Small Molecule Metabolite Biomarker Candidates in Urine from Mice Exposed to Formaldehyde
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of FA on Immune Organs and Function
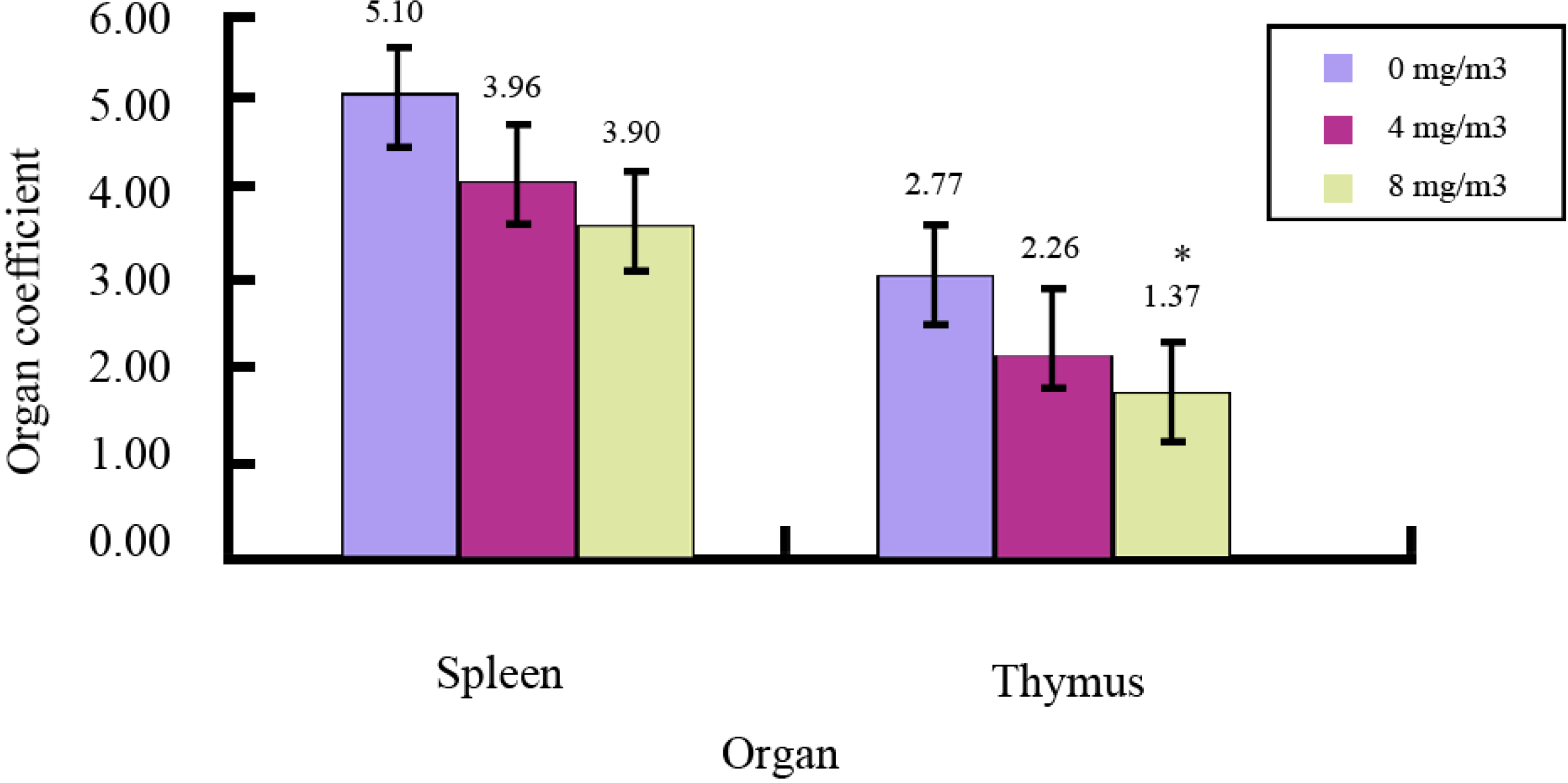
| Dose (mg/m3) | Absorbance (A) | Proliferation Rate |
|---|---|---|
| 0 | 0.485 ± 0.022 | 1 |
| 4 | 0.235 ± 0.038 * | 0.485 |
| 8 | 0.208 ± 0.032 * | 0.429 |
| Dose (mg/m3) | ROS Level (MFI) | SOD (U/mg prot) |
|---|---|---|
| 0 | 2718.60 ± 355.47 | 10.80 ± 3.22 |
| 4 | 2988.67 ± 394.04 | 5.33 ± 1.06 * |
| 8 | 3683.50 ± 332.06 * | 2.26 ± 0.61 ** |
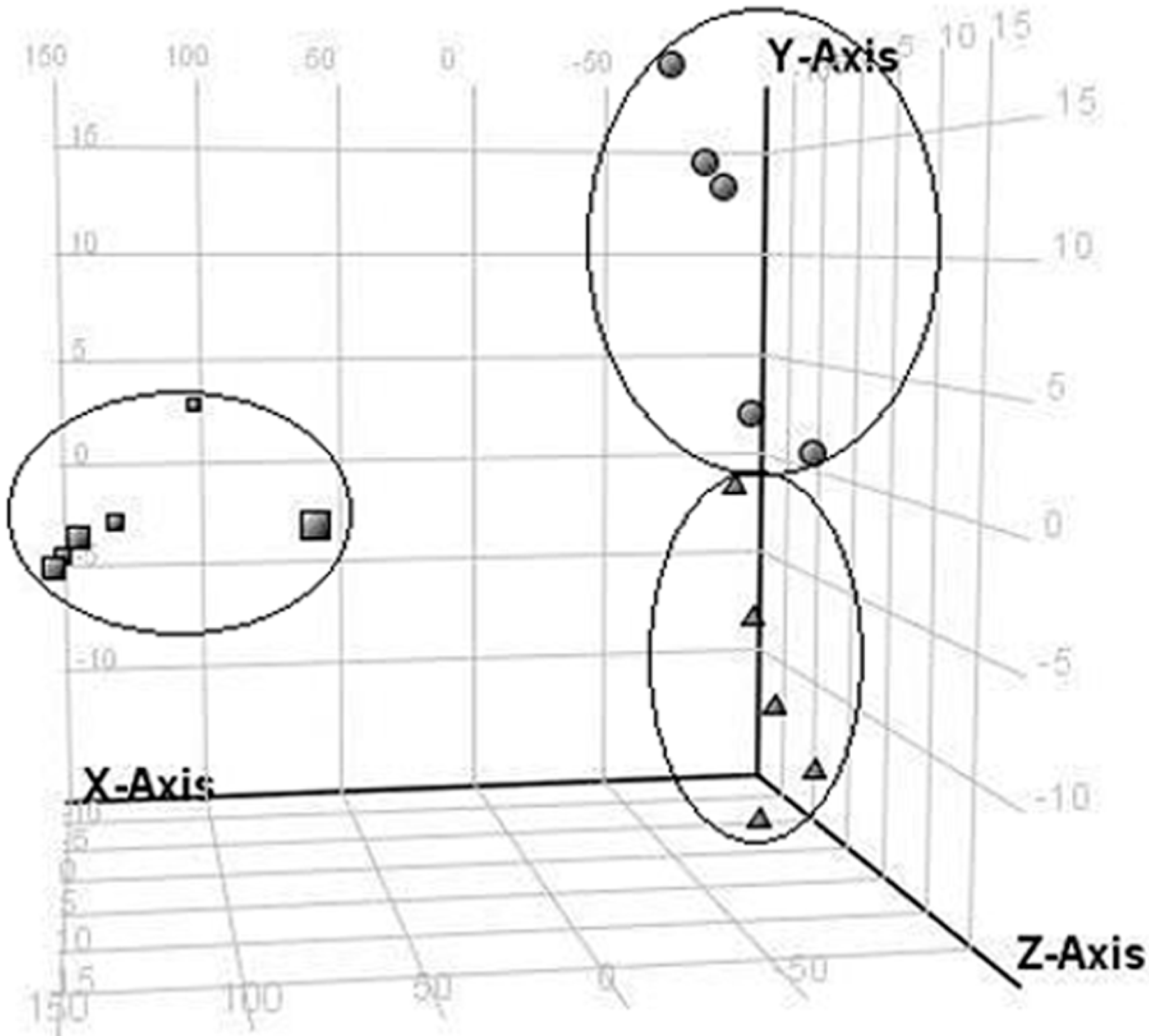
2.2. LC/MS Fingerprinting of Urine from Mice Exposed to FA, and Principal Component Analysis
2.3. Discrimination of Changed Endogenous Metabolites
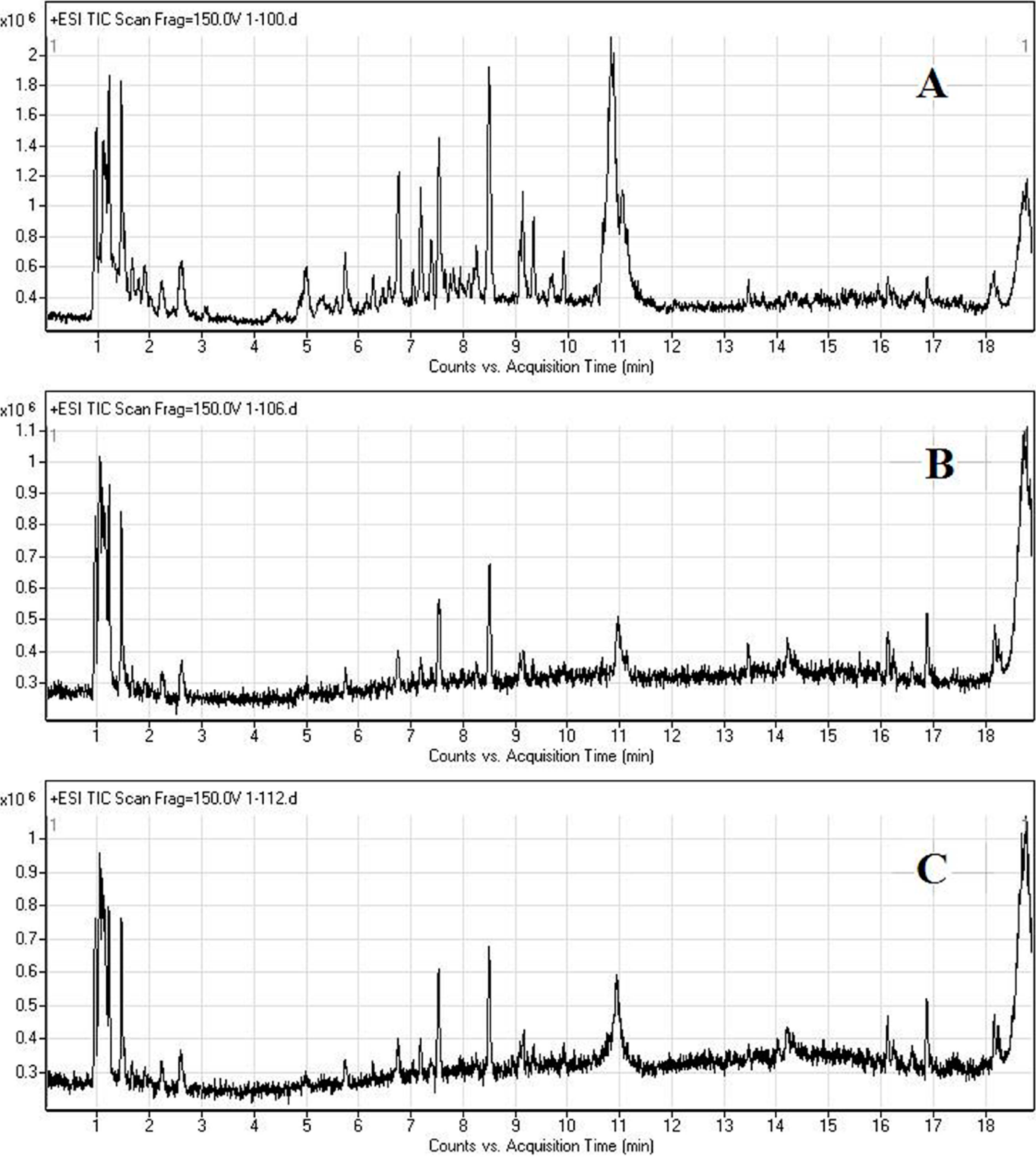
| No. | M + H | Retention Time (min) | Changes in FA Groups | MS/MS Fragmentation (20V) | Metabolites |
|---|---|---|---|---|---|
| 1 | 180.0655 | 6.7560 | down | (10V) 77.0389, 105.0336, 106.0372 | Hippuric acid |
| 2 | 206.0845 | 1.6843 | down | 103.0539, 104.0572, 131.0485, 132.0515 | Cinnamoylglycine |
| 3 | 313.1387 | 1.4592 | down | 85.0284, 120.0439, 189.9947, 190.9969, 191.9917 | unknown |
| 4 | 396.1673 | 8.5375 | down | 97.1007, 115.0574, 125.0948, 203.1085, 397.1463 | unknown |
| 5 | 467.1609 | 7.0492 | down | 261.1174, 305.1073, 306.1103, 467.1604, 468.162 | unknown |
| 6 | 202.1184 | 1.2264 | down | 60.0812, 70.0658, 71.0599, 85.0289, 100.0757 | unknown |
| 7 | 461.2593 | 7.1166 | down | 86.0953, 102.0535, 183.1111, 217.0805, 243.1319 | unknown |
| 8 | 473.2956 | 8.5641 | down | 112.0533, 201.0343, 203.0139, 215.998, 257.1149 | unknown |
| 9 | 105.0369 | 6.7562 | down | (low abundance) | unknown |
| 10 | 326.1070 | 1.7726 | down | 149.0443, 166.0714, 194.0662, 195.068 | unknown |
| 11 | 204.1244 | 1.2209 | down | 57.036, 58.0683, 60.0835, 71.0605, 85.0308 | unknown |
| 12 | 568.27 | 1.4622 | down | (low abundance) | unknown |
3. Experimental Section
3.1. Animals and FA Exposure
3.2. FA Induced Immune Toxicity to Spleen Cells
3.3. Sample Preparation and HPLC/MS Analysis
3.4. Processing of Metabolite Data
3.5. Statistics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Reuss, G.; Disteldorf, W.; Gamer, A.O; Hilt, A. Formaldehyde. In Ullmann’s Encyclopedia of Industrial Chemistry, 6th ed.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2003; Volume 115, pp. 1–34. [Google Scholar]
- Bono, R.; Vincenti, M.; Schiliro, T.; Scursatone, E.; Pignata, C.; Gilli, G. N-Methylenvaline in a group of subjects occupationally exposed to formaldehyde. Toxicol. Lett. 2006, 161, 10–17. [Google Scholar]
- Zhong, W.; Que Hee, S.S. Formaldehyde-induced DNA adducts as biomarkers of in vitro human nasal epithelial cell exposure to formaldehyde. Mutat. Res. 2004, 563, 13–24. [Google Scholar] [CrossRef]
- Hodgson, A.T.; Beal, D.; McIlvane, J.E.R. Sources of formaldehyde, other aldehydes and terpenes in a new manufactured house. Indoor Air 2002, 12, 235–242. [Google Scholar]
- Salthammer, T. Formaldehyde in the ambient atmosphere: From an indoor pollutant to an outdoor pollutant? Angew. Chem. Int. Ed. Engl. 2013, 52, 3320–3327. [Google Scholar]
- Straif, K. The IARC monographs, Vol: 100: A review and update on occupational carcinogens. In Proceedings of the International Agency for Research on Cancer, Lyon, France, 23 October 2010.
- Poirier, M.; Fournier, M.; Brousseau, P.; Morin, A. Effects of volatilearomatics, formaldehydes, and phenols in tobacco smoke on viability and proliferation of mouse lymphocytes. J. Toxicol. Environ. Health A 2002, 65, 1437–1451. [Google Scholar]
- Baj, Z.; Majewska, E.; Zeman, K.; Pokoca, L.; Dworniak, D.; Paradowski, M.; Tchórzewski, H. The effect of chronic exposure to formaldehyde, phenol and organic chlorohydro carbons on peripheral blood cells and the immune system in humans. J. Investig. Allergol. Clin. Immunol. 1994, 4, 186–1891. [Google Scholar]
- Overton, J.H.; Kimbell, J.S.; Miller, F.J. Dosimetry modeling of inhaled formaldehyde: The human respiratory tract. Toxicol. Sci. 2001, 64, 122–134. [Google Scholar]
- Heck, H.d’A.; White, E.L.; Casanova-Schmitz, M. Determination of formaldehyde in biological tissues by gas chromatography/mass spectrometry. Biomed. Mass Spectrom. 1982, 9, 347–353. [Google Scholar]
- Heck, H.d’A.; Chin, T.Y.; Schmitz, M.C. Distribution of (14C) formaldehyde in rats after inhalation exposure. In Formaldehyde Toxicity; Gibson, J.E., Ed.; Hemisphere: Washington, DC, USA, 1983; pp. 26–37. [Google Scholar]
- Kleinnijenhuis, A.J.; Staal, Y.C.; Duistermaat, E.; Engel, R.; Woutersen, R.A. The determination of exogenous formaldehyde in blood of rats during and after inhalation exposure. Food Chem. Toxicol. 2013, 52, 105–112. [Google Scholar]
- Costa, S.; García-Lestón, J.; Coelho, M.; Coelho, P.; Costa, C.; Silva, S.; Porto, B.; Laffon, B.; Teixeira, J.P. Cytogenetic and immunological effects associated with occupational formaldehyde exposure. J. Toxicol. Environ. Health A 2013, 76, 217–229. [Google Scholar]
- Schlosser, P.M.; Lilly, P.D.; Conolly, R.B.; Janszen, D.B.; Kimbell, J.S. Benchmark dose risk assessment for formaldehyde using airflow modeling and a single-compartment, DNA–protein cross-link dosimetry model to estimate human equivalent doses. Risk Anal. 2003, 23, 473–487. [Google Scholar]
- Zhang, L.; Tang, X.; Rothman, N.; Vermeulen, R.; Ji, Z.; Shen, M.; Qiu, C.; Guo, W.; Liu, S.; Reiss, B.; et al. Occupational exposure to formaldehyde, hematotoxicity, and leukemia-specific chromosome changes in cultured myeloid progenitor cells. Cancer Epidemiol. Biomark. Prev. 2010, 19, 80–88. [Google Scholar]
- Lu, K.; Collins, L.B.; Ru, H.; Bermudez, E.; Swenberg, J.A. Distribution of DNA adducts caused by inhaled formaldehyde, is consistent with induction of nasal carcinoma but not leukemia. Toxicol. Sci. 2010, 16, 441–451. [Google Scholar]
- Bouhifd, M.; Hartung, T.; Hogberg, H.T.; Kleensang, A.; Zhao, L. Review: Toxico metabolomics. J. Appl. Toxicol. 2013, 33, 1365–1383. [Google Scholar]
- Nicholson, J.K.; Lindon, J.C. Systems biology: Metabonomics. Nature 2008, 455, 1054–1056. [Google Scholar]
- Robertson, D.G.; Watkins, P.B.; Reily, M.D. Metabolomics in toxicology: Preclinical and clinical applications. Toxicol. Sci. 2011, 120 (Suppl. 1), S146–S170. [Google Scholar]
- Roux, A.; Lison, D.; Junot, C.; Heilier, J.F. Applications of liquid chromatography coupled to mass spectrometry-based metabolomics in clinical chemistry and toxicology: A review. Clin. Biochem. 2011, 44, 119–135. [Google Scholar] [CrossRef]
- Bujak, R.; Daghir, E.; Rybka, J.; Koslinski, P.; Markuszewski, M.J. Metabolomics in urogenital cancer. Bioanalysis 2011, 3, 913–923. [Google Scholar]
- Hosgood, H.D.; Zhang, L.; Tang, X.; Vermeulen, R.; Hao, Z.; Shen, M.; Qiu, C.; Ge, Y.; Hua, M.; Ji, Z.; et al. Occupational exposure to formaldehyde and alterations in lymphocyte subsets. Am. J. Ind. Med. 2013, 56, 252–257. [Google Scholar]
- Ye, X.; Ji, Z.; Wei, C.; McHale, C.M.; Ding, S.; Thomas, R.; Yang, X.; Zhang, L. Inhaled formaldehyde induces DNA-protein crosslinks and oxidative stress in bone marrow and other distant organs of exposed mice. Environ. Mol. Mutagen. 2013, 54, 705–718. [Google Scholar]
- Zhang, Y.; Liu, X.; McHale, C.; Li, R.; Zhang, L.; Wu, Y.; Ye, X.; Yang, X.; Ding, S. Bone marrow injury induced via oxidative stress in mice by inhalation exposure to formaldehyde. PLoS One 2013, 8, e74974. [Google Scholar] [CrossRef]
- Gonzalez, K.C.; Sagebin, F.R.; Oliveira, P.G.; Glock, L.; Thiesen, F.V. A retrospective study analysis of urinary hippuric acidlevels in occupational toxicology exams. Cienc. Saude Colet. 2010, 15 (Suppl. 1), 1637–1641. [Google Scholar]
- Yang, Y.D. Simultaneous determination of creatine, uricacid, creatinine and hippuric acid in urine by high performance liquid chromatography. Biomed. Chroma-togr. 1998, 12, 47–49. [Google Scholar]
- Liebich, H.M.; Bubeck, J.I.; Pickert, A.; Wahl, G.; Scheiter, A.J. Hippuric acid and 3-carboxy-4-methyl-5-propyl-2-furanpropionic acid in serum and urine. Analytical approaches and clinical relevance in kidney diseases. Chromatograph 1990, 500, 615–627. [Google Scholar]
- Zhao, Y.Y.; Lei, P.; Chen, D.Q.; Feng, Y.L.; Bai, X. Renal metabolic profiling of early renal injury and renoprotective effects of Poria cocos epidermis using UPLC Q-TOF/HSMS/MSE. J. Pharm. Biomed. Anal. 2013, 81–82, 202–209. [Google Scholar]
- Zhen, Y.; Krausz, K.W.; Chen, C.; Idle, J.R.; Gonzalez, F.J. Metabolomic and genetic analysis of biomarkers for peroxisome proliferator-activated receptor alpha expression and activation. Mol. Endocrinol. 2007, 21, 2136–2151. [Google Scholar]
- Ganti, S.; Taylor, S.L.; Abu Aboud, O.; Yang, J.; Evans, C.; Osier, M.V.; Alexander, D.C.; Kim, K.; Weiss, R.H. Kidney tumor biomarkers revealed by simultaneous multiple matrix metabolomics analysis. Cancer Res. 2012, 72, 3471–3479. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, J.; Sun, R.; Chen, Y.; Tan, K.; Wei, H.; Yin, L.; Pu, Y. Small Molecule Metabolite Biomarker Candidates in Urine from Mice Exposed to Formaldehyde. Int. J. Mol. Sci. 2014, 15, 16458-16468. https://doi.org/10.3390/ijms150916458
Zhang J, Sun R, Chen Y, Tan K, Wei H, Yin L, Pu Y. Small Molecule Metabolite Biomarker Candidates in Urine from Mice Exposed to Formaldehyde. International Journal of Molecular Sciences. 2014; 15(9):16458-16468. https://doi.org/10.3390/ijms150916458
Chicago/Turabian StyleZhang, Juan, Rongli Sun, Yue Chen, Kehong Tan, Haiyan Wei, Lihong Yin, and Yuepu Pu. 2014. "Small Molecule Metabolite Biomarker Candidates in Urine from Mice Exposed to Formaldehyde" International Journal of Molecular Sciences 15, no. 9: 16458-16468. https://doi.org/10.3390/ijms150916458





