Novel Sustained-Release of Propafenone through Pellets: Preparation and in Vitro/in Vivo Evaluation
Abstract
:1. Introduction
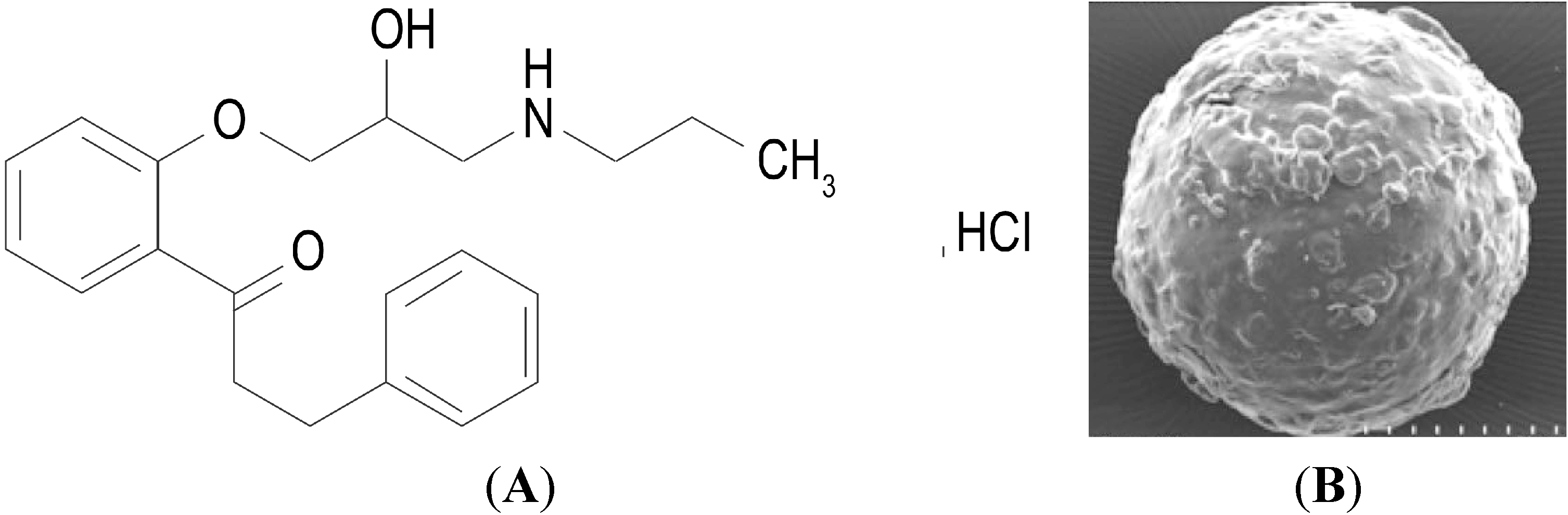
2. Results and Discussion
2.1. Characteristics of the Pellets
2.2. In Vitro Release
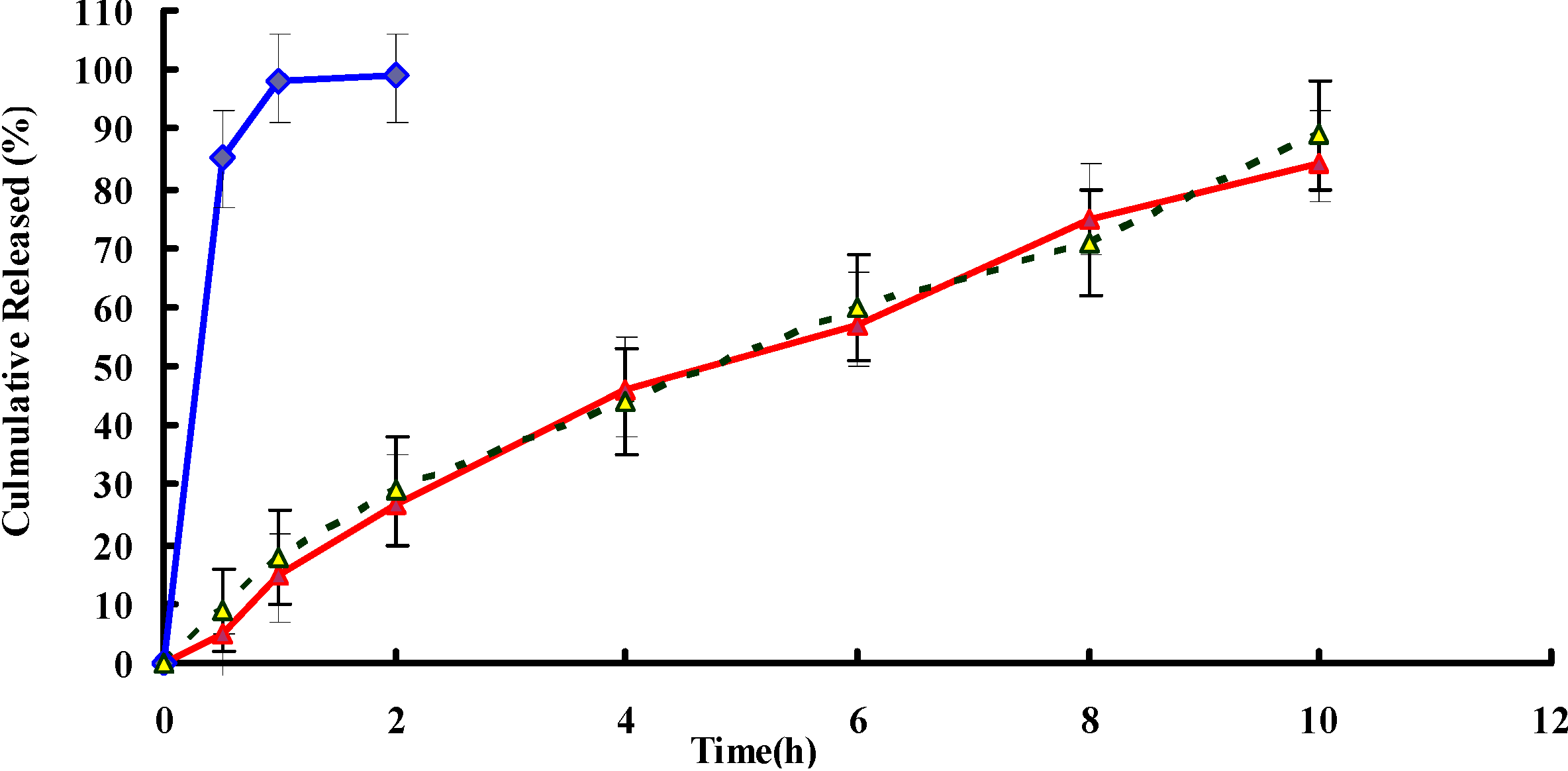
2.3. Stability Studies

2.4. In Vivo Study
| Parameter | Formulations | |
|---|---|---|
| Tablets | Pellets | |
| t1/2 (h) | 1.67 ± 0.7 | 5.72 ± 2.1 Δ |
| Cmax (ng/mL) | 1864.2 ± 354.6 | 1288.3 ± 386.6 Δ |
| AUC0–t (ng·h/mL) | 7863.7 ± 446.3 | 9337.5 ± 463.1 Δ |
| AUC0–∞ (ng·h/mL) | 8272.4 ± 476.4 | 9891.7 ± 479.6 Δ |
| MRT (h) | 1.28 ± 4.6 | 3.82 ± 3.9 Δ |
| CL (L/h) | 4.54 ± 2.7 | 1.33 ± 2.4 Δ |
3. Materials and Methods
3.1. Materials
3.2. Preparation of Propafenone Hydrochloride (PPF) Pellets
3.3. Analysis of Drug Content
3.4. Characteristics of the Pellets
3.5. In Vitro Release Studies
3.6. Stability Study
3.7. HPLC Analysis
3.8. Pharmacokinetic Study
3.8.1. Animals
3.8.2. Study Design
3.8.3. Blood Sampling
3.8.4. Statistical Analysis
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Funck-Brentano, C.; Kroemer, H.K.; Lee, J.T.; Roden, D.M. Propafenone. N. Engl. J. Med. 1990, 322, 518–525. [Google Scholar] [CrossRef]
- Harron, D.W.; Brogden, R.N. Propafenone. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in the treatment of arrhythmias. Drugs 1987, 34, 617–647. [Google Scholar] [CrossRef]
- Sestito, A.; Molina, E. A trial fibrillation and the pharmacological treatment: The role of propafenone. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 242–253. [Google Scholar]
- Ni, G.S. Drugs for Clinical Application; 81 Publishing House: Beijing, China, 1994; p. 270. [Google Scholar]
- Pritchett, E.L.; Page, R.L.; Carlson, M.; Undesser, K.; Fava, G. Rythmol atrial fibrillation trial (RAFT) investigators. Efficacy and safety of sustained-release propafenone (propafenone SR) for patients with atrial fibrillation. Am. J. Cardiol. 2003, 92, 941–946. [Google Scholar] [CrossRef]
- Volz, M.; Mitrovic, V.; Thiemer, J.; Schlepper, M. Steady-state plasma kinetics of slow-release propafenone, its two isomers and its main metabolites. Arzneim. Forsch. 1995, 45, 246–249. [Google Scholar]
- Meinertz, T.; Lip, G.H.Y.; Lombardi, F.; Sadowski, Z.P.; Kalsch, B.; Camez, A.; Hewkin, A.; Eberle, S. The ERAFT investigators. Efficacy, and safety of propafenone sustained release in the prophylaxis of symptomatic paroxysmal atrial fibrillation. Am. J. Cardiol. 2002, 90, 1300–1306. [Google Scholar]
- Lu, B. New Drug Formulations and New Technology; People’s Medical Publishing House: Beijing, China, 1998; p. 288. [Google Scholar]
- Tripurasundari, P.; Prabhakar, B. Review on the production of pellets via extrusion–spheronisation exclusive of microcrystalline cellulose. Int. J. Pharm. Rev. Res. 2012, 2, 1–10. [Google Scholar]
- Abdalla, A.; Mader, K. Preparation and characterization of a selfemulsifying pellet formulation. Eur. J. Pharm. Biopharm. 2007, 66, 220–226. [Google Scholar]
- Fu, J.; Wang, X.; Xu, L.; Meng, J.; Weng, Y.; Li, G.; He, H.; Tang, X. Preparation and in vitro–in vivo evaluation of double layer coated and matrix sustained release pellet formulations of diclofenac potassium. Int. J. Pharm. 2011, 406, 84–90. [Google Scholar]
- Bhaskaran, S.; Lakshmi, P.K. Extrusion spheronization—A review. Int. J. Pharm. Tech. Res. 2010, 2, 2429–2433. [Google Scholar]
- Wang, Z.; Sun, J.; Wang, Y.; Liu, X.; Liu, Y. Solid self-emulsifying Nitrendipine pellets: Preparation and in vitro/in vivo evaluation. Int. J. Pharm. 2010, 383, 1–6. [Google Scholar]
- Trivedi, N.R.; Rajan, M.G.; Johnson, J.R.; Shukla, A.J. Pharmaceutical approaches to preparing pelletized dosage forms using the extrusionspheronization process. Crit. Rev. Ther. Drug Carr. Syst. 2007, 24, 1–40. [Google Scholar] [CrossRef]
- Shah, R.D.; Kabadi, M.; Pope, D.G.; Augsburger, I. Physico-mechanical characterization of the extrusion–spheronization process. Part II: Rheological determinants for successful extrusion and spheronization. Pharm. Res. 1995, 4, 496–507. [Google Scholar]
- Dukic-Ott, A.; Thommes, M.; Remon, J.P.; Kleinebudde, P.; Vervaet, C. Production of pellets via extrusion–spheronisation without the incorporation of microcrystalline cellulose: A critical review. Eur. J. Pharm. Biopharm. 2009, 71, 38–46. [Google Scholar] [CrossRef]
- Gupta, N.; Gowda, D.V.; Balamuralidhara, V.; Mohammed Khan, S. Formulation and evaluation of olanzapine matrix pellets for controlled release. DARU 2011, 4, 249–256. [Google Scholar]
- Wells, J.I. Pharmaceutical Preformulation: The Physicochemical Properties of Drug Substances; Wiley: New York, NY, USA, 1988; pp. 209–211. [Google Scholar]
- O’Connor, R.E.; Schwartz, J.B. Spheronization II: Drug release from drugdiluents’ mixtures. Drug. Dev. Ind. Pharm. 1985, 11, 1837–1857. [Google Scholar] [CrossRef]
- Paul, D.R. Elaborations on the Higuchi model for drug delivery. Int. J. Pharm. 2011, 418, 13–17. [Google Scholar] [CrossRef]
- Newton, J.M.; Pinto, M.R.; Podczeck, F. The preparation of pellets containing a surfactant or a mixture of mono- and di-gylcerides by extrusion/spheronisation. Eur. J. Pharm. Sci. 2007, 30, 333–342. [Google Scholar] [CrossRef]
- Rajesh, N. Design and evaluation of controlled release of Piroxicam from the pellets of microcrystalline cellulose and hydroxypropylmethyl cellulose blends. Int. J. Pharm. Tech. Res. 2010, 2, 1456–1473. [Google Scholar]
- Tavakoli, N.; Varshosaz, J.; Ghannadi, A.R.; Bavarsad, N. Evaluation of trigonella foenum-graecum seeds gum as a novel tablet binder. Int. J. Pharm. Pharm. Sci. 2007, 4, 97–101. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, L.; Jiang, P.; Liu, J. Novel Sustained-Release of Propafenone through Pellets: Preparation and in Vitro/in Vivo Evaluation. Int. J. Mol. Sci. 2014, 15, 15503-15511. https://doi.org/10.3390/ijms150915503
Zhang L, Jiang P, Liu J. Novel Sustained-Release of Propafenone through Pellets: Preparation and in Vitro/in Vivo Evaluation. International Journal of Molecular Sciences. 2014; 15(9):15503-15511. https://doi.org/10.3390/ijms150915503
Chicago/Turabian StyleZhang, Li, Ping Jiang, and Ji Liu. 2014. "Novel Sustained-Release of Propafenone through Pellets: Preparation and in Vitro/in Vivo Evaluation" International Journal of Molecular Sciences 15, no. 9: 15503-15511. https://doi.org/10.3390/ijms150915503




