The Aging Kidney: Increased Susceptibility to Nephrotoxicity
Abstract
:1. Introduction
2. Aging and AKI: Clinical Evidence
3. Aging and AKI: Experimental Models
4. Aging and AKI: Causes
4.1. Chronic Kidney Disease (CKD)
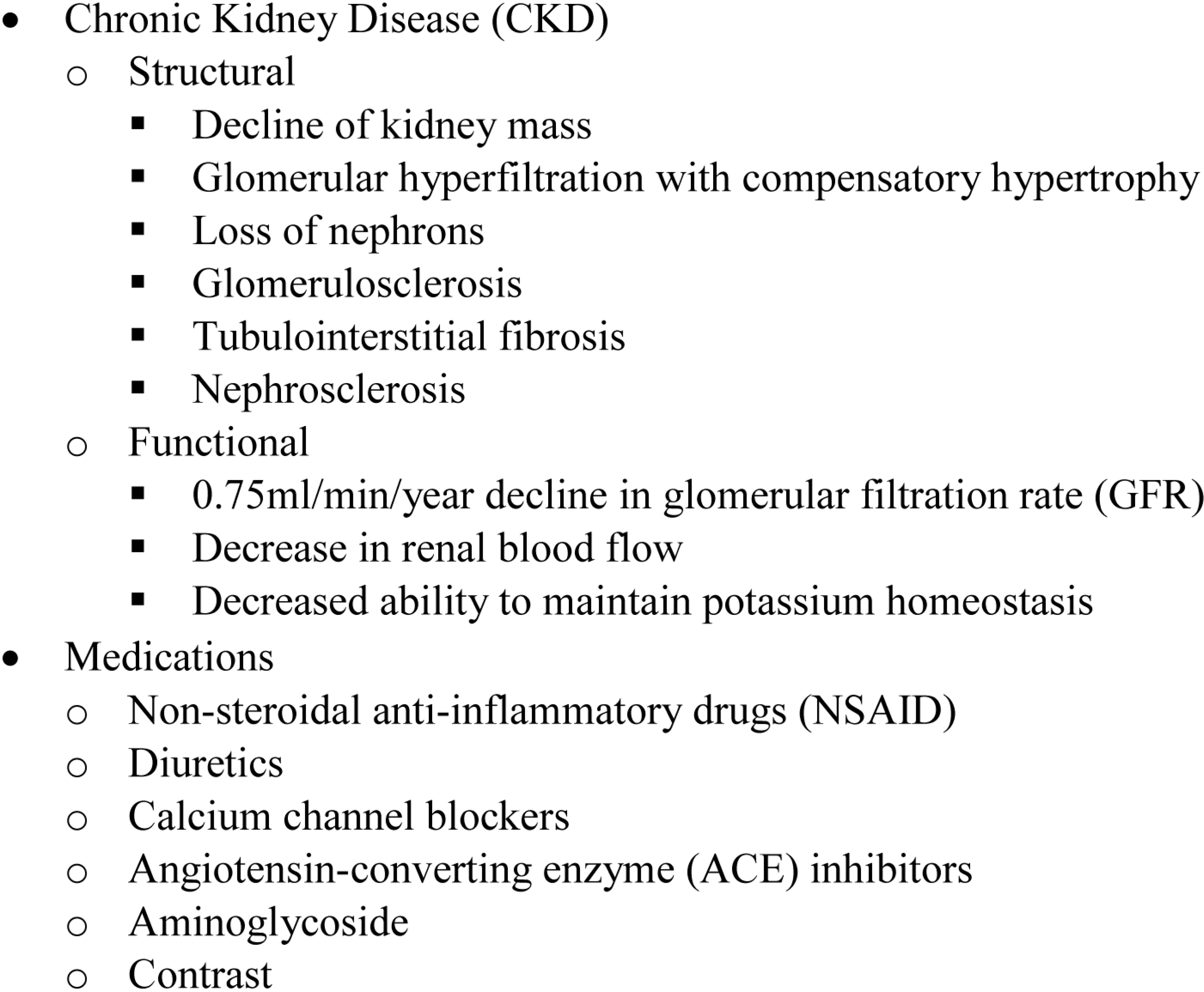
4.2. Medications
5. Aging and AKI: Mechanisms
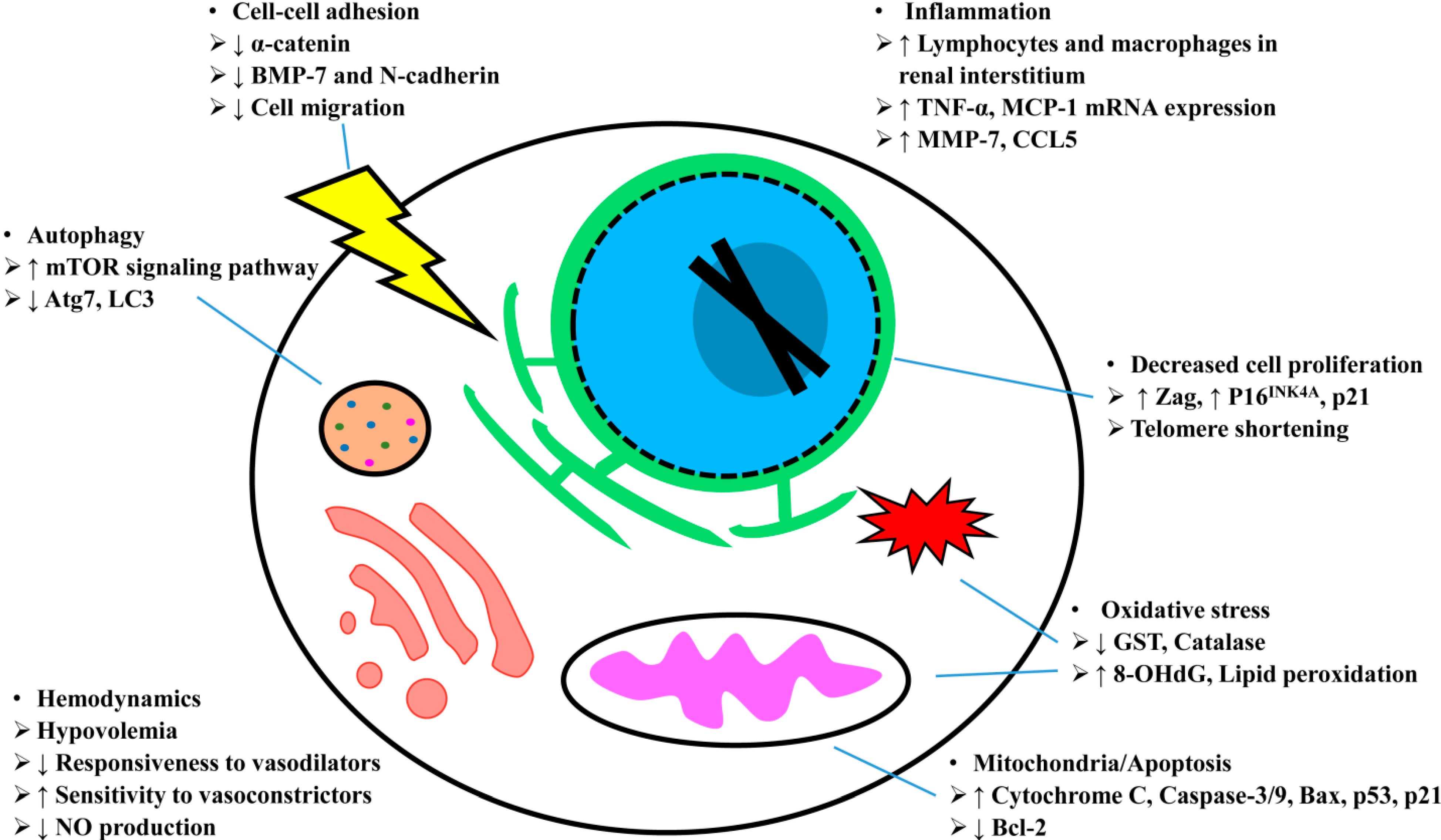
5.1. Hemodynamics
5.2. Oxidative Stress
5.3. Mitochondria/Apoptosis
5.4. Autophagy
5.5. Inflammation
5.6. Repair
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bolignano, D.; Mattace-Raso, F.; Sijbrands, E.J.; Zoccali, C. The aging kidney revisited: A systematic review. Ageing Res. Rev. 2014. [Google Scholar] [CrossRef]
- United States Census Bureau. United States—Age and Sex. Available online: http://www.aoa.gov/AOARoot/Aging_statistics/profile/2009/docs/2009profile_508.pdf (accessed on 26 August 2014).
- Coresh, J.; Astor, B.C.; Greene, T.; Eknoyan, G.; Levey, A.S. Prevalence of chronic kidney disease and decreased kidney function in the adult us population: Third national health and nutrition examination survey. Am. J. Kidney Dis. 2003, 41, 1–12. [Google Scholar]
- Costello, D.; Carone, G. Can europe afford to grow old? Int. Monet. Fund Finance Dev. Mag. 2006, 43, 28. [Google Scholar]
- World Health Organization. Facts: Global Health Situation and Trends 1955–2025; World Health Organization: Geneva, Switzerland, 1998. [Google Scholar]
- Webb, S.; Dobb, G. Arf, atn or aki? It’s now acute kidney injury. Anaesth. Intensive Care 2007, 35, 843–844. [Google Scholar]
- Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C.; Warnock, D.G.; Levin, A. Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit. Care 2007, 11, R31. [Google Scholar] [CrossRef]
- Endre, Z.H. Acute kidney injury: Definitions and new paradigms. Adv. Chron. Kidney Dis. 2008, 15, 213–221. [Google Scholar] [CrossRef]
- Kellum, J.A.; Bellomo, R.; Ronco, C. Definition and classification of acute kidney injury. Nephron Clin. Pract. 2008, 109, c182–c187. [Google Scholar] [CrossRef]
- De Almeida, D.C.; Donizetti-Oliveira, C.; Barbosa-Costa, P.; Origassa, C.S.; Camara, N.O. In search of mechanisms associated with mesenchymal stem cell-based therapies for acute kidney injury. Clin. Biochem. Rev./Aust. Assoc. Clin. Biochem. 2013, 34, 131–144. [Google Scholar]
- Bellomo, R.; Kellum, J.A.; Ronco, C. Acute kidney injury. Lancet 2012, 380, 756–766. [Google Scholar] [CrossRef]
- Waikar, S.S.; Curhan, G.C.; Wald, R.; McCarthy, E.P.; Chertow, G.M. Declining mortality in patients with acute renal failure, 1988 to 2002. J. Am. Soc. Nephrol. 2006, 17, 1143–1150. [Google Scholar] [CrossRef]
- Xue, J.L.; Daniels, F.; Star, R.A.; Kimmel, P.L.; Eggers, P.W.; Molitoris, B.A.; Himmelfarb, J.; Collins, A.J. Incidence and mortality of acute renal failure in medicare beneficiaries, 1992 to 2001. J. Am. Soc. Nephrol. 2006, 17, 1135–1142. [Google Scholar] [CrossRef]
- Lameire, N.; van Biesen, W.; Vanholder, R. The rise of prevalence and the fall of mortality of patients with acute renal failure: What the analysis of two databases does and does not tell us. J. Am. Soc. Nephrol. 2006, 17, 923–925. [Google Scholar] [CrossRef]
- Stott, R.B.; Cameron, J.S.; Ogg, C.S.; Bewick, M. Why the persistently high mortality in acute renal failure. Lancet 1972, 2, 75–79. [Google Scholar]
- Kumar, R.; Hill, C.M.; McGeown, M.G. Acute renal failure in the elderly. Lancet 1973, 1, 90–91. [Google Scholar] [CrossRef]
- Rosenfeld, J.B.; Shohat, J.; Grosskopf, I.; Boner, G. Acute renal failure: A disease of the elderly? Adv. Nephrol. Necker Hosp. 1987, 16, 159–167. [Google Scholar]
- Turney, J.H.; Marshall, D.H.; Brownjohn, A.M.; Ellis, C.M.; Parsons, F.M. The evolution of acute renal failure, 1956–1988. Q. J. Med. 1990, 74, 83–104. [Google Scholar]
- Abdel-Kader, K.; Palevsky, P.M. Acute kidney injury in the elderly. Clin. Geriatr. Med. 2009, 25, 331–358. [Google Scholar] [CrossRef]
- Rosner, M.H. The pathogenesis of susceptibility to acute kidney injury in the elderly. Curr. Aging Sci. 2009, 2, 158–164. [Google Scholar] [CrossRef]
- Himmelfarb, J. Acute kidney injury in the elderly: Problems and prospects. Semin. Nephrol. 2009, 29, 658–664. [Google Scholar] [CrossRef]
- Coca, S.G. Acute kidney injury in elderly persons. Am. J. Kidney Dis. 2010, 56, 122–131. [Google Scholar] [CrossRef]
- Chronopoulos, A.; Cruz, D.N.; Ronco, C. Hospital-acquired acute kidney injury in the elderly. Nat. Rev. Nephrol. 2010, 6, 141–149. [Google Scholar] [CrossRef]
- Lameire, N.; Matthys, E.; Vanholder, R.; de Keyser, K.; Pauwels, W.; Nachtergaele, H.; Lambrecht, L.; Ringoir, S. Causes and prognosis of acute renal failure in elderly patients. Nephrol. Dial. Transplant. 1987, 2, 316–322. [Google Scholar]
- Neild, G.H. Multi-organ renal failure in the elderly. Int. Urol. Nephrol. 2001, 32, 559–565. [Google Scholar] [CrossRef]
- Pascual, J.; Orofino, L.; Liano, F.; Marcen, R.; Naya, M.T.; Orte, L.; Ortuno, J. Incidence and prognosis of acute renal failure in older patients. J. Am. Geriatr. Soc. 1990, 38, 25–30. [Google Scholar]
- Uchino, S.; Kellum, J.A.; Bellomo, R.; Doig, G.S.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; et al. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 2005, 294, 813–818. [Google Scholar] [CrossRef]
- Baraldi, A.; Ballestri, M.; Rapana, R.; Lucchi, L.; Borella, P.; Leonelli, M.; Furci, L.; Lusvarghi, E. Acute renal failure of medical type in an elderly population. Nephrol. Dial. Transplant. 1998, 13 (Suppl. S7), 25–29. [Google Scholar]
- Hsu, C.Y.; Chertow, G.M.; McCulloch, C.E.; Fan, D.; Ordonez, J.D.; Go, A.S. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin. J. Am. Soc. Nephrol. 2009, 4, 891–898. [Google Scholar] [CrossRef]
- Zhang, L.; Fu, P.; Wang, L.; Cai, G.; Chen, D.; Guo, D.; Sun, X.; Chen, F.; Bi, W.; Zeng, X.; et al. The clinical features and outcome of crush patients with acute kidney injury after the wenchuan earthquake: Differences between elderly and younger adults. Injury 2012, 43, 1470–1475. [Google Scholar] [CrossRef]
- Yang, H.; Fogo, A.B. Cell senescence in the aging kidney. J. Am. Soc. Nephrol. 2010, 21, 1436–1439. [Google Scholar] [CrossRef]
- Rosner, M.H. Acute kidney injury in the elderly. Clin. Geriatr. Med. 2013, 29, 565–578. [Google Scholar] [CrossRef]
- Hsu, R.K.; McCulloch, C.E.; Dudley, R.A.; Lo, L.J.; Hsu, C.Y. Temporal changes in incidence of dialysis-requiring aki. J. Am. Soc. Nephrol. 2013, 24, 37–42. [Google Scholar] [CrossRef]
- Pascual, J.; Liano, F. Causes and prognosis of acute renal failure in the very old. Madrid acute renal failure study group. J. Am. Geriatr. Soc. 1998, 46, 721–725. [Google Scholar]
- Van den Noortgate, N.; Mouton, V.; Lamot, C.; van Nooten, G.; Dhondt, A.; Vanholder, R.; Afschrift, M.; Lameire, N. Outcome in a post-cardiac surgery population with acute renal failure requiring dialysis: Does age make a difference? Nephrol. Dial. Transplant. 2003, 18, 732–736. [Google Scholar] [CrossRef]
- Gentric, A.; Cledes, J. Immediate and long-term prognosis in acute renal failure in the elderly. Nephrol. Dial. Transplant. 1991, 6, 86–90. [Google Scholar] [CrossRef]
- Arora, P.; Kher, V.; Kohli, H.S.; Sharma, R.K.; Gupta, A.; Jha, R. Acute renal failure in the elderly: Experience from a single centre in india. Nephrol. Dial. Transplant. 1993, 8, 827–830. [Google Scholar]
- Bagshaw, S.M.; Laupland, K.B.; Doig, C.J.; Mortis, G.; Fick, G.H.; Mucenski, M.; Godinez-Luna, T.; Svenson, L.W.; Rosenal, T. Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: A population-based study. Crit. Care 2005, 9, R700–R709. [Google Scholar] [CrossRef]
- Trevisan, A.; Nicolli, A.; Chiara, F. Are rats the appropriate experimental model to understand age-related renal drug metabolism and toxicity? Expert Opin. Drug Metab. Toxicol. 2010, 6, 1451–1459. [Google Scholar] [CrossRef]
- Beierschmitt, W.P.; Keenan, K.P.; Weiner, M. Age-related increased susceptibility of male fischer 344 rats to acetaminophen nephrotoxicity. Life Sci. 1986, 39, 2335–2342. [Google Scholar] [CrossRef]
- McMartin, D.N.; Engel, S.G. Effect of aging on gentamicin nephrotoxicity and pharmacokinetics in rats. Res. Commun. Chem. Pathol. Pharmacol. 1982, 38, 193–207. [Google Scholar]
- Zager, R.A.; Alpers, C.E. Effects of aging on expression of ischemic acute renal failure in rats. Lab. Investig. 1989, 61, 290–294. [Google Scholar]
- Miura, K.; Goldstein, R.S.; Morgan, D.G.; Pasino, D.A.; Hewitt, W.R.; Hook, J.B. Age-related differences in susceptibility to renal ischemia in rats. Toxicol. Appl. Pharmacol. 1987, 87, 284–296. [Google Scholar] [CrossRef]
- Chen, G.; Bridenbaugh, E.A.; Akintola, A.D.; Catania, J.M.; Vaidya, V.S.; Bonventre, J.V.; Dearman, A.C.; Sampson, H.W.; Zawieja, D.C.; Burghardt, R.C.; et al. Increased susceptibility of aging kidney to ischemic injury: Identification of candidate genes changed during aging, but corrected by caloric restriction. American journal of physiology. Ren. Physiol. 2007, 293, F1272–F1281. [Google Scholar] [CrossRef]
- Peters-Volleberg, G.W.; Dortant, P.M.; Speijers, G.J. Comparison of tobramycin nephrotoxicity in young adult and aged female rats. Pharmacol. Toxicol. 1999, 84, 147–153. [Google Scholar] [CrossRef]
- Clements, M.E.; Chaber, C.J.; Ledbetter, S.R.; Zuk, A. Increased cellular senescence and vascular rarefaction exacerbate the progression of kidney fibrosis in aged mice following transient ischemic injury. PLoS One 2013, 8, e70464. [Google Scholar]
- Miyaji, T.; Hu, X.; Yuen, P.S.; Muramatsu, Y.; Iyer, S.; Hewitt, S.M.; Star, R.A. Ethyl pyruvate decreases sepsis-induced acute renal failure and multiple organ damage in aged mice. Kidney Int. 2003, 64, 1620–1631. [Google Scholar] [CrossRef]
- Zhou, X.J.; Rakheja, D.; Yu, X.; Saxena, R.; Vaziri, N.D.; Silva, F.G. The aging kidney. Kidney Int. 2008, 74, 710–720. [Google Scholar] [CrossRef]
- Tauchi, H.; Tsuboi, K.; Okutomi, J. Age changes in the human kidney of the different races. Gerontologia 1971, 17, 87–97. [Google Scholar] [CrossRef]
- Long, D.A.; Mu, W.; Price, K.L.; Johnson, R.J. Blood vessels and the aging kidney. Nephron Exp. Nephrol. 2005, 101, e95–e99. [Google Scholar] [CrossRef]
- McLachlan, M.; Wasserman, P. Changes in sizes and distensibility of the aging kidney. Br. J. Radiol. 1981, 54, 488–491. [Google Scholar] [CrossRef]
- Rao, U.V.; Wagner, H.N., Jr. Normal weights of human organs. Radiology 1972, 102, 337–339. [Google Scholar] [CrossRef]
- Goyal, V.K. Changes with age in the human kidney. Exp. Gerontol. 1982, 17, 321–331. [Google Scholar] [CrossRef]
- Jassal, S.V.; Oreopoulos, D.G. The aging kidney. Geriatr. Nephrol. Urol. 1998, 8, 141–147. [Google Scholar] [CrossRef]
- Karam, Z.; Tuazon, J. Anatomic and physiologic changes of the aging kidney. Clin. Geriatr. Med. 2013, 29, 555–564. [Google Scholar] [CrossRef]
- Rule, A.D.; Amer, H.; Cornell, L.D.; Taler, S.J.; Cosio, F.G.; Kremers, W.K.; Textor, S.C.; Stegall, M.D. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann. Intern. Med. 2010, 152, 561–567. [Google Scholar] [CrossRef]
- Tonelli, M.; Riella, M.C. World kidney day 2014: Ckd and the aging population. Am. J. Kidney Dis. 2014, 63, 349–353. [Google Scholar] [CrossRef]
- Lindeman, R.D.; Tobin, J.; Shock, N.W. Longitudinal studies on the rate of decline in renal function with age. J. Am. Geriatr. Soc. 1985, 33, 278–285. [Google Scholar]
- Jiang, S.; Sun, X.; Gu, H.; Chen, Y.; Xi, C.; Qiao, X.; Chen, X. Age-related change in kidney function, its influencing factors, and association with asymptomatic carotid atherosclerosis in healthy individuals—A 5-year follow-up study. Maturitas 2012, 73, 230–238. [Google Scholar] [CrossRef]
- Grace B, H.K.; McDonald, S. New patients commencing treatment in 2011. In 2012 Annual Report, 35th ed.; ANZDATA Registry: Adelaide, Australia, 2012; Chapter 2. [Google Scholar]
- Marcum, Z.A.; Fried, L.F. Aging and antihypertensive medication-related complications in the chronic kidney disease patient. Curr. Opin. Nephrol. Hypertens. 2011, 20, 449–456. [Google Scholar] [CrossRef]
- Del Giudice, A.; Aucella, F. Acute renal failure in the elderly: Epidemiology and clinical features. J. Nephrol. 2012, 25, S48–S57. [Google Scholar] [CrossRef]
- Anderson, S.; Eldadah, B.; Halter, J.B.; Hazzard, W.R.; Himmelfarb, J.; Horne, F.M.; Kimmel, P.L.; Molitoris, B.A.; Murthy, M.; O’Hare, A.M.; et al. Acute kidney injury in older adults. J. Am. Soc. Nephrol. 2011, 22, 28–38. [Google Scholar] [CrossRef]
- Ishani, A.; Xue, J.L.; Himmelfarb, J.; Eggers, P.W.; Kimmel, P.L.; Molitoris, B.A.; Collins, A.J. Acute kidney injury increases risk of esrd among elderly. J. Am. Soc. Nephrol. 2009, 20, 223–228. [Google Scholar] [CrossRef]
- Venkatachalam, M.A.; Griffin, K.A.; Lan, R.; Geng, H.; Saikumar, P.; Bidani, A.K. Acute kidney injury: A springboard for progression in chronic kidney disease. Am. J. Physiol. Ren. Physiol. 2010, 298, F1078–F1094. [Google Scholar] [CrossRef]
- Yang, L.; Besschetnova, T.Y.; Brooks, C.R.; Shah, J.V.; Bonventre, J.V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 2010, 16, 535–543. [Google Scholar] [CrossRef]
- Peres, L.A.; da Cunha, A.D., Jr. Acute nephrotoxicity of cisplatin: Molecular mechanisms. J. Bras. Nefrol. 2013, 35, 332–340. [Google Scholar] [CrossRef]
- Kohli, H.S.; Bhaskaran, M.C.; Muthukumar, T.; Thennarasu, K.; Sud, K.; Jha, V.; Gupta, K.L.; Sakhuja, V. Treatment-related acute renal failure in the elderly: A hospital-based prospective study. Nephrol. Dial. Transplant. 2000, 15, 212–217. [Google Scholar] [CrossRef]
- Mulkerrin, E.; Epstein, F.H.; Clark, B.A. Aldosterone responses to hyperkalemia in healthy elderly humans. J. Am. Soc. Nephrol. 1995, 6, 1459–1462. [Google Scholar]
- Lawson, D.H.; Henderson, A.K.; McGeachy, R.R. Amoxycillin: Pharmacokinetic studies in normal subjects, patients with pernicious anaemia and those with renal failure. Postgrad. Med. J. 1974, 50, 500–503. [Google Scholar] [CrossRef]
- Henry, D.; Page, J.; Whyte, I.; Nanra, R.; Hall, C. Consumption of non-steroidal anti-inflammatory drugs and the development of functional renal impairment in elderly subjects. Results of a case-control study. Br. J. Clin. Pharmacol. 1997, 44, 85–90. [Google Scholar]
- Blackshear, J.L.; Davidman, M.; Stillman, M.T. Identification of risk for renal insufficiency from nonsteroidal anti-inflammatory drugs. Arch. Intern. Med. 1983, 143, 1130–1134. [Google Scholar] [CrossRef]
- Huerta, C.; Castellsague, J.; Varas-Lorenzo, C.; Garcia Rodriguez, L.A. Nonsteroidal anti-inflammatory drugs and risk of arf in the general population. Am. J. Kidney Dis. 2005, 45, 531–539. [Google Scholar]
- Adhiyaman, V.; Asghar, M.; Oke, A.; White, A.D.; Shah, I.U. Nephrotoxicity in the elderly due to co-prescription of angiotensin converting enzyme inhibitors and nonsteroidal anti-inflammatory drugs. J. R. Soc. Med. 2001, 94, 512–514. [Google Scholar]
- Moore, R.D.; Smith, C.R.; Lipsky, J.J.; Mellits, E.D.; Lietman, P.S. Risk factors for nephrotoxicity in patients treated with aminoglycosides. Ann. Intern. Med. 1984, 100, 352–357. [Google Scholar] [CrossRef]
- Muhlberg, W.; Platt, D. Age-dependent changes of the kidneys: Pharmacological implications. Gerontology 1999, 45, 243–253. [Google Scholar] [CrossRef]
- Baciewicz, A.M.; Sokos, D.R.; Cowan, R.I. Aminoglycoside-associated nephrotoxicity in the elderly. Ann. Pharmacother. 2003, 37, 182–186. [Google Scholar]
- Rich, M.W.; Crecelius, C.A. Incidence, risk factors, and clinical course of acute renal insufficiency after cardiac catheterization in patients 70 years of age or older. A prospective study. Arch. Intern. Med. 1990, 150, 1237–1242. [Google Scholar] [CrossRef]
- McGillicuddy, E.A.; Schuster, K.M.; Kaplan, L.J.; Maung, A.A.; Lui, F.Y.; Maerz, L.L.; Johnson, D.C.; Davis, K.A. Contrast-induced nephropathy in elderly trauma patients. J. Trauma 2010, 68, 294–297. [Google Scholar] [CrossRef]
- Rudnick, M.R.; Goldfarb, S.; Tumlin, J. Contrast-induced nephropathy: Is the picture any clearer? Clin. J. Am. Soc. Nephrol. 2008, 3, 261–262. [Google Scholar] [CrossRef]
- Cheung, C.M.; Ponnusamy, A.; Anderton, J.G. Management of acute renal failure in the elderly patient: A clinician’s guide. Drugs Aging 2008, 25, 455–476. [Google Scholar] [CrossRef]
- Aymanns, C.; Keller, F.; Maus, S.; Hartmann, B.; Czock, D. Review on pharmacokinetics and pharmacodynamics and the aging kidney. Clin. J. Am. Soc. Nephrol. 2010, 5, 314–327. [Google Scholar] [CrossRef]
- Goldstein, R.S.; Pasino, D.A.; Hook, J.B. Cephaloridine nephrotoxicity in aging male fischer-344 rats. Toxicology 1986, 38, 43–53. [Google Scholar] [CrossRef]
- Samiy, A.H. Renal disease in the elderly. Med. Clin. N. Am. 1983, 67, 463–480. [Google Scholar]
- Davies, D.F.; Shock, N.W. Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J. Clin. Investig. 1950, 29, 496–507. [Google Scholar] [CrossRef]
- Thomas, S.E.; Anderson, S.; Gordon, K.L.; Oyama, T.T.; Shankland, S.J.; Johnson, R.J. Tubulointerstitial disease in aging: Evidence for underlying peritubular capillary damage, a potential role for renal ischemia. J. Am. Soc. Nephrol. 1998, 9, 231–242. [Google Scholar]
- Miya, M.; Maeshima, A.; Mishima, K.; Sakurai, N.; Ikeuchi, H.; Kuroiwa, T.; Hiromura, K.; Nojima, Y. Age-related decline in label-retaining tubular cells: Implication for reduced regenerative capacity after injury in the aging kidney. Am. J. Physiol. Ren. Physiol. 2012, 302, F694–F702. [Google Scholar] [CrossRef]
- Heyman, S.N.; Khamaisi, M.; Rosen, S.; Rosenberger, C. Renal parenchymal hypoxia, hypoxia response and the progression of chronic kidney disease. Am. J. Nephrol. 2008, 28, 998–1006. [Google Scholar] [CrossRef]
- Weinstein, J.R.; Anderson, S. The aging kidney: Physiological changes. Adv. Chronic. Kidney Dis. 2010, 17, 302–307. [Google Scholar] [CrossRef]
- Sabbatini, M.; Sansone, G.; Uccello, F.; de Nicola, L.; Giliberti, A.; Sepe, V.; Margri, P.; Conte, G.; Andreucci, V.E. Functional vs. structural changes in the pathophysiology of acute ischemic renal failure in aging rats. Kidney Int. 1994, 45, 1355–1361. [Google Scholar] [CrossRef]
- Long, D.A.; Newaz, M.A.; Prabhakar, S.S.; Price, K.L.; Truong, L.D.; Feng, L.; Mu, W.; Oyekan, A.O.; Johnson, R.J. Loss of nitric oxide and endothelial-derived hyperpolarizing factor-mediated responses in aging. Kidney Int. 2005, 68, 2154–2163. [Google Scholar] [CrossRef]
- Sabbatini, M.; Pisani, A.; Uccello, F.; Serio, V.; Seru, R.; Paterno, R.; Cianciaruso, B.; Fuiano, G.; Andreucci, M. Atorvastatin improves the course of ischemic acute renal failure in aging rats. J. Am. Soc. Nephrol. 2004, 15, 901–909. [Google Scholar] [CrossRef]
- Xiong, Y.; Yuan, L.W.; Deng, H.W.; Li, Y.J.; Chen, B.M. Elevated serum endogenous inhibitor of nitric oxide synthase and endothelial dysfunction in aged rats. Clin. Exp. Pharmacol. Physiol. 2001, 28, 842–847. [Google Scholar] [CrossRef]
- Kielstein, J.T.; Bode-Boger, S.M.; Frolich, J.C.; Ritz, E.; Haller, H.; Fliser, D. Asymmetric dimethylarginine, blood pressure, and renal perfusion in elderly subjects. Circulation 2003, 107, 1891–1895. [Google Scholar] [CrossRef]
- Valdivielso, J.; Reverte, M.; Rivas-Cabanero, L.; Lopez-Novoa, J. Increased severity of gentamicin nephrotoxicity in aging rats is mediated by a reduced glomerular nitric oxide production. Environ. Toxicol. Pharmacol. 1996, 2, 73–75. [Google Scholar] [CrossRef]
- Esposito, C.; Plati, A.; Mazzullo, T.; Fasoli, G.; de Mauri, A.; Grosjean, F.; Mangione, F.; Castoldi, F.; Serpieri, N.; Cornacchia, F.; et al. Renal function and functional reserve in healthy elderly individuals. J. Nephrol. 2007, 20, 617–625. [Google Scholar]
- Jerkic, M.; Vojvodic, S.; Lopez-Novoa, J.M. The mechanism of increased renal susceptibility to toxic substances in the elderly. Part i. The role of increased vasoconstriction. Int. Urol. Nephrol. 2001, 32, 539–547. [Google Scholar] [CrossRef]
- Hollenberg, N.K.; Adams, D.F.; Solomon, H.S.; Rashid, A.; Abrams, H.L.; Merrill, J.P. Senescence and the renal vasculature in normal man. Circ. Res. 1974, 34, 309–316. [Google Scholar] [CrossRef]
- Mulkerrin, E.C.; Brain, A.; Hampton, D.; Penney, M.D.; Sykes, D.A.; Williams, J.D.; Coles, G.A.; Woodhouse, K.W. Reduced renal hemodynamic response to atrial natriuretic peptide in elderly volunteers. Am. J. Kidney Dis. 1993, 22, 538–544. [Google Scholar] [CrossRef]
- Fuiano, G.; Sund, S.; Mazza, G.; Rosa, M.; Caglioti, A.; Gallo, G.; Natale, G.; Andreucci, M.; Memoli, B.; de Nicola, L.; et al. Renal hemodynamic response to maximal vasodilating stimulus in healthy older subjects. Kidney Int. 2001, 59, 1052–1058. [Google Scholar] [CrossRef]
- Campo, C.; Lahera, V.; Garcia-Robles, R.; Cachofeiro, V.; Alcazar, J.M.; Andres, A.; Rodicio, J.L.; Ruilope, L.M. Aging abolishes the renal response to l-arginine infusion in essential hypertension. Kidney Int. Suppl. 1996, 55, S126–S128. [Google Scholar]
- Hajduczok, G.; Chapleau, M.W.; Johnson, S.L.; Abboud, F.M. Increase in sympathetic activity with age. I. Role of impairment of arterial baroreflexes. Am. J. Physiol. 1991, 260, H1113–H1120. [Google Scholar]
- Zhang, X.Z.; Qiu, C.; Baylis, C. Sensitivity of the segmental renal arterioles to angiotensin ii in the aging rat. Mech. Ageing Dev. 1997, 97, 183–192. [Google Scholar] [CrossRef]
- Akcetin, Z.; Erdemli, G.; Bromme, H.J. Experimental study showing a diminished cytosolic antioxidative capacity in kidneys of aged rats. Urol. Int. 2000, 64, 70–73. [Google Scholar] [CrossRef]
- Kusaka, J.; Koga, H.; Hagiwara, S.; Hasegawa, A.; Kudo, K.; Noguchi, T. Age-dependent responses to renal ischemia-reperfusion injury. J. Surg. Res. 2012, 172, 153–158. [Google Scholar] [CrossRef]
- Shimizu, M.H.; Araujo, M.; Borges, S.M.; de Tolosa, E.M.; Seguro, A.C. Influence of age and vitamin e on post-ischemic acute renal failure. Exp. Gerontol. 2004, 39, 825–830. [Google Scholar] [CrossRef]
- Ferenbach, D.A.; Nkejabega, N.C.; McKay, J.; Choudhary, A.K.; Vernon, M.A.; Beesley, M.F.; Clay, S.; Conway, B.C.; Marson, L.P.; Kluth, D.C.; et al. The induction of macrophage hemeoxygenase-1 is protective during acute kidney injury in aging mice. Kidney Int. 2011, 79, 966–976. [Google Scholar] [CrossRef]
- Hasegawa, K.; Wakino, S.; Yoshioka, K.; Tatematsu, S.; Hara, Y.; Minakuchi, H.; Sueyasu, K.; Washida, N.; Tokuyama, H.; Tzukerman, M.; et al. Kidney-specific overexpression of sirt1 protects against acute kidney injury by retaining peroxisome function. J. Biol. Chem. 2010, 285, 13045–13056. [Google Scholar] [CrossRef]
- Kyle, M.E.; Kocsis, J.J. The effect of age on salicylate-induced nephrotoxicity in male rats. Toxicol. Appl. Pharmacol. 1985, 81, 337–347. [Google Scholar] [CrossRef]
- Qiao, X.; Chen, X.; Wu, D.; Ding, R.; Wang, J.; Hong, Q.; Shi, S.; Li, J.; Xie, Y.; Lu, Y.; et al. Mitochondrial pathway is responsible for aging-related increase of tubular cell apoptosis in renal ischemia/reperfusion injury. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2005, 60, 830–839. [Google Scholar] [CrossRef]
- Patiyal, S.N.; Katoch, S.S. Beta-adrenoceptor agonist clenbuterol down-regulates matrix metalloproteinase (mmp-9) and results in an impairment of collagen turnover in mice left ventricle. Jpn. J. Physiol. 2005, 55, 165–172. [Google Scholar] [CrossRef]
- Schmitt, R.; Cantley, L.G. The impact of aging on kidney repair. Am. J. Physiol. Ren. Physiol. 2008, 294, F1265–F1272. [Google Scholar] [CrossRef]
- Lee, J.H.; Jung, K.J.; Kim, J.W.; Kim, H.J.; Yu, B.P.; Chung, H.Y. Suppression of apoptosis by calorie restriction in aged kidney. Exp. Gerontol. 2004, 39, 1361–1368. [Google Scholar] [CrossRef]
- Ding, G.; Franki, N.; Kapasi, A.A.; Reddy, K.; Gibbons, N.; Singhal, P.C. Tubular cell senescence and expression of tgf-beta1 and p21(waf1/cip1) in tubulointerstitial fibrosis of aging rats. Exp. Mol. Pathol. 2001, 70, 43–53. [Google Scholar] [CrossRef]
- Lin, N.Y.; Beyer, C.; Giessl, A.; Kireva, T.; Scholtysek, C.; Uderhardt, S.; Munoz, L.E.; Dees, C.; Distler, A.; Wirtz, S.; et al. Autophagy regulates tnfalpha-mediated joint destruction in experimental arthritis. Ann. Rheum. Dis. 2013, 72, 761–768. [Google Scholar] [CrossRef]
- Howell, G.M.; Gomez, H.; Collage, R.D.; Loughran, P.; Zhang, X.; Escobar, D.A.; Billiar, T.R.; Zuckerbraun, B.S.; Rosengart, M.R. Augmenting autophagy to treat acute kidney injury during endotoxemia in mice. PLoS One 2013, 8, e69520. [Google Scholar] [CrossRef]
- Kimura, T.; Takabatake, Y.; Takahashi, A.; Kaimori, J.Y.; Matsui, I.; Namba, T.; Kitamura, H.; Niimura, F.; Matsusaka, T.; Soga, T.; et al. Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J. Am. Soc. Nephrol. 2011, 22, 902–913. [Google Scholar] [CrossRef]
- Cui, J.; Bai, X.Y.; Shi, S.; Cui, S.; Hong, Q.; Cai, G.; Chen, X. Age-related changes in the function of autophagy in rat kidneys. Age (Dordr) 2012, 34, 329–339. [Google Scholar] [CrossRef]
- Jiang, M.; Wei, Q.; Dong, G.; Komatsu, M.; Su, Y.; Dong, Z. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int. 2012, 82, 1271–1283. [Google Scholar] [CrossRef]
- Periyasamy-Thandavan, S.; Jiang, M.; Wei, Q.; Smith, R.; Yin, X.M.; Dong, Z. Autophagy is cytoprotective during cisplatin injury of renal proximal tubular cells. Kidney Int. 2008, 74, 631–640. [Google Scholar] [CrossRef]
- Mei, C.; Zheng, F. Chronic inflammation potentiates kidney aging. Semin. Nephrol. 2009, 29, 555–568. [Google Scholar] [CrossRef]
- Vlassara, H.; Torreggiani, M.; Post, J.B.; Zheng, F.; Uribarri, J.; Striker, G.E. Role of oxidants/inflammation in declining renal function in chronic kidney disease and normal aging. Kidney Int. Suppl. 2009, S3–S11. [Google Scholar] [CrossRef]
- Winsryg, M.D.; Arambel, M.J.; Kent, B.A.; Walters, J.L. Effect of sometribove on rumen fermentation, rate of passage, digestibility, and milk production responses in dairy cows. J. Dairy Sci. 1991, 74, 3518–3523. [Google Scholar] [CrossRef]
- Akcay, A.; Nguyen, Q.; Edelstein, C.L. Mediators of inflammation in acute kidney injury. Mediat. Inflamm. 2009, 2009, 137072. [Google Scholar]
- Kinsey, G.R.; Li, L.; Okusa, M.D. Inflammation in acute kidney injury. Nephron Exp. Nephrol. 2008, 109, e102–e107. [Google Scholar] [CrossRef]
- Melk, A.; Mansfield, E.S.; Hsieh, S.C.; Hernandez-Boussard, T.; Grimm, P.; Rayner, D.C.; Halloran, P.F.; Sarwal, M.M. Transcriptional analysis of the molecular basis of human kidney aging using cdna microarray profiling. Kidney Int. 2005, 68, 2667–2679. [Google Scholar] [CrossRef]
- Rodwell, G.E.; Sonu, R.; Zahn, J.M.; Lund, J.; Wilhelmy, J.; Wang, L.; Xiao, W.; Mindrinos, M.; Crane, E.; Segal, E.; et al. A transcriptional profile of aging in the human kidney. PLoS Biol. 2004, 2, e427. [Google Scholar] [CrossRef]
- Wielockx, B.; Libert, C.; Wilson, C. Matrilysin (matrix metalloproteinase-7): A new promising drug target in cancer and inflammation? Cytokine Growth Factor Rev. 2004, 15, 111–115. [Google Scholar] [CrossRef]
- Furuichi, K.; Kaneko, S.; Wada, T. Chemokine/chemokine receptor-mediated inflammation regulates pathologic changes from acute kidney injury to chronic kidney disease. Clin. Exp. Nephrol. 2009, 13, 9–14. [Google Scholar] [CrossRef]
- Izquierdo, M.C.; Sanz, A.B.; Sanchez-Nino, M.D.; Perez-Gomez, M.V.; Ruiz-Ortega, M.; Poveda, J.; Ruiz-Andres, O.; Ramos, A.M.; Moreno, J.A.; Egido, J.; et al. Acute kidney injury transcriptomics unveils a relationship between inflammation and ageing. Nefrologia 2012, 32, 715–723. [Google Scholar]
- Schmitt, R.; Marlier, A.; Cantley, L.G. Zag expression during aging suppresses proliferation after kidney injury. J. Am. Soc. Nephrol. 2008, 19, 2375–2383. [Google Scholar] [CrossRef]
- Melk, A. Senescence of renal cells: Molecular basis and clinical implications. Nephrol. Dial. Transplant. 2003, 18, 2474–2478. [Google Scholar] [CrossRef]
- Melk, A.; Schmidt, B.M.; Vongwiwatana, A.; Rayner, D.C.; Halloran, P.F. Increased expression of senescence-associated cell cycle inhibitor p16ink4a in deteriorating renal transplants and diseased native kidney. Am. J. Transplant. 2005, 5, 1375–1382. [Google Scholar] [CrossRef]
- Braun, H.; Schmidt, B.M.; Raiss, M.; Baisantry, A.; Mircea-Constantin, D.; Wang, S.; Gross, M.L.; Serrano, M.; Schmitt, R.; Melk, A. Cellular senescence limits regenerative capacity and allograft survival. J. Am. Soc. Nephrol. 2012, 23, 1467–1473. [Google Scholar] [CrossRef]
- Suzuki, A.; Sakaguchi, T.; Inaba, K.; Suzuki, S.; Konno, H. Impact of cell cycle disruption on impaired hepatic regeneration in aged livers with ischemic insult. J. Surg. Res. 2012, 173, 267–277. [Google Scholar] [CrossRef]
- Leontieva, O.V.; Lenzo, F.; Demidenko, Z.N.; Blagosklonny, M.V. Hyper-mitogenic drive coexists with mitotic incompetence in senescent cells. Cell Cycle 2012, 11, 4642–4649. [Google Scholar] [CrossRef]
- Melk, A.; Ramassar, V.; Helms, L.M.; Moore, R.; Rayner, D.; Solez, K.; Halloran, P.F. Telomere shortening in kidneys with age. J. Am. Soc. Nephrol. 2000, 11, 444–453. [Google Scholar]
- Melk, A.; Halloran, P.F. Cell senescence and its implications for nephrology. J. Am. Soc. Nephrol. 2001, 12, 385–393. [Google Scholar]
- Karihaloo, A.; Nickel, C.; Cantley, L.G. Signals which build a tubule. Nephron Exp. Nephrol. 2005, 100, e40–e45. [Google Scholar] [CrossRef]
- Chou, J.S.; Reiser, I.W.; Porush, J.G. Aging and urinary excretion of epidermal growth factor. Ann. Clin. Lab. Sci. 1997, 27, 116–122. [Google Scholar]
- Kang, D.H.; Anderson, S.; Kim, Y.G.; Mazzalli, M.; Suga, S.; Jefferson, J.A.; Gordon, K.L.; Oyama, T.T.; Hughes, J.; Hugo, C.; et al. Impaired angiogenesis in the aging kidney: Vascular endothelial growth factor and thrombospondin-1 in renal disease. Am. J. Kidney Dis. 2001, 37, 601–611. [Google Scholar] [CrossRef]
- Shurin, G.V.; Yurkovetsky, Z.R.; Chatta, G.S.; Tourkova, I.L.; Shurin, M.R.; Lokshin, A.E. Dynamic alteration of soluble serum biomarkers in healthy aging. Cytokine 2007, 39, 123–129. [Google Scholar] [CrossRef]
- Li, Z.; Chen, X.; Xie, Y.; Shi, S.; Feng, Z.; Fu, B.; Zhang, X.; Cai, G.; Wu, C.; Wu, D.; et al. Expression and significance of integrin-linked kinase in cultured cells, normal tissue, and diseased tissue of aging rat kidneys. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 984–996. [Google Scholar] [CrossRef]
- Thakar, C.V.; Zahedi, K.; Revelo, M.P.; Wang, Z.; Burnham, C.E.; Barone, S.; Bevans, S.; Lentsch, A.B.; Rabb, H.; Soleimani, M. Identification of thrombospondin 1 (tsp-1) as a novel mediator of cell injury in kidney ischemia. J. Clin. Investig. 2005, 115, 3451–3459. [Google Scholar] [CrossRef]
- Nichols, L.A.; Slusarz, A.; Grunz-Borgmann, E.A.; Parrish, A.R. Alpha(e)-catenin regulates bmp-7 expression and migration in renal epithelial cells. Am. J. Nephrol. 2014, 39, 409–417. [Google Scholar] [CrossRef]
- Nichols, L.A.; Grunz-Borgmann, E.A.; Wang, X.; Parrish, A.R. A role for the age-dependent loss of alpha(e)-catenin in regulation of n-cadherin expression and cell migration. Physiol. Rep. 2014, 2. [Google Scholar] [CrossRef]
- Yang, L.; Humphreys, B.D.; Bonventre, J.V. Pathophysiology of acute kidney injury to chronic kidney disease: Maladaptive repair. Contrib. Nephrol. 2011, 174, 149–155. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, X.; Bonventre, J.V.; Parrish, A.R. The Aging Kidney: Increased Susceptibility to Nephrotoxicity. Int. J. Mol. Sci. 2014, 15, 15358-15376. https://doi.org/10.3390/ijms150915358
Wang X, Bonventre JV, Parrish AR. The Aging Kidney: Increased Susceptibility to Nephrotoxicity. International Journal of Molecular Sciences. 2014; 15(9):15358-15376. https://doi.org/10.3390/ijms150915358
Chicago/Turabian StyleWang, Xinhui, Joseph V. Bonventre, and Alan R. Parrish. 2014. "The Aging Kidney: Increased Susceptibility to Nephrotoxicity" International Journal of Molecular Sciences 15, no. 9: 15358-15376. https://doi.org/10.3390/ijms150915358



