Quantification of Drug Transport Function across the MultipleResistance-Associated Protein 2 (Mrp2) in Rat Livers
Abstract
:1. Introduction
2.Results
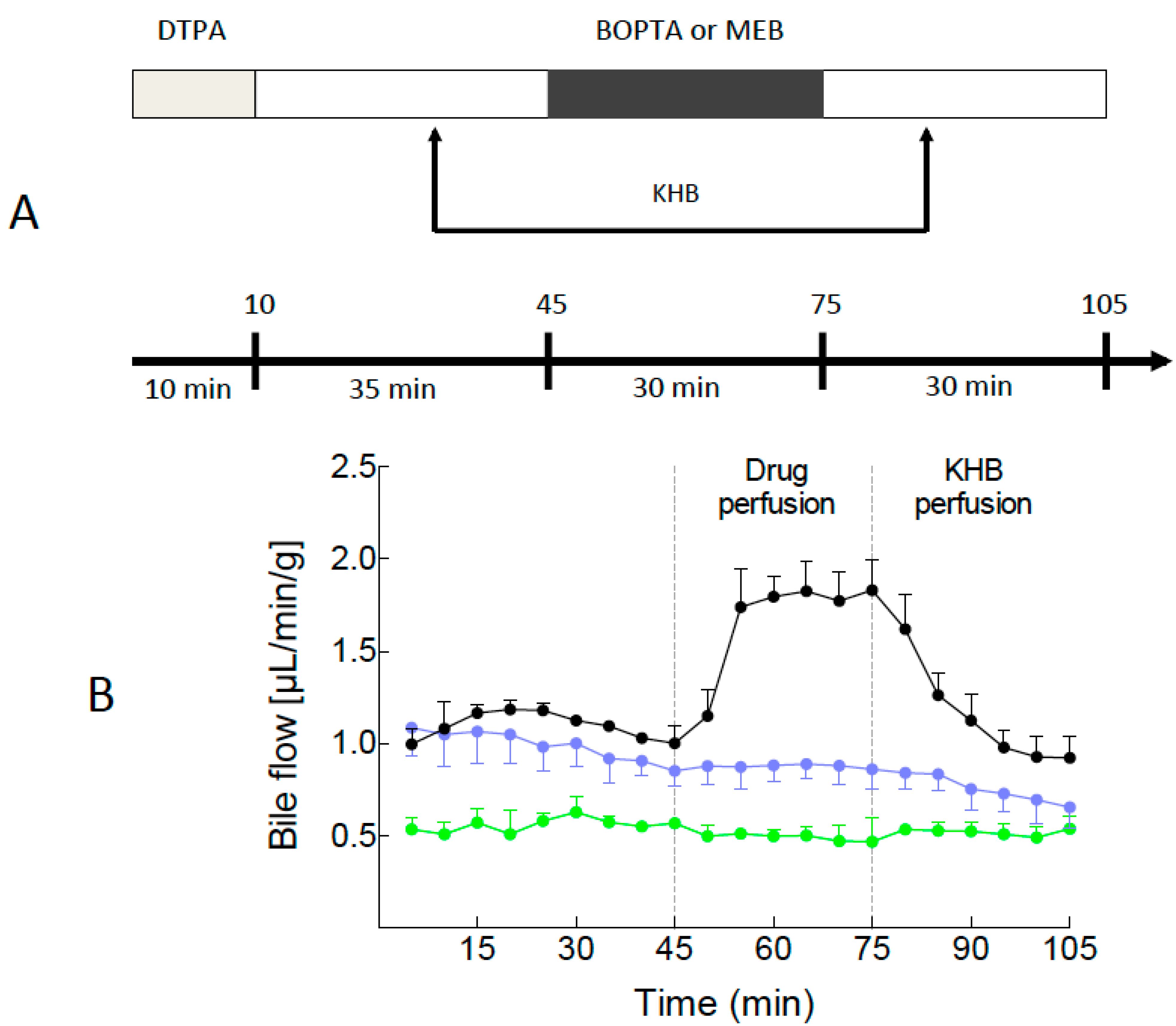
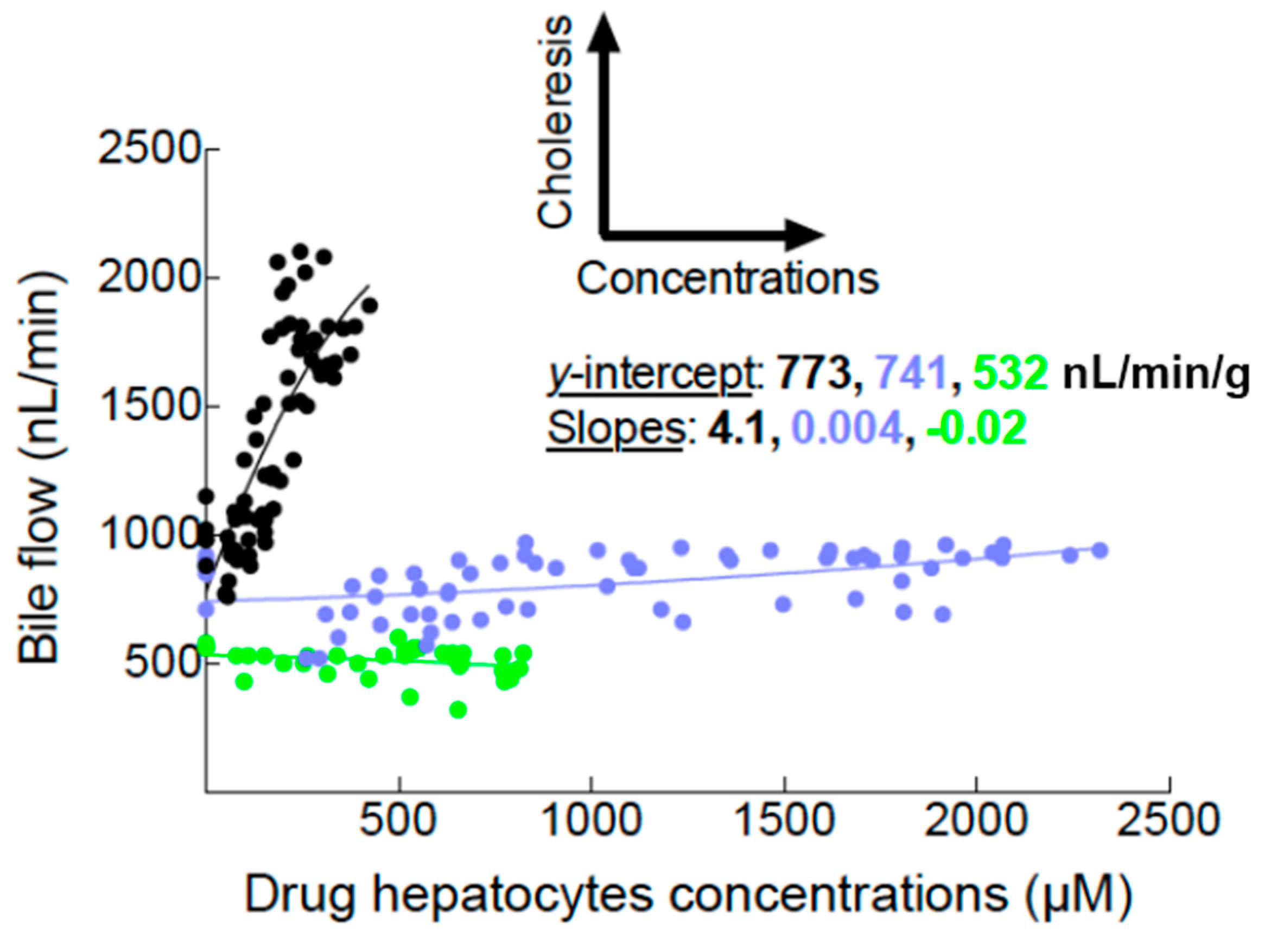
2.1. Bile Flows during Drug Perfusion
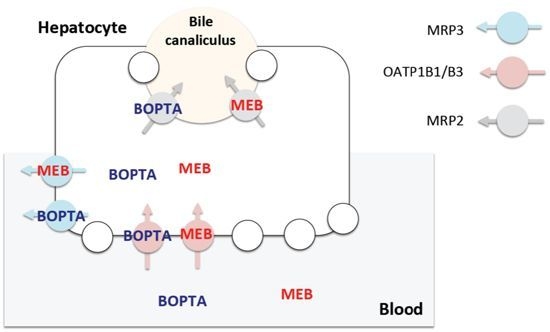
2.2. Canalicular Transport of BOPTA and MEB


3. Discussion
3.1. Assessment of Mrp2 Function in the Entire Rat Liver
3.2. Drug Hepatic Pharmacokinetics in Isolated Perfused Rodent Livers
3.3. Calculation of Hepatocyte Concentrations
3.4. Transport Function of Rat Mrp2
4. Experimental Section
4.1. Experimental Groups
4.2. Isolation and Perfusion of Rat Livers
4.3. Drugs and Experimental Protocol (Figure 1A)
4.4. Drug Concentrations in Hepatocytes
4.5. Statistics
5. Clinical Relevance of the Study
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Esteller, A. Physiology of bile secretion. World J. Gastroenterol. 2008, 14, 5641–5649. [Google Scholar]
- Boyer, J.L. Bile formation and secretion. Compr. Physiol. 2013, 3, 1035–1078. [Google Scholar]
- Köck, K.; Brouwer, K.L. A perspective on efflux transport proteins in the liver. Clin. Pharmacol. Ther. 2012, 92, 599–612. [Google Scholar]
- Yang, K.; Pfeifer, N.D.; Hardwick, R.N.; Yue, W.; Stewart, P.W.; Brouwer, K.L. An experimental approach to evaluate the impact of impaired transport function on hepatobiliary drug disposition using Mrp2-deficient TR-rat sandwich-cultured hepatocytes in combination with BCRP knockdown. Mol. Pharm. 2014, 11, 766–775. [Google Scholar]
- Pastor, C.M.; Müllhaupt, B.; Stieger, B. The role of organic anion transporters in diagnosing liver diseases by magnetic resonance imaging. Drug Metab. Dispos. 2014, 42, 675–684. [Google Scholar]
- Morana, G.; Grazioli, L.; Kirchin, M.A.; Bondioni, M.P.; Faccioli, N.; Guarise, A.; Schneider, G. Solid hypervascular liver lesions: Accurate identification of true benign lesions on enhanced dynamic and hepatobiliary phase magnetic resonance imaging after gadobenate dimeglumine administration. Investig. Radiol. 2011, 46, 225–239. [Google Scholar]
- Planchamp, C.; Hadengue, A.; Stieger, B.; Bourquin, J.; Vonlaufen, A.; Frossard, J.L.; Quadri, R.; Becker, C.D.; Pastor, C.M. Function of both sinusoidal and canalicular transporters controls the concentration of organic anions within hepatocytes. Mol. Pharmacol. 2007, 71, 1089–1097. [Google Scholar]
- De Graaf, W.; van Lienden, K.P.; van Gulik, T.M.; Bennink, R.J. 99mTc-mebrofenin hepatobiliary scintigraphy with SPECT for the assessment of hepatic function and liver functional volume before partial hepatectomy. J. Nucl. Med. 2010, 51, 229–236. [Google Scholar]
- Tavoloni, N.; Reed, J.S.; Boyer, J.L. Hemodynamic effects on determinants of bile secretion in isolated rat liver. Am. J. Physiol. 1978, 234, E584–E592. [Google Scholar]
- Ferrigno, A.; Richelmi, P.; Vairetti, M. Troubleshooting and improving the mouse and rat isolated perfused liver preparation. J. Pharmacol. Toxicol. Methods 2013, 67, 107–114. [Google Scholar]
- Parasrampuria, R.; Mehvar, R. Dose-dependent inhibition of transporter-mediated hepatic uptake and biliary excretion of methotrexate by cyclosporine A in an isolated perfused rat liver model. J. Pharm. Sci. 2010, 99, 5060–5069. [Google Scholar]
- Daidoji, T.; Inoue, H.; Kato, S.; Yokota, H. Glucuronidation and excretion of nonylphenol in perfused rat liver. Drug Metab. Dispos. 2003, 31, 993–998. [Google Scholar]
- Pastor, C.M.; Planchamp, C.; Pochon, S.; Lorusso, V.; Montet, X.; Mayer, J.; Terrier, F.; Vallée, J.P. Kinetics of gadobenate dimeglumine in isolated perfused rat liver: MR imaging evaluation. Radiology 2003, 229, 119–125. [Google Scholar]
- Planchamp, C.; Montet, X.; Frossard, J.L.; Quadri, R.; Stieger, B.; Meier, P.J.; Ivancevic, M.K.; Vallée, J.P.; Terrier, F.; Pastor, C.M. Magnetic resonance imaging with hepatospecific contrast agents in cirrhotic rat livers. Investig. Radiol. 2005, 40, 187–194. [Google Scholar]
- Blouin, A.; Bolender, R.P.; Weibel, E.R. Distribution of organelles and membranes between hepatocytes and nonhepatocytes in the rat liver parenchyma: A stereological study. J. Cell Biol. 1977, 72, 441–455. [Google Scholar]
- Doo, E.; Krishnamurthy, G.T.; Eklem, M.J.; Gilbert, S.; Brown, P.H. Quantification of hepatobiliary function as an integral part of imaging with technetium-99m-mebrofenin in health and disease. J. Nucl. Med. 1991, 32, 48–57. [Google Scholar]
- Erdogan, D.; Heijnen, B.H.; Bennink, R.J.; Kok, M.; Dinant, S.; Straatsburg, I.H.; Gouma, D.J.; van Gulik, T.M. Preoperative assessment of liver function: A comparison of 99mTc-mebrofenin scintigraphy with indocyanine green clearance test. Liver Int. 2004, 24, 117–123. [Google Scholar]
- Bennink, R.J.; Vetelainen, R.; de Bruin, K.; van Vliet, A.K.; van Gulik, T.M. Imaging of liver function with dedicated animal dynamic pinhole scintigraphy in rats. Nucl. Med. Commun. 2005, 26, 1005–1012. [Google Scholar]
- Swift, B.; Pfeifer, N.D.; Brouwer, K.L. Sandwich-cultured hepatocytes: An in vitro model to evaluate hepatobiliary transporter-based drug interactions and hepatotoxicity. Drug Metab. Rev. 2010, 42, 446–471. [Google Scholar]
- De Graaf, W.; Häusler, S.; Heger, M.; van Ginhoven, T.M.; van Cappellen, G.; Bennink, R.J.; Kullak-Ublick, G.A.; Hesselmann, R.; van Gulik, T.M.; Stieger, B. Transporters involved in the hepatic uptake of 99mTc-mebrofenin and indocyanine green. J. Hepatol. 2011, 54, 738–745. [Google Scholar]
- Ghibellini, G.; Leslie, E.M.; Pollack, G.M.; Brouwer, K.L. Use of Tc-99m mebrofenin as a clinical probe to assess altered hepatobiliary transport: Integration of in vitro, pharmacokinetic modeling, and simulation studies. Pharm. Res. 2008, 25, 1851–1860. [Google Scholar]
- Swift, B.; Yue, W.; Brouwer, K.L. Evaluation of 99mtechnetium-mebrofenin and 99mechnetium-sestamibi as specific probes for hepatic transport protein function in rat and human hepatocytes. Pharm. Res. 2010, 27, 1987–1998. [Google Scholar]
- Neyt, S.; Huisman, M.T.; Vanhove, C.; de Man, H.; Vliegen, M.; Moerman, L.; Dumolyn, C.; Mannens, G.; de Vos, F. In vivo visualization and quantification of (disturbed) Oatp-mediated hepatic uptake and Mrp2-mediated biliary excretion of 99mTc-mebrofenin in mice. J. Nucl. Med. 2013, 54, 624–630. [Google Scholar]
- Pfeifer, N.D.; Hardwick, R.N.; Brouwer, K.L.R. Role of hepatic efflux transporters in regulating systemic and hepatocyte exposure to xenobiotics. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 509–535. [Google Scholar]
- Takashima, T.; Nagata, H.; Nakae, T.; Cui, Y.; Wada, Y.; Kitamura, S.; Doi, H.; Suzuki, M.; Maeda, K.; Kusuhara, H.; et al. Positron emission tomography studies using (15R)-16-m-[11C]tolyl-17,18,19,20-tetranorisocarbacyclin Methyl ester for the evaluation of hepatobiliary transport. J. Pharmacol. Exp. Ther. 2010, 335, 314–323. [Google Scholar]
- Takashima, T.; Hashizume, Y.; Katayama, Y.; Murai, M.; Wada, Y.; Maeda, K.; Sugiyama, Y.; Watanabe, Y. The involvement of organic anion transporting polypeptide in the hepatic uptake of telmisartan in rats: PET studies with [11C]telmisartan. Mol. Pharm. 2011, 8, 1789–1798. [Google Scholar]
- Yoshida, K.; Maeda, K.; Sugiyama, Y. Hepatic and intestinal drug transporters: Prediction of pharmacokinetic effects caused by drug-drug interactions and genetic polymorphisms. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 581–612. [Google Scholar]
- Kusuhara, H. Imaging in the study of membrane transporters. Clin. Pharmacol. Ther. 2013, 94, 33–36. [Google Scholar]
- Wulkersdorfer, B.; Wanek, T.; Bauer, M.; Zeitlinger, M.; Müller, M.; Langer, O. Using positron emission tomography to study transporter-mediated drug–drug interactions in tissues. Clin. Pharmacol. Ther. 2014, 96, 206–213. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonnaventure, P.; Pastor, C.M. Quantification of Drug Transport Function across the MultipleResistance-Associated Protein 2 (Mrp2) in Rat Livers. Int. J. Mol. Sci. 2015, 16, 135-147. https://doi.org/10.3390/ijms16010135
Bonnaventure P, Pastor CM. Quantification of Drug Transport Function across the MultipleResistance-Associated Protein 2 (Mrp2) in Rat Livers. International Journal of Molecular Sciences. 2015; 16(1):135-147. https://doi.org/10.3390/ijms16010135
Chicago/Turabian StyleBonnaventure, Pierre, and Catherine M. Pastor. 2015. "Quantification of Drug Transport Function across the MultipleResistance-Associated Protein 2 (Mrp2) in Rat Livers" International Journal of Molecular Sciences 16, no. 1: 135-147. https://doi.org/10.3390/ijms16010135