Electricity Generation and Wastewater Treatment of Oil Refinery in Microbial Fuel Cells Using Pseudomonas putida
Abstract
:1. Introduction
2. Results and Discussion
2.1. Current Generation from Refinery Wastewater
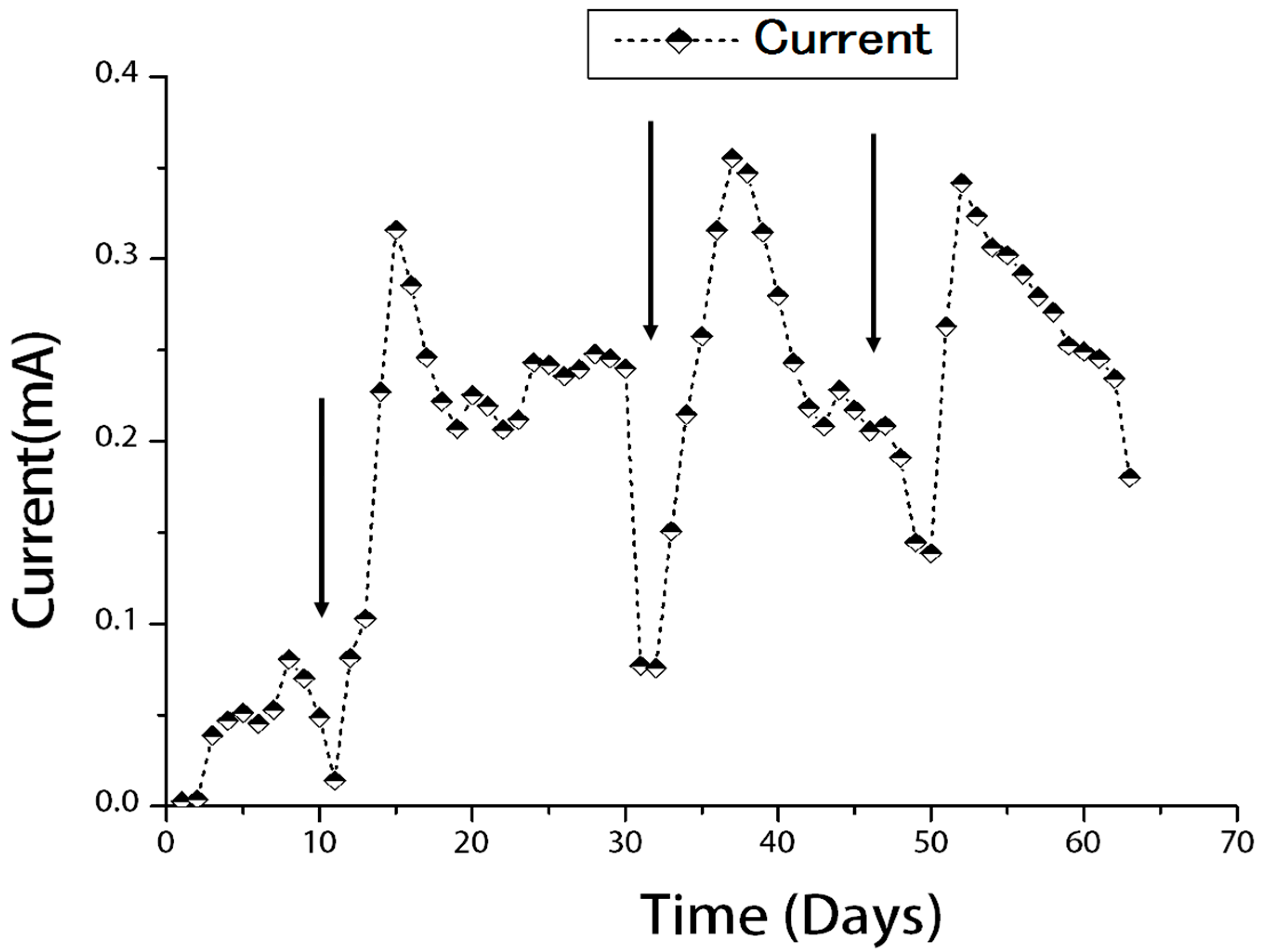
2.2. Characterization of Microbial Fuel Cells (MFC)
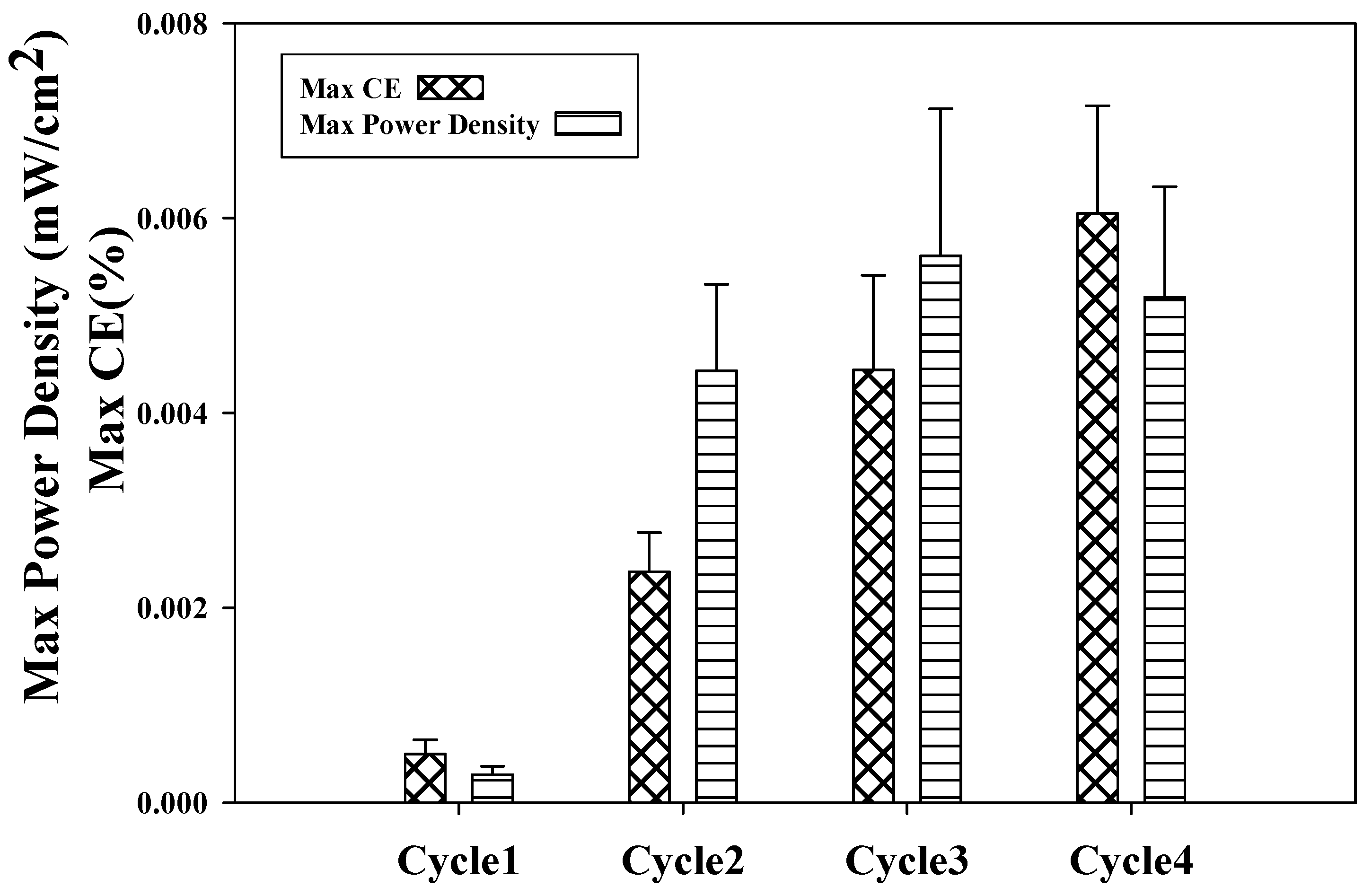
| Cycle Number | Open Circuit Potential (mV) | Maximum Current (mA) | Maximum Power Density (mW/cm2) |
|---|---|---|---|
| Cycle 1 | 312 | 0.079612121 | 0.000092 |
| Cycle 2 | 402 | 0.257575758 | 0.000973 |
| Cycle 3 | 409 | 0.284848485 | 0.00119 |
| Cycle 4 | 401 | 0.321212121 | 0.0015132 |
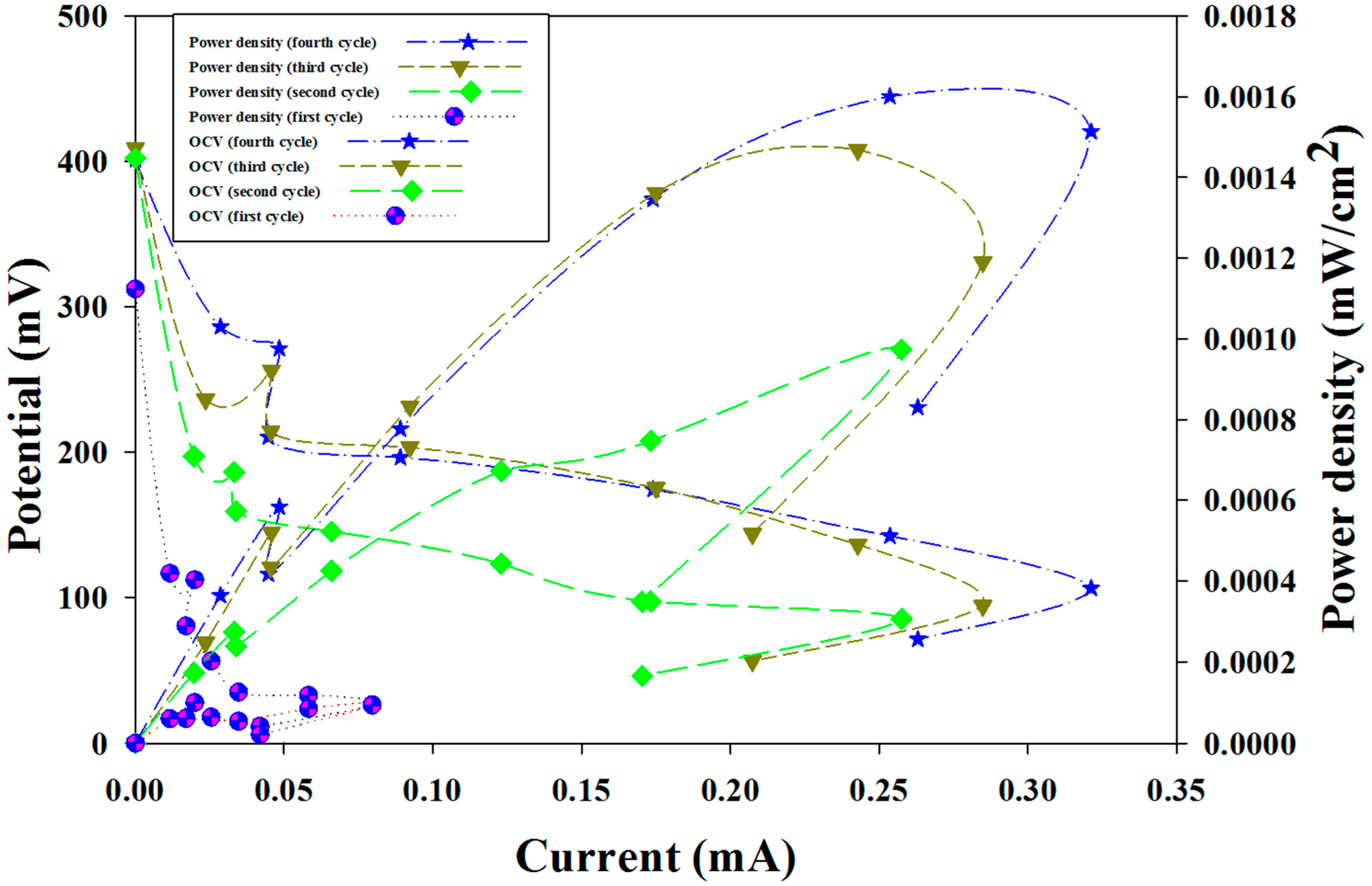
2.3. Cyclic Voltammetry
2.4. Coulombic Efficiency as a Function of Power Density Using Refinery Wastewater

2.5. Chemical Oxygen Demand (COD) Removal and Wastewater Treatment Efficiency


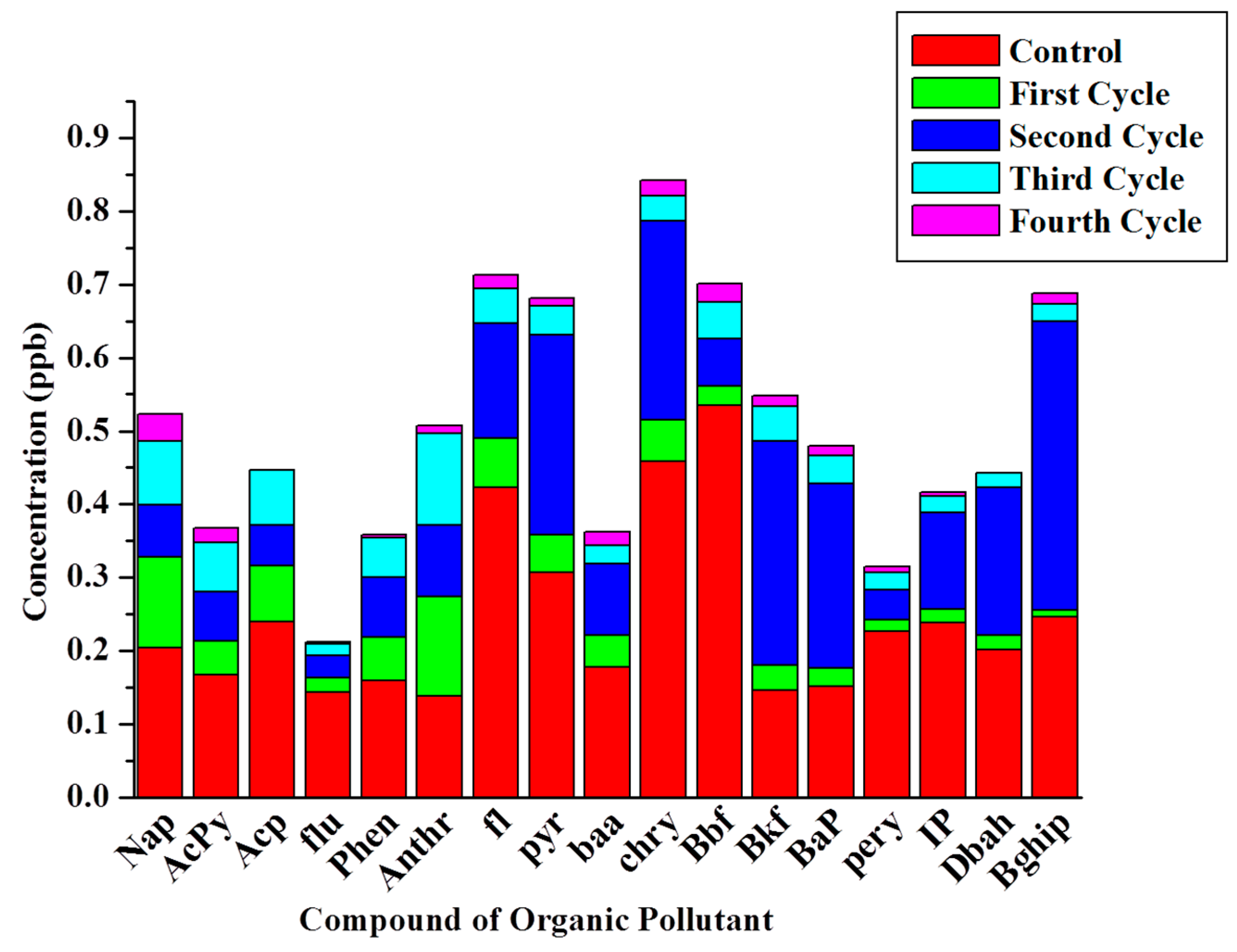
| Abbreviation | Full Name | Formula | Molecular Weight |
|---|---|---|---|
| Nap | Naphthalene | C10H8 | 128 |
| Acpy | Acenaphthylene | C12H8 | 152 |
| Acp | Acenaphthene | C12H10 | 154 |
| Flu | Fluorene | C13H10 | 166 |
| Phen | Phenanthrene | C14H10 | 178 |
| Anthr | Anthracene | C14H10 | 178 |
| Fl | Fluoranthene | C16H10 | 202 |
| Pyr | Pyrene | C16H12 | 202 |
| Baa | Benzo[a]anthracene | C18H12 | 228 |
| Chry | Chrysene | C18H12 | 228 |
| Bbf | Benzo[b]fluoranthene | C20H12 | 252 |
| Bkf | Benzo[k]fluoranthene | C20H12 | 252 |
| Bap | Benzo[a]pyrene | C20H12 | 252 |
| Pery | Perylene | C20H12 | 252 |
| IP | Ideno[1,2,3-c,d]pyrene | C22H12 | 276 |
| Dbah | Dibenzo[a,h]anthracene | C22H14 | 278 |
| Bghip | Benzo[g,h,i] perylene | C22H12 | 276 |
3. Experimental Section
3.1. Oil Refinery Wastewater
3.2. Microorganisms and Medium
3.3. MFC Configuration
3.4. Attainment of Data and Electrochemical Calculation
3.5. Cyclic Voltammetry
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wen, Q.; Wu, Y.; Cao, D.; Zhao, L.; Sun, Q. Electricity generation and modeling of microbial fuel cell from continuous beer brewery wastewater. Bioresour. Technol. 2009, 100, 4171–4175. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Logan, B.E. Effectiveness of domestic wastewater treatment using microbial fuel cells at ambient and mesophilic temperatures. Bioresour. Technol. 2010, 101, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Call, D.; Cheng, S.; Hamelers, H.V.; Sleutels, T.H.; Jeremiasse, A.W.; Rozendal, R.A. Microbial electrolysis cells for high yield hydrogen gas production from organic matter. Environ. Sci. Technol. 2008, 42, 8630–8640. [Google Scholar] [CrossRef] [PubMed]
- Kiely, P.D.; Cusick, R.; Call, D.F.; Selembo, P.A.; Regan, J.M.; Logan, B.E. Anode microbial communities produced by changing from microbial fuel cell to microbial electrolysis cell operation using two different wastewaters. Bioresour. Technol. 2011, 102, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.A.; Surakasi, V.P.; Koul, S.; Ijmulwar, S.; Vivek, A.; Shouche, Y.; Kapadnis, B. Electricity generation using chocolate industry wastewater and its treatment in activated sludge based microbial fuel cell and analysis of developed microbial community in the anode chamber. Bioresour. Technol. 2009, 100, 5132–5139. [Google Scholar] [CrossRef] [PubMed]
- Schröder, U. Anodic electron transfer mechanisms in microbial fuel cells and their energy efficiency. Phys. Chem. Chem. Phys. 2007, 9, 2619–2629. [Google Scholar] [CrossRef] [PubMed]
- Nimje, V.R.; Chen, C.-Y.; Chen, C.-C.; Jean, J.-S.; Reddy, A.S.; Fan, C.-W.; Pan, K.-Y.; Liu, H.-T.; Chen, J.-L. Stable and high energy generation by a strain of Bacillus subtilis in a microbial fuel cell. J. Power Sources 2009, 190, 258–263. [Google Scholar] [CrossRef]
- Nimje, V.R.; Chen, C.-Y.; Chen, C.-C.; Chen, H.-R.; Tseng, M.-J.; Jean, J.-S.; Chang, Y.-F. Glycerol degradation in single-chamber microbial fuel cells. Bioresour. Technol. 2011, 102, 2629–2634. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.C. Electrical effects accompanying the decomposition of organic compounds. Proceed. R. Soc. Lond. Ser. B 1911, 84, 260–276. [Google Scholar] [CrossRef]
- ElMekawya, A.; Srikanth, S.; Vanbroekhoven, K.; Wever, H.D.; Pant, D. Bioelectro-catalytic valorization of dark fermentation effluents by acetate oxidizing bacteria in bioelectrochemical system (BES). J. Power Sources 2014, 262, 183–191. [Google Scholar] [CrossRef]
- Pant, D.; Arslan, D.; van, B.G.; Gallego, Y.A.; de, W.H.; Diels, L.; Vanbroekhoven, K. Integrated conversion of food waste diluted with sewage into volatile fatty acids through fermentation and electricity through a fuel cell. Environ. Technol. 2013, 34, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ramnarayanan, R.; Logan, B.E. Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environ. Sci. Technol. 2004, 38, 2281–2285. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Zhao, Q.; Jiang, J.; Zhang, J. Treatment of domestic wastewater with simultaneous electricity generation in microbial fuel cell under continuous operation. Chem. Biochem. Eng. Q. 2006, 20, 407–412. [Google Scholar]
- Min, B.; Angelidaki, I. Innovative microbial fuel cell for electricity production from anaerobic reactors. J. Power Sources 2008, 180, 641–647. [Google Scholar] [CrossRef]
- Zhan, Y.-L.; Zhang, P.-P.; Yan, G.-X.; Guo, S.-H. Constructing and operating of mediator- and membrane-less microbial fuel cell. J. Chem. Eng. Chin. Univ. 2008, 22, 177–181. [Google Scholar] [CrossRef]
- Huang, L.; Logan, B.E. Electricity generation and treatment of paper recycling wastewater using a microbial fuel cell. Appl. Microbiol. Biotechnol. 2008, 80, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, X.; Logan, B.E.; Lee, H. Brewery wastewater treatment using air-cathode microbial fuel cells. Appl. Microbiol. Biotechnol. 2008, 78, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, Y.; Lee, H. Electricity production from beer brewery wastewater using single chamber microbial fuel cell. Water Sci. Technol. 2008, 57, 1117–1121. [Google Scholar] [PubMed]
- Vijayaraghavan, K.; Ahmad, D.; Lesa, R. Electrolytic treatment of beer brewery wastewater. Ind. Eng. Chem. Res. 2006, 45, 6854–6859. [Google Scholar] [CrossRef]
- Lu, N.; Zhou, S.-G.; Zhuang, L.; Zhang, J.-T.; Ni, J.-R. Electricity generation from starch processing wastewater using microbial fuel cell technology. Biochem. Eng. J. 2009, 43, 246–251. [Google Scholar] [CrossRef]
- Min, B.; Kim, J.; Oh, S.; Regan, J.M.; Logan, B.E. Electricity generation from swine wastewater using microbial fuel cells. Water Res. 2005, 39, 4961–4968. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Dec, J.; Bruns, M.A.; Logan, B.E. Removal of odors from swine wastewater by using microbial fuel cells. Appl. Environ. Microbiol. 2008, 74, 2540–2543. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Kong, F.; Zheng, H.; Cao, D.; Ren, Y.; Yin, J. Electricity generation from synthetic penicillin wastewater in an air-cathode single chamber microbial fuel cell. Chem. Eng. J. 2011, 168, 572–576. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Lei, L. Electricity production during the treatment of real electroplating wastewater containing Cr6+ using microbial fuel cell. Process. Biochem. 2008, 43, 1352–1358. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Mohanakrishna, G.; Reddy, B.P.; Saravanan, R.; Sarma, P. Bioelectricity generation from chemical wastewater treatment in mediatorless (anode) microbial fuel cell (mfc) using selectively enriched hydrogen producing mixed culture under acidophilic microenvironment. Biochem. Eng. J. 2008, 39, 121–130. [Google Scholar] [CrossRef]
- Fan, Y.; Hu, H.; Liu, H. Enhanced coulombic efficiency and power density of air-cathode microbial fuel cells with an improved cell configuration. J. Power Sources 2007, 171, 348–354. [Google Scholar] [CrossRef]
- Sevda, S.; Dominguez-Benetton, X.; Vanbroekhoven, K.; Sreekrishnan, T.R.; Pant, D. Characterization and comparison of the performance of two different separator types in air–cathode microbial fuel cell treating synthetic wastewater. Chem. Eng. J. 2013, 228, 1–11. [Google Scholar] [CrossRef]
- Kim, G.J.; Lee, I.Y.; Yoon, S.C.; Shin, Y.C.; Park, Y.H. Enhanced yield and a high production of medium-chain-length poly (3-hydroxyalkanoates) in a two-step fed-batch cultivation of Pseudomonas putida by combined use of glucose and octanoate. Enzym. Microb. Technol. 1997, 20, 500–505. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, H.; Logan, B.E. Increased performance of single-chamber microbial fuel cells using an improved cathode structure. Electrochem. Commun. 2006, 8, 489–494. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Lin, J.; Han, S.; Lei, L. Azo dye treatment with simultaneous electricity production in an anaerobic–aerobic sequential reactor and microbial fuel cell coupled system. Bioresour. Technol. 2010, 101, 4440–4445. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Majumder, D.; Maity, J.P.; Tseng, M.-J.; Nimje, V.R.; Chen, H.-R.; Chen, C.-C.; Chang, Y.-F.; Yang, T.-C.; Chen, C.-Y. Electricity Generation and Wastewater Treatment of Oil Refinery in Microbial Fuel Cells Using Pseudomonas putida. Int. J. Mol. Sci. 2014, 15, 16772-16786. https://doi.org/10.3390/ijms150916772
Majumder D, Maity JP, Tseng M-J, Nimje VR, Chen H-R, Chen C-C, Chang Y-F, Yang T-C, Chen C-Y. Electricity Generation and Wastewater Treatment of Oil Refinery in Microbial Fuel Cells Using Pseudomonas putida. International Journal of Molecular Sciences. 2014; 15(9):16772-16786. https://doi.org/10.3390/ijms150916772
Chicago/Turabian StyleMajumder, Dip, Jyoti Prakash Maity, Min-Jen Tseng, Vanita Roshan Nimje, Hau-Ren Chen, Chien-Cheng Chen, Young-Fo Chang, Tsui-Chu Yang, and Chen-Yen Chen. 2014. "Electricity Generation and Wastewater Treatment of Oil Refinery in Microbial Fuel Cells Using Pseudomonas putida" International Journal of Molecular Sciences 15, no. 9: 16772-16786. https://doi.org/10.3390/ijms150916772





