Expression of OCT4A: The First Step to the Next Stage of Urothelial Bladder Cancer Progression
Abstract
:1. Introduction
2. Results
2.1. OCT4A and Staging
| Urothelial Tissue | Percentage of Cases | |||
|---|---|---|---|---|
| Low | Moderate | All | ||
| % | p | |||
| Non-neoplastic (normal urothelium) | 25 | 0 | 25 | >0.05 |
| Primary tumor | 81.7 | 18.1 | 84.3 | >0.05 |
| Primary metastasizing | 82.6 | 17.4 | 91.3 | >0.05 |
| Primary non-metastasizing | 80 | 20 | 81.7 | >0.05 |
| Lymph node metastasis | 87 | 10 | 87 | >0.05 |
| Feature | Zero Per cent of OCT4A-Positive Cells (n = 16) | Eighty Per cent of OCT4A-Positive Cells (n = 3) |
|---|---|---|
| pT | a (n = 6), is (n = 1), 1 (n = 1), 2 (n = 2), 3 (n = 6) | is, 3, 4 |
| pN | 1 (n = 1), 2 (n = 1) | 1 |
| G | High (n = 10), low (n = 6) | High |
| NDN | 0 (n = 10), 1 (n = 3), 2 (n = 2), 3 (n = 1) | 2 |
| TIT | Focal (n = 7), styloid (n = 1), dispersive (n = 1) | Focal, dispersive |
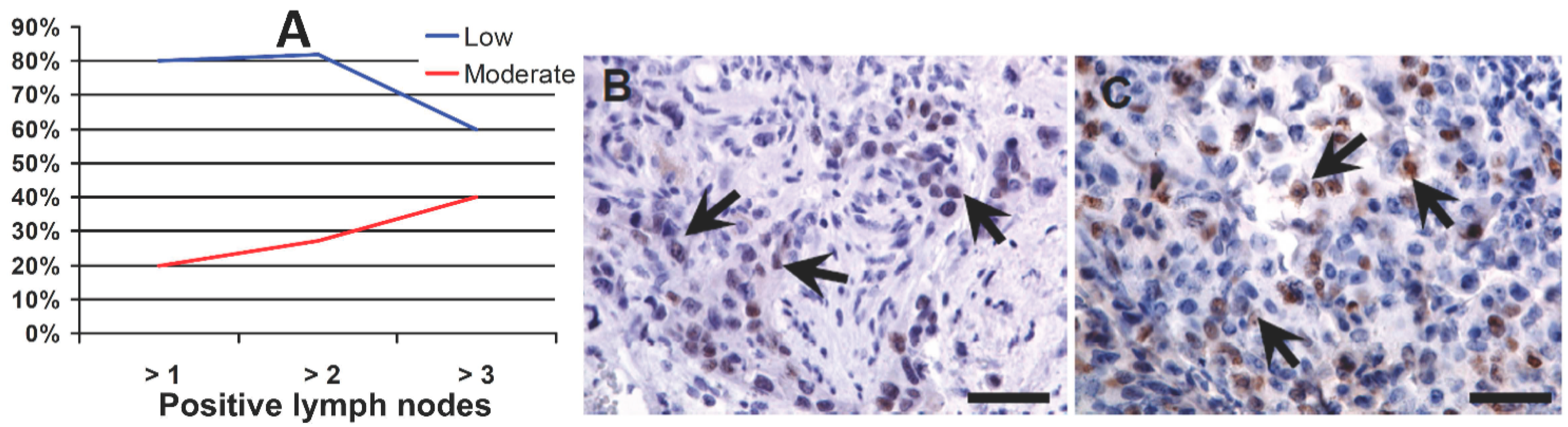
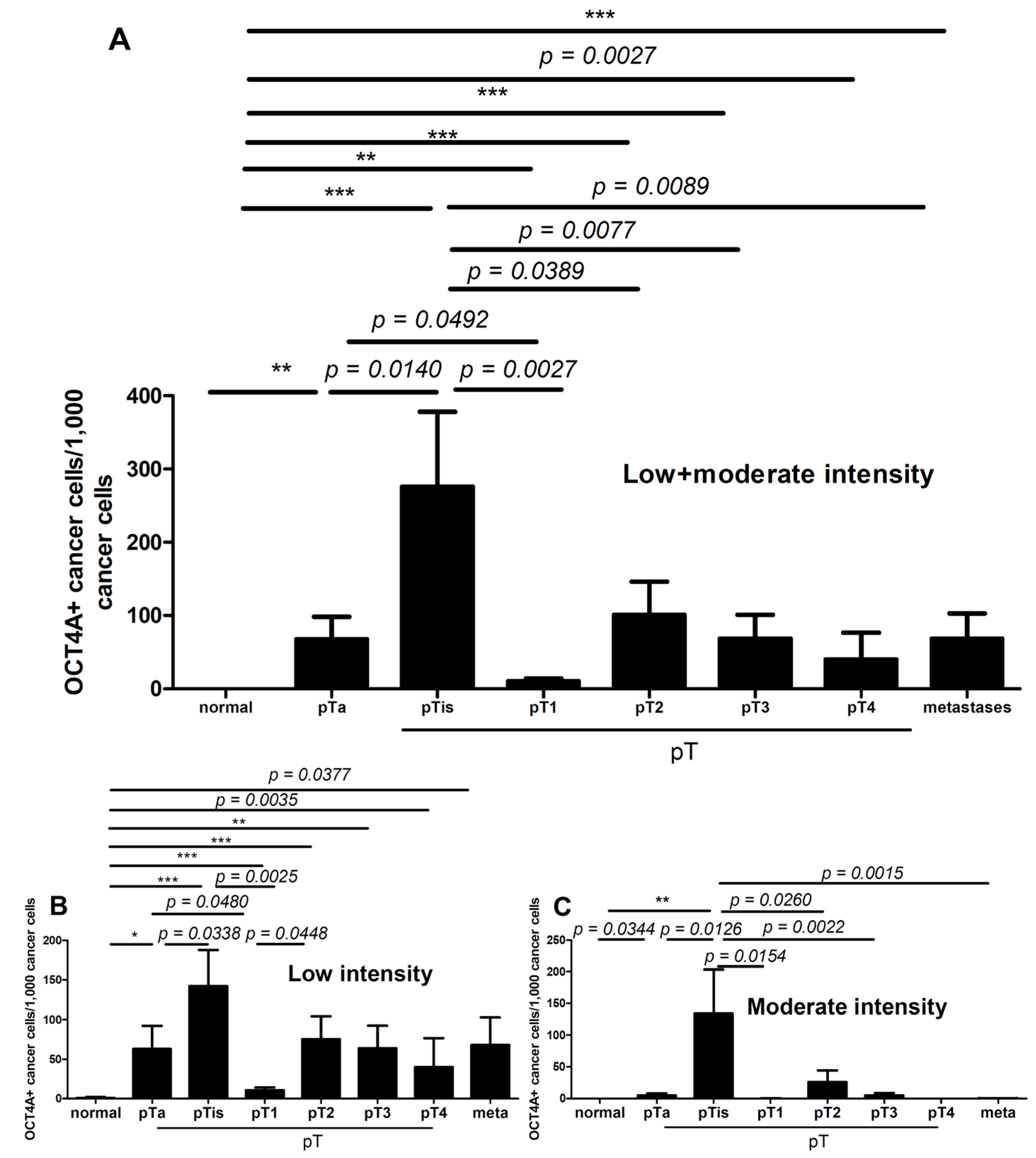

2.2. OCT4A and Grading
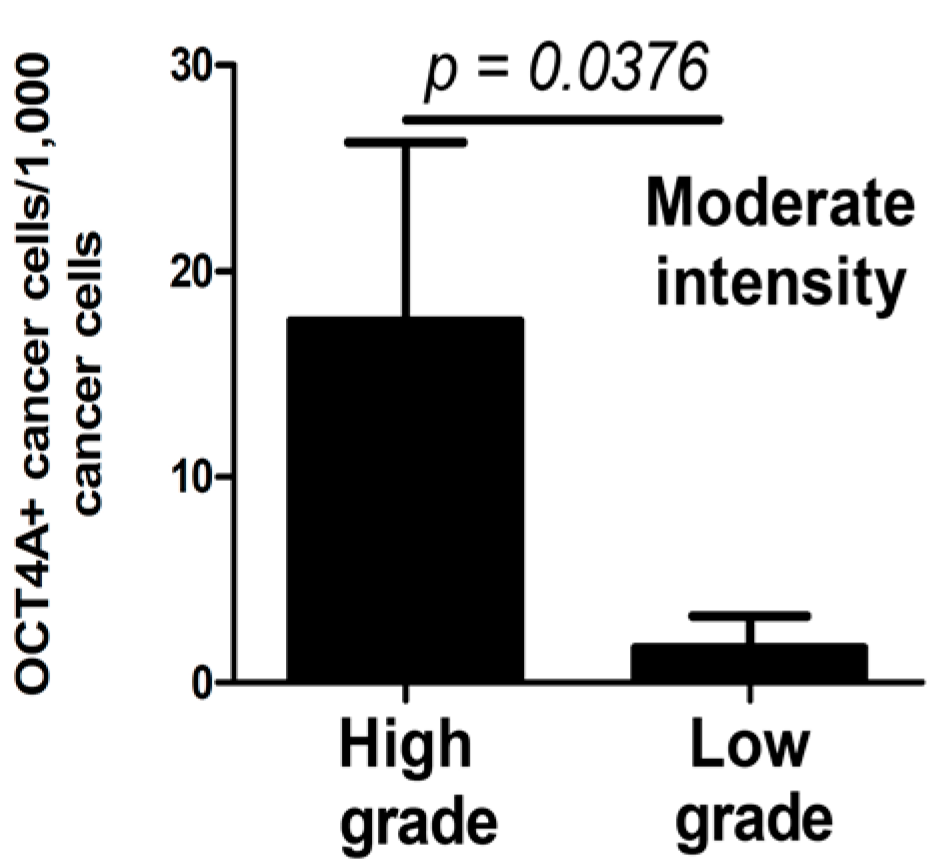
2.3. OCT4A and Tissue Invasion Type
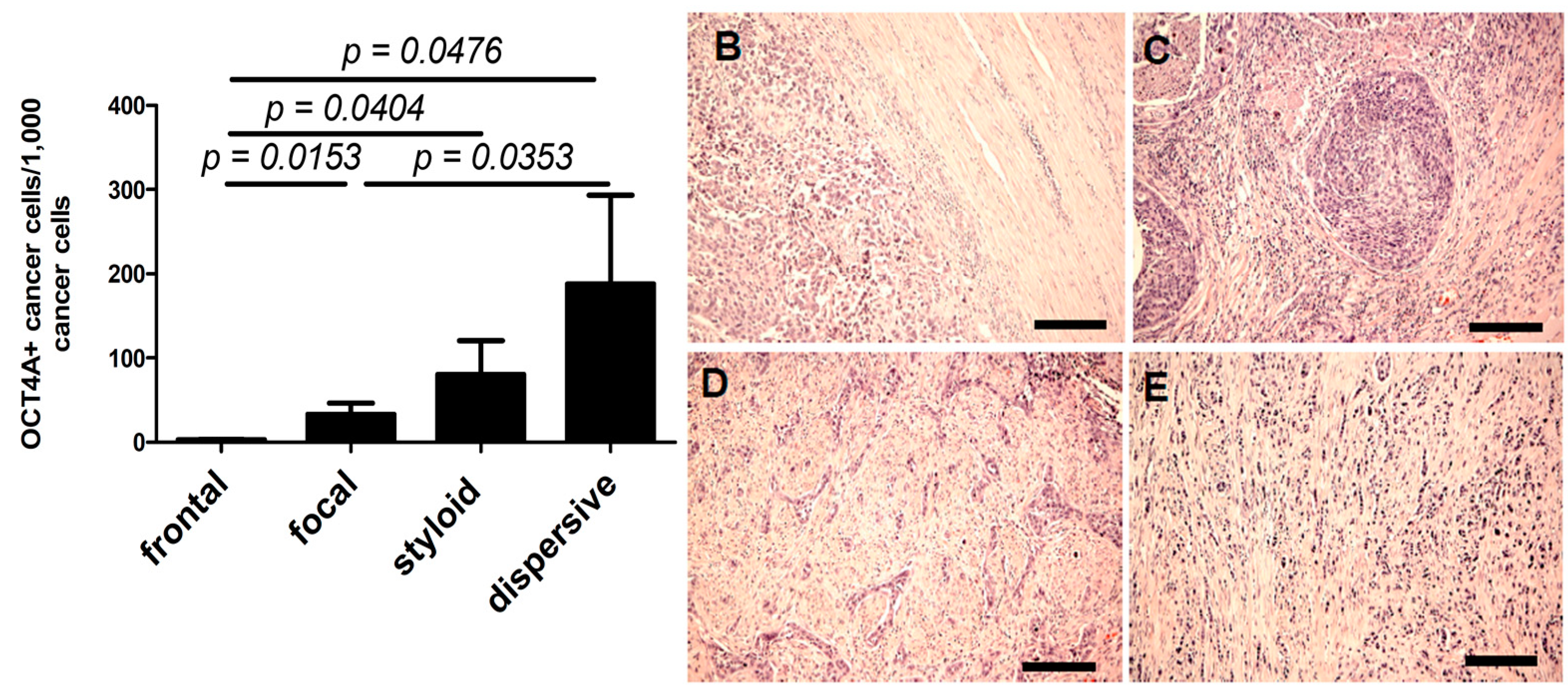
2.4. OCT4A and NDN
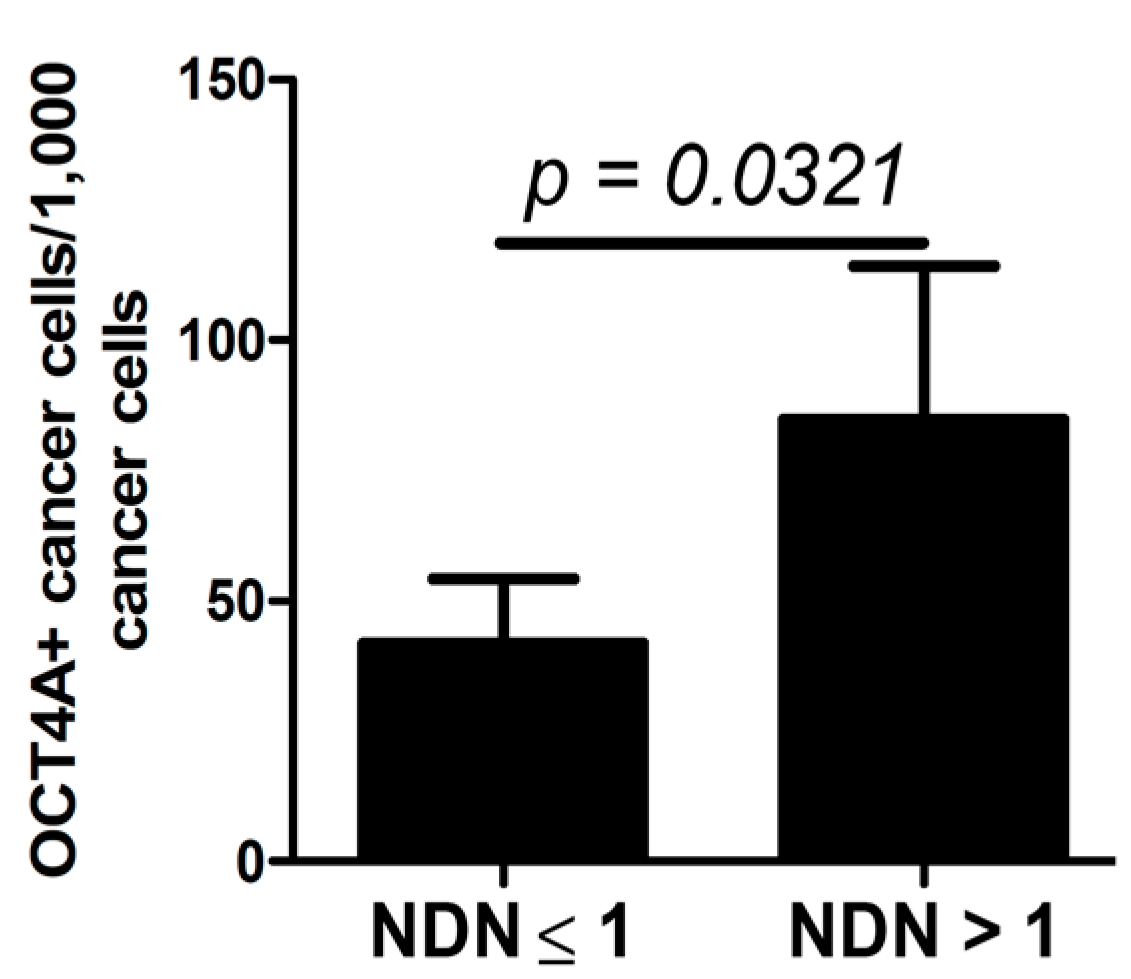
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Immunohistochemical Staining of Sections
4.3. Evaluation of Immunohistochemically Stained Sections
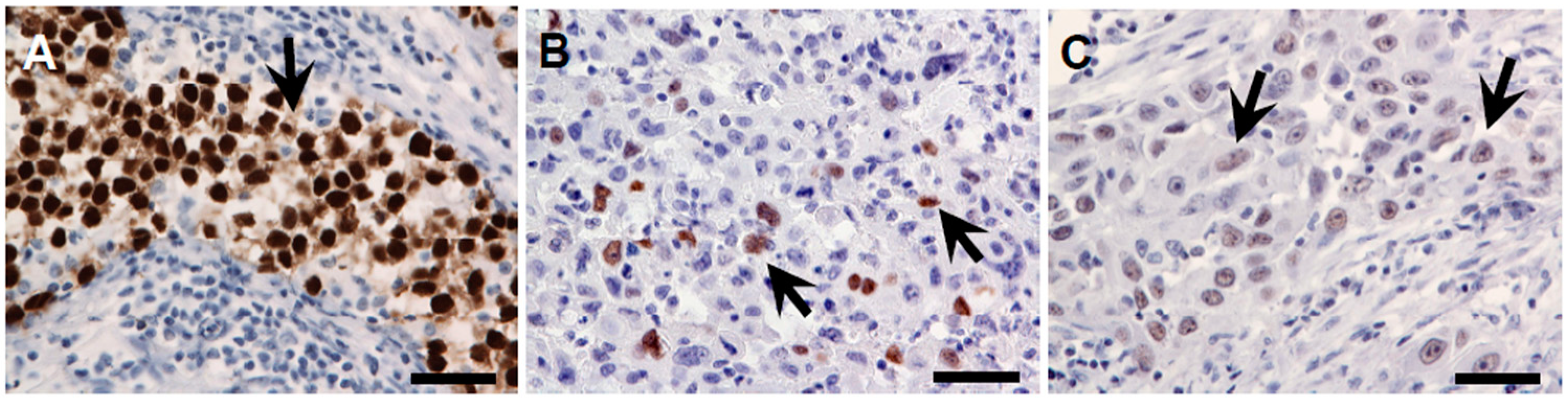
4.4. Tissue Invasion Type
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lopez-Beltran, A.; Sauter, G.; Gasser, T.; Hartmann, A.; Schmitz-Dräger, B.J.; Helpap, B.; Ayala, A.G.; Tamboli, P.; Knowles, M.A.; Sidransky, D.; et al. Tumours of the urinary system. Infiltrating urothelial carcinoma. In World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs; Eble, J.N., Sauter, G., Epstein, J.I., Sesterhenn, I.A., Eds.; IARC Press: Lyon, France, 2004; pp. 93–109. [Google Scholar]
- Jozwicki, W.; Domaniewski, J.; Skok, Z.; Wolski, Z.; Domanowska, E.; Jozwicka, G. Usefulness of histologic homogeneity estimation of muscle-invasive urinary bladder cancer in an individual prognosis: A mapping study. Urology 2005, 66, 1122–1126. [Google Scholar] [CrossRef]
- Jozwicki, W.; Skok, Z.; Brożyna, A.; Siekiera, J.; Wolski, Z.; Domaniewski, J. Prognostic and diagnostic implications of histological differentiation in invasive urothelial cell carcinoma of the bladder: Variant or non-classic differentiation number. Central Eur. J. Urol. 2010, 63, 112–116. [Google Scholar] [CrossRef]
- Behnsawy, H.M.; Miyake, H.; Abdalla, M.A.; Sayed, M.A.; Ahmed Ael, F.; Fujisawa, M. Expression of cell cycle-associated proteins in non-muscle-invasive bladder cancer: Correlation with intravesical recurrence following transurethral resection. Urol. Oncol. 2011, 29, 495–501. [Google Scholar] [CrossRef]
- Shariat, S.F.; Palapattu, G.S.; Karakiewicz, P.I.; Rogers, C.G.; Vazina, A.; Bastian, P.J.; Schoenberg, M.P.; Lerner, S.P.; Sagalowsky, A.I.; Lotan, Y. Concomitant carcinoma in situ is a feature of aggressive disease in patients with organ-confined TCC at radical cystectomy. Eur. Urol. 2007, 51, 152–160. [Google Scholar] [CrossRef]
- Shariat, S.F.; Passoni, N.; Bagrodia, A.; Rachakonda, V.; Xylinas, E.; Robinson, B.; Kapur, P.; Sagalowsky, A.I.; Lotan, Y. Prospective evaluation of a preoperative biomarker panel for prediction of upstaging at radical cystectomy. BJU Int. 2014, 113, 70–76. [Google Scholar] [CrossRef]
- Xylinas, E.; Kluth, L.A.; Lotan, Y.; Daneshmand, S.; Rieken, M.; Karakiewicz, P.I.; Shariat, S.F. Blood- and tissue-based biomarkers for prediction of outcomes in urothelial carcinoma of the bladder. Urol. Oncol. 2014, 32, 230–242. [Google Scholar] [CrossRef]
- Shin, K.; Lim, A.; Odegaard, J.I.; Honeycutt, J.D.; Kawano, S.; Hsieh, M.H.; Beachy, P.A. Cellular origin of bladder neoplasia and tissue dynamics of its progression to invasive carcinoma. Nat. Cell Biol. 2014, 16, 469–478. [Google Scholar] [CrossRef]
- Luanpitpong, S.; Wang, L.; Castranova, V.; Rojanasakul, Y. Induction of stem-like cells with malignant properties by chronic exposure of human lung epithelial cells to single-walled carbon nanotubes. Part. Fibre Toxicol. 2014. [Google Scholar] [CrossRef]
- Molofsky, A.V.; Pardal, R.; Morrison, S.J. Diverse mechanisms regulate stem cell self-renewal. Curr. Opin. Cell Biol. 2004, 16, 700–707. [Google Scholar] [CrossRef]
- Liedtke, S.; Stephan, M.; Koegler, G. Oct4 expression revisited: Potential pitfalls for data misinterpretation in stem cell research. Biol. Chem. 2008, 389, 845–850. [Google Scholar] [CrossRef]
- Farashahi Yazd, E.; Rafiee, M.R.; Soleimani, M.; Tavallaei, M.; Salmani, M.K.; Mowla, S.J. OCT4b1, a novel spliced variant of OCT4, generates a stable truncated protein with a potential role in stress response. Cancer Lett. 2011, 309, 170–175. [Google Scholar]
- Lee, J.; Kim, H.K.; Rho, J.Y.; Han, Y.M.; Kim, J. The human OCT-4 isoforms differ in their ability to confer self-renewal. J. Biol. Chem. 2006, 281, 33554–33565. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Xiao, Z.; Chen, B.; Wei, Z.; Wang, B.; Zhang, J.; Han, J.; Gao, Y.; Li, L.; et al. Alternative translation of OCT4 by an internal ribosome entry site and its novel function in stress response. Stem Cells 2009, 27, 1265–1275. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Xu, Z.; Ahmad, A.; Li, E.; Wang, Y.; Qin, S.; Wang, Q. Expression of sox2 and oct4 and their clinical significance in human non-small-cell lung cancer. Int. J. Mol. Sci. 2012, 13, 7663–7675. [Google Scholar] [CrossRef]
- Yasuda, H.; Tanaka, K.; Okita, Y.; Araki, T.; Saigusa, S.; Toiyama, Y.; Yokoe, T.; Yoshiyama, S.; Kawamoto, A.; Inoue, Y.; et al. CD133, OCT4, and NANOG in ulcerative colitis-associated colorectal cancer. Oncol. Lett. 2011, 2, 1065–1071. [Google Scholar]
- Yin, X.; Li, Y.W.; Zhang, B.H.; Ren, Z.G.; Qiu, S.J.; Yi, Y.; Fan, J. Coexpression of stemness factors Oct4 and Nanog predict liver resection. Ann. Surg. Oncol. 2012, 19, 2877–2887. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, G.R.; Hu, P. Over-expression of Oct4 in human esophageal squamous cell carcinoma. Mol. Cells 2011, 32, 39–45. [Google Scholar] [CrossRef]
- He, W.; Li, K.; Wang, F.; Qin, Y.R.; Fan, Q.X. Expression of OCT4 in human esophageal squamous cell carcinoma is significantly associated with poorer prognosis. World J. Gastroenterol. 2012, 18, 712–719. [Google Scholar] [CrossRef]
- Kumar, S.M.; Liu, S.; Lu, H.; Zhang, H.; Zhang, P.J.; Gimotty, P.A.; Guerra, M.; Guo, W.; Xu, X. Acquired cancer stem cell phenotypes through Oct4-mediated dedifferentiation. Oncogene 2012, 31, 4898–4911. [Google Scholar] [CrossRef]
- Koukourakis, M.I.; Giatromanolaki, A.; Tsakmaki, V.; Danielidis, V.; Sivridis, E. Cancer stem cell phenotype relates to radio-chemotherapy outcome in locally advanced squamous cell head-neck cancer. Br. J. Cancer 2012, 106, 846–853. [Google Scholar] [CrossRef]
- Linn, D.E.; Yang, X.; Sun, F.; Xie, Y.; Chen, H.; Jiang, R.; Chumsri, S.; Burger, A.M.; Qiu, Y. A role for OCT4 in tumor initiation of drug-resistant prostate cancer cells. Genes Cancer 2010, 1, 908–916. [Google Scholar] [CrossRef]
- Tsai, L.L.; Yu, C.C.; Chang, Y.C.; Yu, C.H.; Chou, M.Y. Markedly increased Oct4 and Nanog expression correlates with cisplatin resistance in oral squamous cell carcinoma. J. Oral Pathol. Med. 2011, 40, 621–628. [Google Scholar] [CrossRef]
- Atlasi, Y.; Mowla, S.J.; Ziaee, S.A.; Bahrami, A.R. OCT-4, an embryonic stem cell marker, is highly expressed in bladder cancer. Int. J. Cancer 2007, 120, 1598–1602. [Google Scholar] [CrossRef]
- Huang, P.; Chen, J.; Wang, L.; Na, Y.; Kaku, H.; Ueki, H.; Sasaki, K.; Yamaguchi, K.; Zhang, K.; Saika, T.; et al. Implications of transcriptional factor, Oct-4, in human bladder malignancy and tumor recurrence. Med. Oncol. 2012, 29, 829–834. [Google Scholar] [CrossRef]
- Kruger, S.; Noack, F.; Bohle, A.; Feller, A.C. Histologic tumor growth pattern is significantly associated with disease-related survival in muscle-invasive transitional cell carcinoma of the urinary bladder. Oncol. Rep. 2004, 12, 609–613. [Google Scholar]
- Jóźwicki, W.; Brożyna, A. Does stem cell marker Oct4a expression affect urothelial bladder cancer phenotype? In Civilization and Social Diseases; Sokolowska, B., Ed.; Pope John II University in Biała Podlaska, Institute of Health: Podlaska, Poland, 2010; pp. 43–53. [Google Scholar]
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef]
- Huntly, B.J.; Gilliland, D.G. Leukaemia stem cells and the evolution of cancer-stem-cell research. Nat. Rev. Cancer 2005, 5, 311–321. [Google Scholar] [CrossRef]
- Tai, M.H.; Chang, C.C.; Kiupel, M.; Webster, J.D.; Olson, L.K.; Trosko, J.E. Oct4 expression in adult human stem cells: Evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis 2005, 26, 495–502. [Google Scholar] [CrossRef]
- Dontu, G.; Al-Hajj, M.; Abdallah, W.M.; Clarke, M.F.; Wicha, M.S. Stem cells in normal breast development and breast cancer. Cell Prolif. 2003, 36, 59–72. [Google Scholar] [CrossRef]
- Burger, P.E.; Xiong, X.; Coetzee, S.; Salm, S.N.; Moscatelli, D.; Goto, K.; Wilson, E.L. Sca-1 expression identifies stem cells in the proximal region of prostatic ducts with high capacity to reconstitute prostatic tissue. Proc. Natl. Acad. Sci. USA 2005, 102, 7180–7185. [Google Scholar] [CrossRef]
- Singh, S.K.; Yadav, R.P.; Tiwari, S.; Singh, A. Toxic effect of stem bark and leaf of euphorbia hirta plant against freshwater vector snail Lymnaea acuminata. Chemosphere 2005, 59, 263–270. [Google Scholar] [CrossRef]
- Kim, C.F.; Jackson, E.L.; Woolfenden, A.E.; Lawrence, S.; Babar, I.; Vogel, S.; Crowley, D.; Bronson, R.T.; Jacks, T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 2005, 121, 823–835. [Google Scholar] [CrossRef]
- Xin, L.; Lawson, D.A.; Witte, O.N. The Sca-1 cell surface marker enriches for a prostate-regenerating cell subpopulation that can initiate prostate tumorigenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 6942–6947. [Google Scholar] [CrossRef]
- Yao, J.; Cai, H.H.; Wei, J.S.; An, Y.; Ji, Z.L.; Lu, Z.P.; Wu, J.L.; Chen, P.; Jiang, K.R.; Dai, C.C.; et al. Side population in the pancreatic cancer cell lines SW1990 and CFPAC-1 is enriched with cancer stem-like cells. Oncol. Rep. 2010, 23, 1375–1382. [Google Scholar]
- Rycaj, K.; Tang, D.G. Cancer stem cells and radioresistance. Int. J. Radiat. Biol. 2014, 90, 615–621. [Google Scholar] [CrossRef]
- Yang, J.; Yue, J.B.; Liu, J.; Yu, J.M. Repopulation of tumor cells during fractionated radiotherapy and detection methods (review). Oncol. Lett. 2014, 7, 1755–1760. [Google Scholar]
- Rodini, C.O.; Suzuki, D.E.; Saba-Silva, N.; Cappellano, A.; de Souza, J.E.; Cavalheiro, S.; Toledo, S.R.; Okamoto, O.K. Expression analysis of stem cell-related genes reveal OCT4 as a predictor of poor clinical outcome in medulloblastoma. J. Neurooncol. 2012, 106, 71–79. [Google Scholar] [CrossRef]
- Wanggou, S.; Jiang, X.; Yuan, X.; Ren, C.; Zeng, Y.; Li, G.; Li, Q. Prognostic value of OCT4 in primary intracranial germinoma: a single institute analysis of 31 cases. Br. J. Neurosurg. 2012, 26, 237–246. [Google Scholar] [CrossRef]
- Hara, T.; Takahashi, M.; Gondo, T.; Nagao, K.; Ohmi, C.; Sakano, S.; Naito, K.; Matsuyama, H. Risk of concomitant carcinoma in situ determining biopsy candidates among primary non-muscle-invasive bladder cancer patients: retrospective analysis of 173 japanese cases. Int. J. Urol. 2009, 16, 293–298. [Google Scholar] [CrossRef]
- Zigeuner, R.; Shariat, S.F.; Margulis, V.; Karakiewicz, P.I.; Roscigno, M.; Weizer, A.; Kikuchi, E.; Remzi, M.; Raman, J.D.; Bolenz, C.; et al. Tumour necrosis is an indicator of aggressive biology in patients with urothelial carcinoma of the upper urinary tract. Eur. Urol. 2010, 57, 575–581. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jóźwicki, W.; Brożyna, A.A.; Siekiera, J. Expression of OCT4A: The First Step to the Next Stage of Urothelial Bladder Cancer Progression. Int. J. Mol. Sci. 2014, 15, 16069-16082. https://doi.org/10.3390/ijms150916069
Jóźwicki W, Brożyna AA, Siekiera J. Expression of OCT4A: The First Step to the Next Stage of Urothelial Bladder Cancer Progression. International Journal of Molecular Sciences. 2014; 15(9):16069-16082. https://doi.org/10.3390/ijms150916069
Chicago/Turabian StyleJóźwicki, Wojciech, Anna A. Brożyna, and Jerzy Siekiera. 2014. "Expression of OCT4A: The First Step to the Next Stage of Urothelial Bladder Cancer Progression" International Journal of Molecular Sciences 15, no. 9: 16069-16082. https://doi.org/10.3390/ijms150916069




