tumor Models. Int. J. Mol. Sci. 2015, 16, 148-158. https://doi.org/10.3390/ijms16010148
Kawano T, Murata M, Piao JS, Narahara S, Hamano N, Kang J-H, Hashizume M. Systemic Delivery of Protein Nanocages Bearing CTT Peptides for Enhanced Imaging of MMP-2 Expression in Metastatic Tumor Models. International Journal of Molecular Sciences. 2015; 16(1):148-158. https://doi.org/10.3390/ijms16010148
Chicago/Turabian StyleKawano, Takahito, Masaharu Murata, Jing Shu Piao, Sayoko Narahara, Nobuhito Hamano, Jeong-Hun Kang, and Makoto Hashizume. 2015. "Systemic Delivery of Protein Nanocages Bearing CTT Peptides for Enhanced Imaging of MMP-2 Expression in Metastatic Tumor Models" International Journal of Molecular Sciences 16, no. 1: 148-158. https://doi.org/10.3390/ijms16010148




