Function of SREBP1 in the Milk Fat Synthesis of Dairy Cow Mammary Epithelial Cells
Abstract
:1. Introduction
2. Results and Discussion
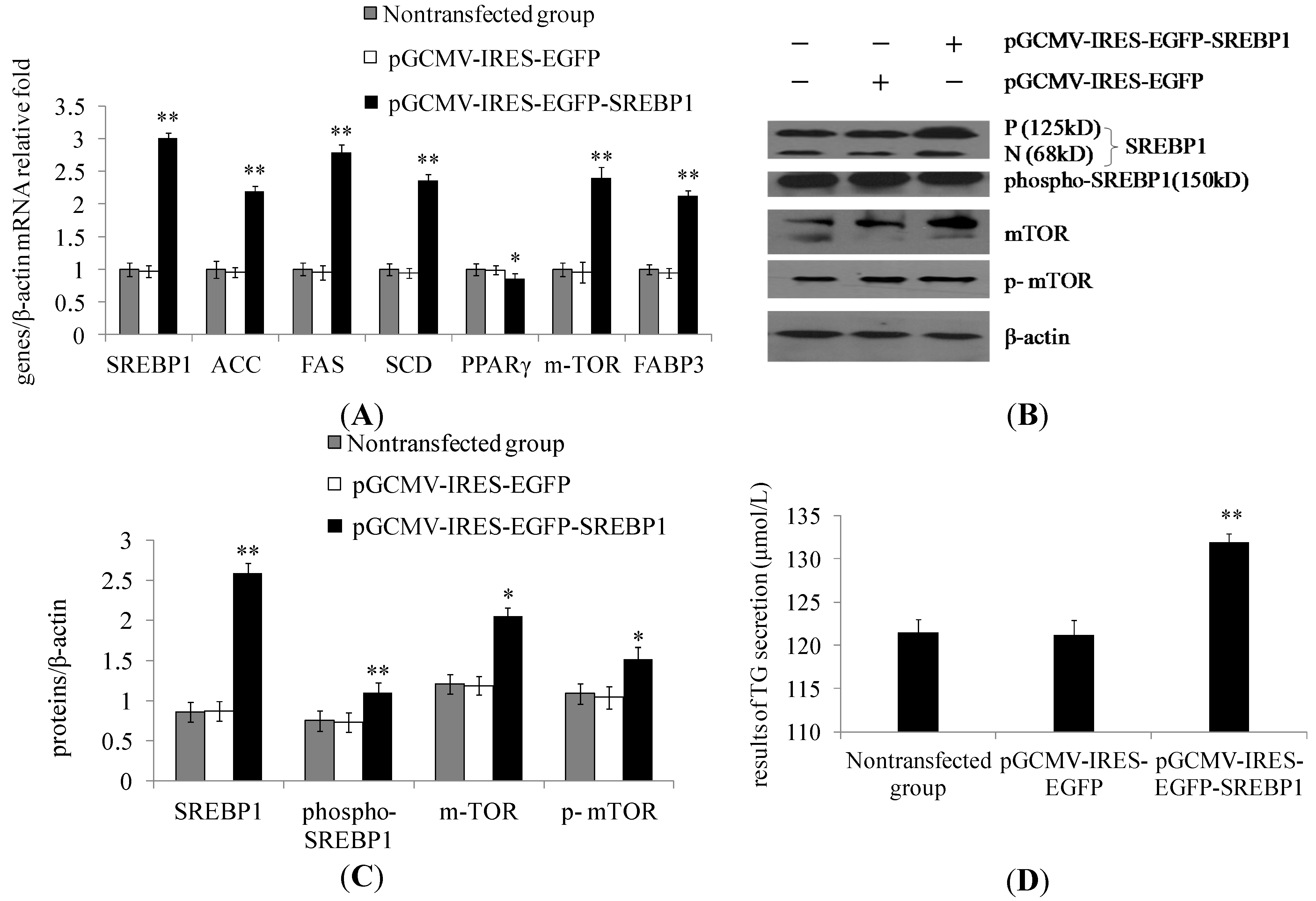
2.1. Sterol Regulatory Element-Binding Protein 1 (SREBP1) Overexpression in Dairy Cow Mammary Epithelial Cells (DCMECs) Increases the Expression of Lipogenic Genes and Key Enzymes of Fatty Acid Synthesis as Well as Triglyceride Secretion
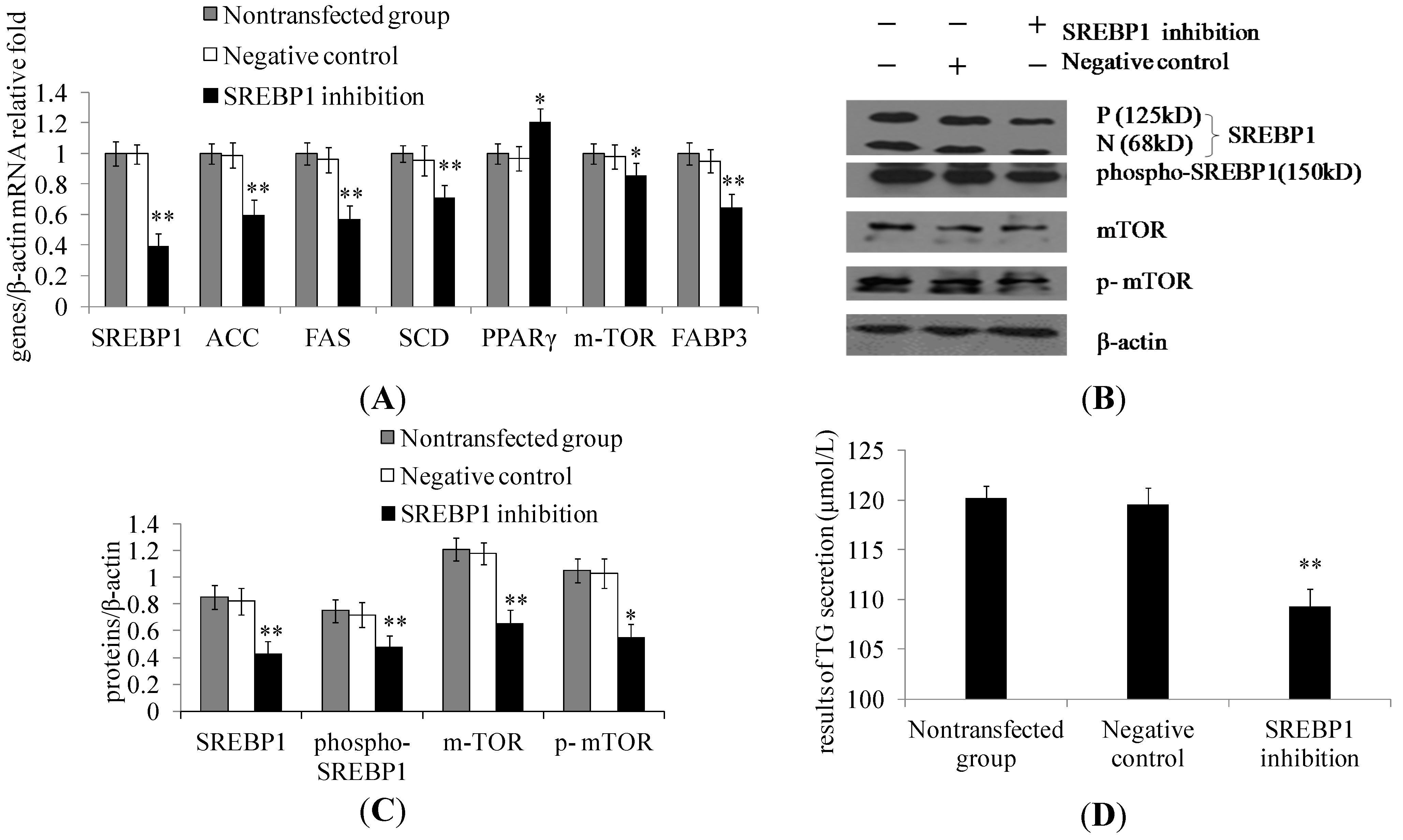
2.2. SREBP1 Gene Silencing Decreases the Expression of Lipogenic Genes and Key Enzymes of Fatty Acid Synthesis and Decreases Triglyceride Secretion in DCMECs
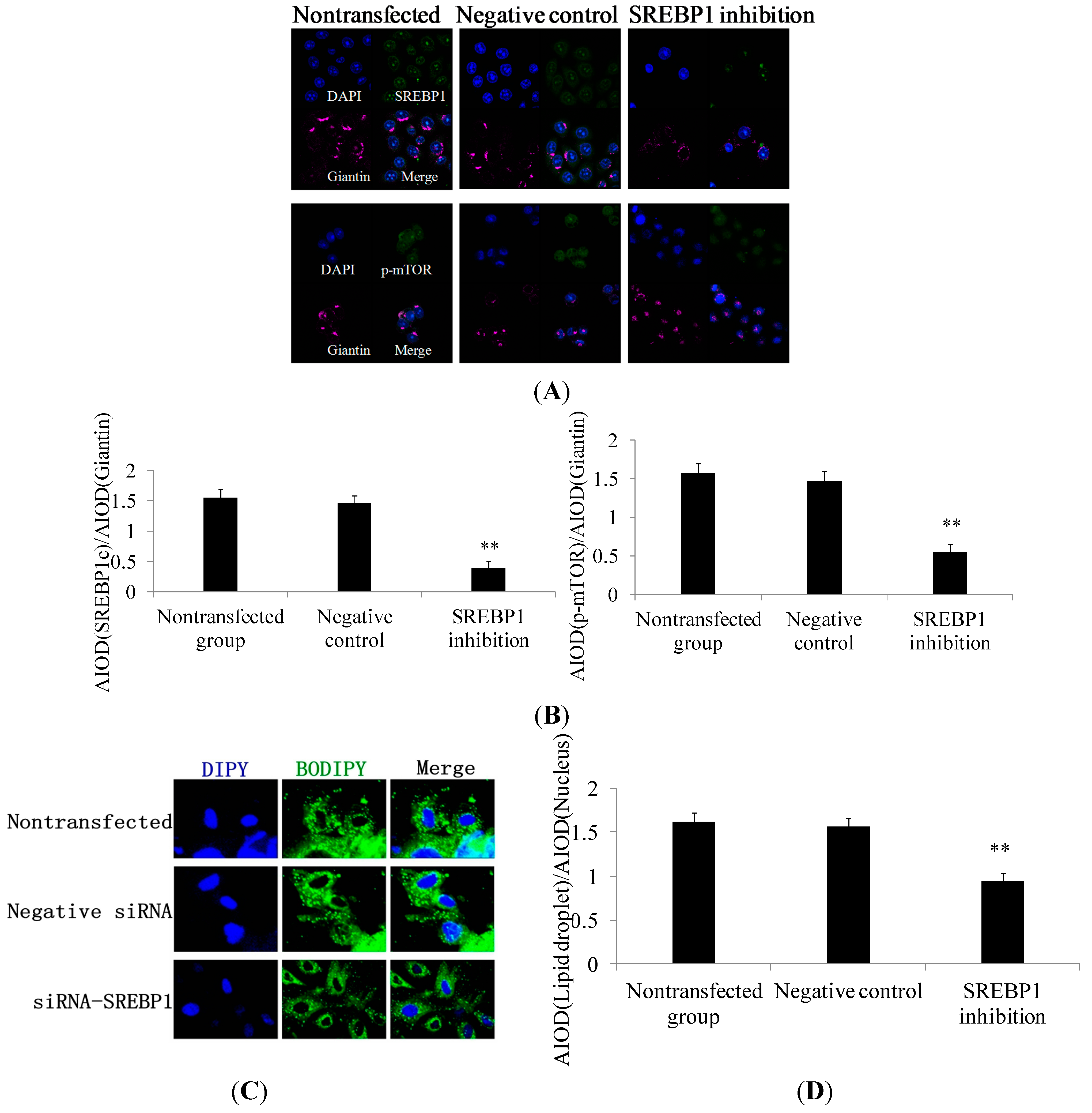
2.3. The Effect of Stearic Acid and Serum on SREBP1 and mTOR Expression in the Regulation of Milk Fat Synthesis
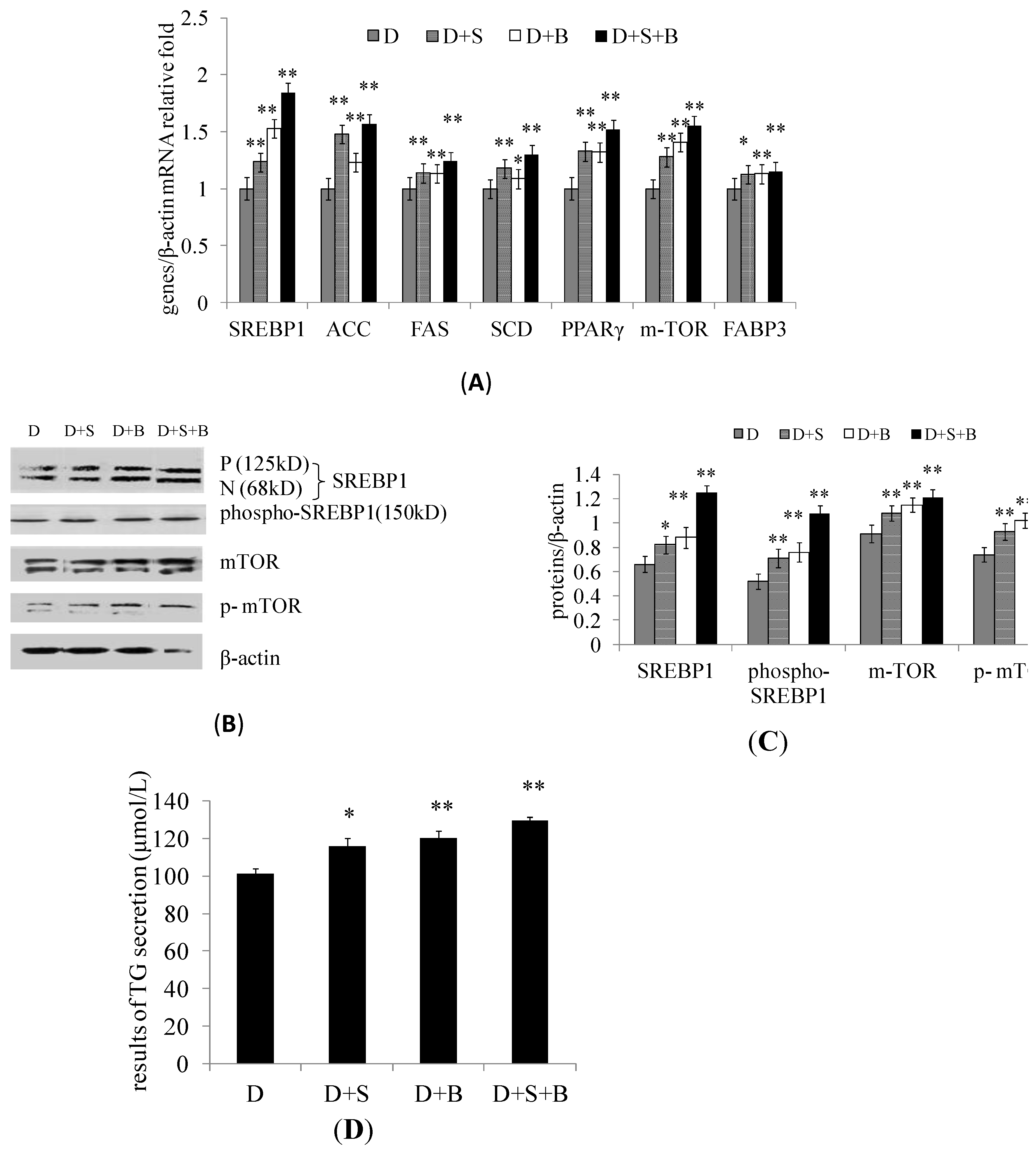
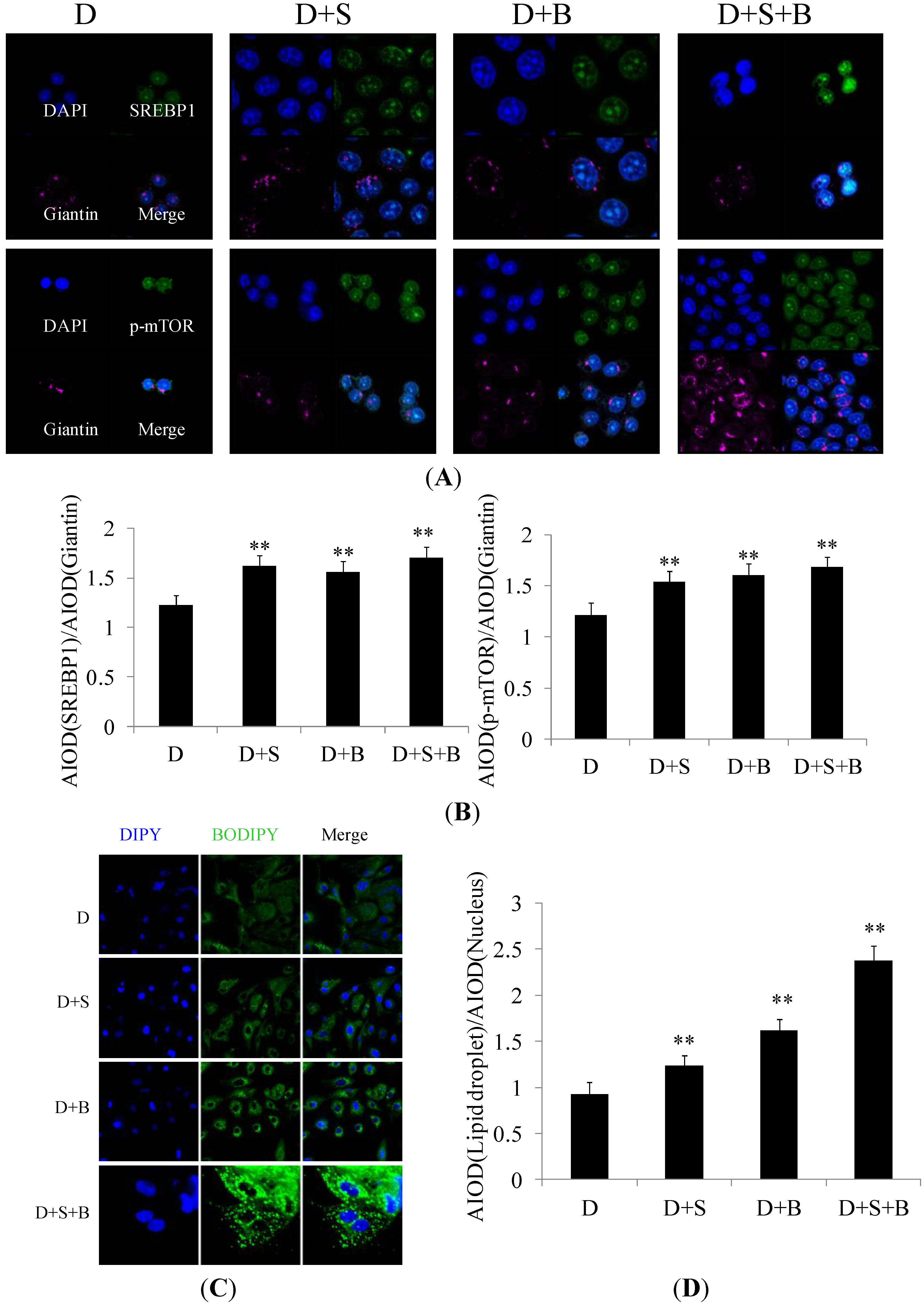
2.4. Discussion
3. Experimental Section
3.1. Experimental Materials
3.2. Primary DCMEC Culture and Treatment
3.3. Gene Silencing of SREBP1
3.4. pGCMV–IRES–EGFP–SREBP1 Construction and Transfection
3.5. BODIPY Staining of Lipid Droplets
3.6. Detection of Triglyceride Secretion
3.7. RNA Extraction and Real-Time Quantitative PCR
3.8. Immunofluorescence Assay
3.9. Western Blotting Analysis
3.10. Bioinformatics Analysis
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hua, X.; Wu, J.; Goldstein, J.L.; Brown, M.S.; Hobbs, H.H. Structure of the human gene encoding sterol regulatoryelement binding protein 1 (SREBF1) and localization of SREBF1 and SREBF2 to chromosomes 17p11.2 and 22q13. Genomics 1995, 25, 667–673. [Google Scholar] [CrossRef]
- Yokoyama, C.; Wang, X.; Briggs, M.R.; Admon, A.; Wu, J.; Hua, X.; Goldstein, J.L.; Brown, M.S. SREBP1, a basic helixloop-helix leucine zipper protein that controls transcription of the LDL receptor gene. Cell 1993, 75, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Yang, J.; Wang, X.; Evans, M.J.; Ho, Y.K.; Goldstein, J.L.; Brown, M.S. Assignment of the membrane attachment, DNA binding, and transcriptional activation domains of sterol regulatory element binding protein l (SREBP1). J. Biol. Chem. 1994, 269, 17267–17273. [Google Scholar] [PubMed]
- Naar, A.M.; Beaurang, P.A.; Robinson, K.M.; Oliner, J.D.; Avizonis, D.; Scheek, S.; Zwicker, J.; Kadonaga, J.T.; Tjian, R. Chromatin, TAFs, and a novel multiprotein coactivator are required for synergistic activation by Sp1 and SREBP1a in vitro. Genes Dev. 1998, 12, 3020–3031. [Google Scholar] [CrossRef] [PubMed]
- McPherson, R.; Gauthier, A. Molecular regulation of SREBP function: The Insig-SCAP connection and isoform-specific modulation of lipid synthesis. Biochem. Cell Biol. 2004, 2, 201–211. [Google Scholar] [CrossRef]
- Bennett, M.K.; Toth, J.I.; Osborne, T.F. Selective association of sterol regulatory element-binding protein isoforms with target promoters in vivo. J. Biol. Chem. 2004, 279, 37360–37367. [Google Scholar] [CrossRef] [PubMed]
- Llorente-Cortés, V.; Costales, P.; Bernués, J.; Camino-Lopez, S.; Badimon, L. Sterol regulatory element-binding protein-2 negatively regulates low density lipoprotein receptor-related protein transcription. J. Mol. Biol. 2006, 359, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Robichon, C.; Varret, M.; Le, L.X.; Lasnier, F.; Hajduch, E.; Ferré, P.; Dugail, I. DnaJA4 is a SREBP-regulated chaperone involved in the cholesterol biosynthesis pathway. Biochim. Biophys. Acta 2006, 1761, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, R.; Thelen, K.M.; Paiva, H.; Matinheikki, J.; Vesalainen, R.; Janatuinen, T.; Knuuti, J.; Rontu, R.; von Bergmann, K.; Lütjohann, D.; et al. Genetic variant ofthe SREBF1 gene is significantly related to cholesterol synthesis in man. Atherosclerosis 2006, 185, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Corl, B.A. Transcriptional regulation of lipid synthesis in bovine mammary epithelial cells by sterol regulatory element binding protein 1. J. Dairy Sci. 2012, 95, 3743–3755. [Google Scholar] [CrossRef] [PubMed]
- Bionaz, M.; Loor, J.J. Gene networks driving bovine milk fat synthesis during the lactation cycle. BMC Genomics 2008, 9, 366. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.Y.; Tong, H.L.; Li, Q.Z.; Gao, X.J. Influence on cellular signal transduction pathway in dairy cow mammary gland epithelial cells by galactopoietic compound isolated from Vaccariae segetalis. Agric. Sci. China 2011, 10, 101–105. [Google Scholar] [CrossRef]
- Huang, Y.L.; Zhao, F.; Luo, C.C.; Zhang, X.; Si, Y.; Sun, Z.; Zhang, L.; Li, Q.Z.; Gao, X.J. SOCS3-mediated blockade reveals major contribution of JAK2/STAT5 signaling pathway to lactation and proliferation of dairy cow mammary epithelial cells in vitro. Molecules 2013, 18, 12987–13000. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, Y.; Xu, D.; Zhang, R.; Cheng, Y.; Pan, Y.; Huang, C.; Chen, Y. Luteolin alleviates alcoholic liver disease induced by chronic and binge ethanol feeding in mice. J. Nutr. 2014. [Google Scholar] [CrossRef]
- Bakan, I.; Laplante, M. Connecting mTORC1 signaling to SREBP1 activation. Curr. Opin. Lipidol. 2012, 23, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Yecies, J.L.; Zhang, H.H.; Menon, S.; Liu, S.; Yecies, D.; Lipovsky, A.I.; Gorgun, C.; Kwiatkowski, D.J.; Hotamisligil, G.S.; Lee, C.H.; et al. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 2011, 14, 21–32. [Google Scholar]
- Li, S.; Ogawa, W.; Emi, A.; Hayashi, K.; Senga, Y.; Nomura, K.; Hara, K.; Yu, D.; Kasuga, M. Role of S6K1 in regulation of SREBP1c expression in the liver. Biochem. Biophys. Res. Commun. 2011, 412, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Brown, M.S.; Goldstein, J.L. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 3441–3446. [Google Scholar] [CrossRef] [PubMed]
- Dü vel, K.; Yecies, J.L.; Menon, S.; Raman, P.; Lipovsky, A.I.; Souza, A.L.; Triantafellow, E.; Ma, Q.; Gorski, R.; Cleaver, S.; et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell. 2010, 39, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Porstmann, T.; Santos, C.R.; Griffiths, B.; Cully, M.; Wu, M.; Leevers, S.; Griffiths, J.R.; Chung, Y.L.; Schulze, A. SREBP activity is regulatedby mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008, 8, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Moule, S.K.; Edgell, N.J.; Welsh, G.I.; Diggle, T.A.; Foulstone, E.J.; Heesom, K.J.; Proud, C.G.; Denton, R.M. Multiple signalling pathways involved in the stimulation of fatty acid and glycogen synthesis by insulin in rat epididymal fat cells. Biochem. J. 1995, 311, 595–601. [Google Scholar] [PubMed]
- Peterson, T.R.; Sengupta, S.S.; Harris, T.E.; Carmack, A.E.; Kang, S.A.; Balderas, E.; Guertin, D.A.; Madden, K.L.; Carpenter, A.E.; Finck, B.N.; et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell 2011, 146, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activatorsof the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Goldstein, J.L. Cholesterol feedback: From Schoenheimer’s bottle to Scap’s MELADL. J. Lipid Res. Suppl. 2009, 50, S15–S27. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.; et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011, 13, 376–388. [Google Scholar]
- McArthur, M.J.; Atshaves, B.P.; Frolov, A.; Foxworth, W.D.; Kier, A.B.; Schroeder, F. Cellular uptake and intracellular trafficking of long chain fatty acids. J. Lipid Res. 1999, 40, 1371–1383. [Google Scholar] [PubMed]
- Frolov, A.; Cho, T.H.; Murphy, E.J.; Schroeder, F. Isoforms of rat liver fatty acid binding protein differ in structure and affinity forfatty acids and fatty acyl CoAs. Biochemistry 1997, 36, 6545–6555. [Google Scholar] [CrossRef] [PubMed]
- Whetstone, H.D.; Hurley, W.L.; Davis, C.L. Identification and characterization of a fatty acid binding protein in bovine mammary gland. Comp. Biochem. Physiol. B 1986, 85, 687–692. [Google Scholar]
- Kawaiand, M.; Rosen, C.J. PPARγ: A circadian transcription factor in adipogenesis and osteogenesis. Nat. Rev. Endocrinol. 2010, 6, 629–636. [Google Scholar] [CrossRef] [PubMed]
- König, B.; Koch, A.; Spielmann, J.; Hilgenfeld, C.; Hirche, F.; Stangl, G.I.; Eder, K. Activation of PPARα and PPARγ reduces triacylglycerol synthesis in rat hepatoma cells by reduction of nuclear SREBP1. Eur. J. Pharmacol. 2009, 605, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Shin, H.T.; Seo, Y.H.; Byun, H.O.; Yoon, S.H.; Lee, I.K.; Hyun, D.H.; Chung, H.Y.; Yoon, G. Sterol regulatory element-binding protein (SREBP) 1 mediated lipogenesis is involved in cell senescence. J. Biol. Chem. 2010, 285, 29069–29077. [Google Scholar] [CrossRef] [PubMed]
- Kadegowda, A.K.; Bionaz, M.; Piperova, L.S.; Erdman, R.A.; Loor, J.J. Peroxisome proliferator-activated receptor-γ activation and long-chain fatty acids alter lipogenic gene networks in bovine mammary epithelial cells to various extents. J. Dairy Sci. 2009, 92, 4276–4289. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.-L.; Gao, X.-J.; Li, Q.-Z.; Liu, J.; Li, N.; Wan, Z.-Y. Metabolic regulation of mammary gland epithelial cells of dairy cow by galactopoietic compound isolated from Vaccariae segetalis. Agric. Sci. China 2011, 10, 1106–1116. [Google Scholar] [CrossRef]
- Lu, L.-M; Gao, X.-J; Li, Q.-Z; Huang, J.-G; Liu, R.; Li, H. Comparative phosphoproteomics analysis of the effects of l-methionine on dairy cow mammary epithelial cells. Can. J. Anim. Sci. 2012, 92, 433–442. [Google Scholar]
- Lu, L.M.; Li, Q.Z.; Huang, J.G.; Gao, X.J. Proteomic and functional analyses reveal MAPK1 regulates milk protein synthesis. Molecules 2013, 18, 263–275. [Google Scholar] [CrossRef]
- Lee, J.S.; Mendez, R.; Heng, H.H.; Yang, Z.Q.; Zhang, K. Pharmacological ER stress promotes hepatic lipogenesis and lipid droplet formation. Am. J. Transl. Res. 2012, 4, 102–113. [Google Scholar] [PubMed]
- Huang, J.G.; Gao, X.J.; Li, Q.Z.; Lu, L.M.; Liu, R.; Luo, C.C.; Wang, J.L.; Qiao, B.; Jin, X. Proteomic analysis of the nuclear phosphorylated proteins in dairy cowmammary epithelial cells treated with estrogen. Vitro Cell. Dev. Biol. Anim. 2012, 48, 449–457. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, N.; Zhao, F.; Wei, C.; Liang, M.; Zhang, N.; Wang, C.; Li, Q.-Z.; Gao, X.-J. Function of SREBP1 in the Milk Fat Synthesis of Dairy Cow Mammary Epithelial Cells. Int. J. Mol. Sci. 2014, 15, 16998-17013. https://doi.org/10.3390/ijms150916998
Li N, Zhao F, Wei C, Liang M, Zhang N, Wang C, Li Q-Z, Gao X-J. Function of SREBP1 in the Milk Fat Synthesis of Dairy Cow Mammary Epithelial Cells. International Journal of Molecular Sciences. 2014; 15(9):16998-17013. https://doi.org/10.3390/ijms150916998
Chicago/Turabian StyleLi, Nan, Feng Zhao, Chenjie Wei, Mengyao Liang, Na Zhang, Chunmei Wang, Qing-Zhang Li, and Xue-Jun Gao. 2014. "Function of SREBP1 in the Milk Fat Synthesis of Dairy Cow Mammary Epithelial Cells" International Journal of Molecular Sciences 15, no. 9: 16998-17013. https://doi.org/10.3390/ijms150916998



