Phylogenetic and Transcription Analysis of Chrysanthemum WRKY Transcription Factors
Abstract
:1. Introduction
2. Results and Discussion
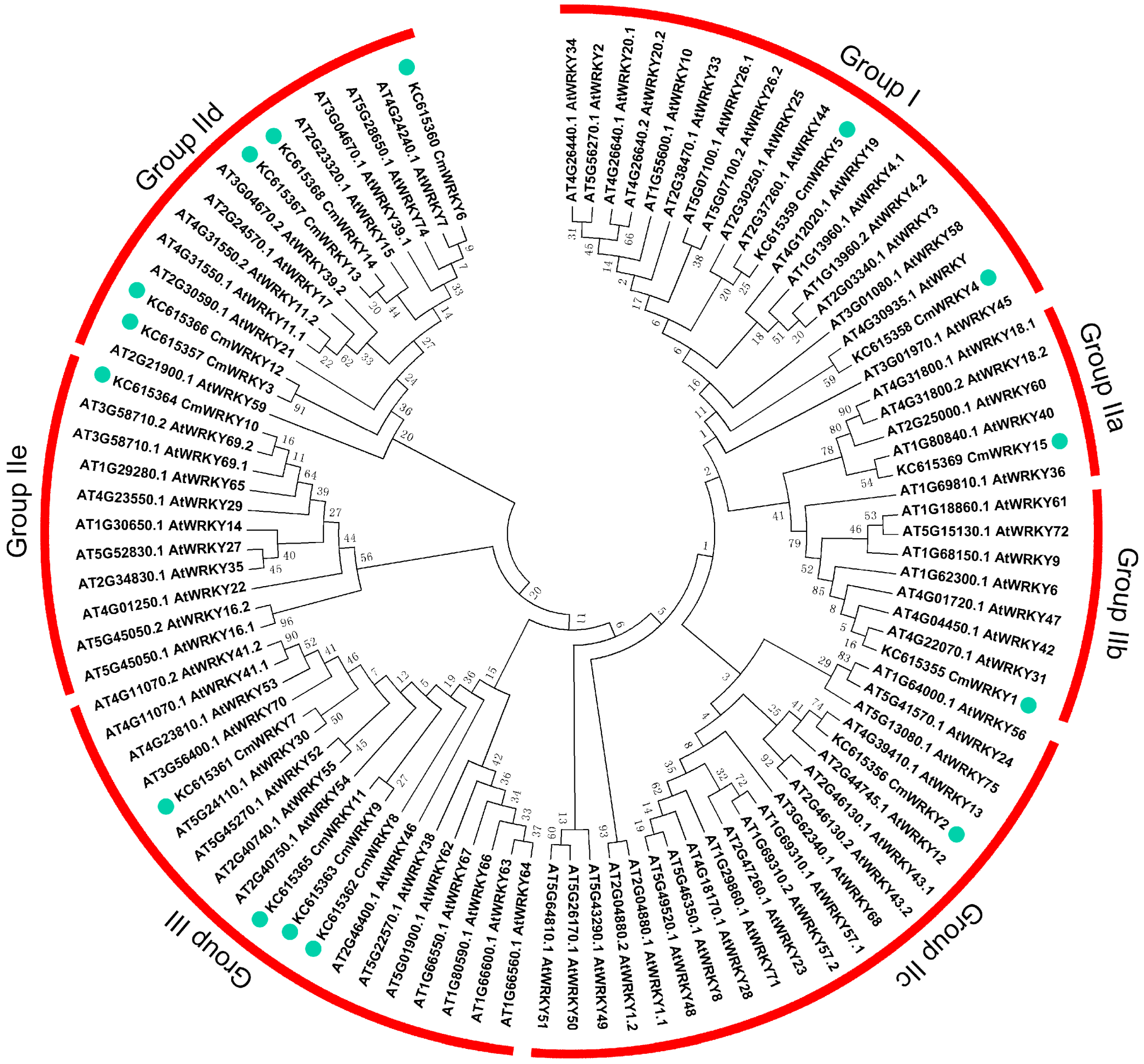
2.1. The WRKY Gene Content of Chrysanthemum
| Gene | GenBank Accession No. | cDNA Length (bp) | Amino Acids Length (aa) | AtWRKY Orthologs | Locus Name | E-Value |
|---|---|---|---|---|---|---|
| CmWRKY1 | KC615355 | 1750 | 504 | AtWRKY6 | AT1G62300 | 5e-86 |
| CmWRKY2 | KC615356 | 823 | 200 | AtWRKY13 | AT4G39410 | 2e-47 |
| CmWRKY3 | KC615357 | 928 | 248 | AtWRKY11 | AT4G31550 | 3e-38 |
| CmWRKY4 | KC615358 | 1608 | 447 | AtWRKY32 | AT4G30935 | 3e-68 |
| CmWRKY5 | KC615359 | 1668 | 410 | AtWRKY44 | AT2G37260 | 5e-74 |
| CmWRKY6 | KC615360 | 1119 | 232 | AtWRKY21 | AT2G30590 | 2e-56 |
| CmWRKY7 | KC615361 | 757 | 193 | AtWRKY41 | AT4G11070 | 2e-31 |
| CmWRKY8 | KC615362 | 1019 | 247 | AtWRKY41 | AT4G11070 | 5e-30 |
| CmWRKY9 | KC615363 | 1331 | 314 | AtWRKY46 | AT2G46400 | 8e-37 |
| CmWRKY10 | KC615364 | 1216 | 287 | AtWRKY65 | AT1G29280 | 3e-49 |
| CmWRKY11 | KC615365 | 1117 | 268 | AtWRKY70 | AT3G56400 | 6e-31 |
| CmWRKY12 | KC615366 | 875 | 229 | AtWRKY17 | AT2G24570 | 3e-24 |
| CmWRKY13 | KC615367 | 936 | 311 | AtWRKY7 | AT4G24240 | 6e-65 |
| CmWRKY14 | KC615368 | 942 | 313 | AtWRKY7 | AT4G24240 | 1e-53 |
| CmWRKY15 | KC615369 | 941 | 268 | AtWRKY40 | AT1G80840 | 1e-43 |
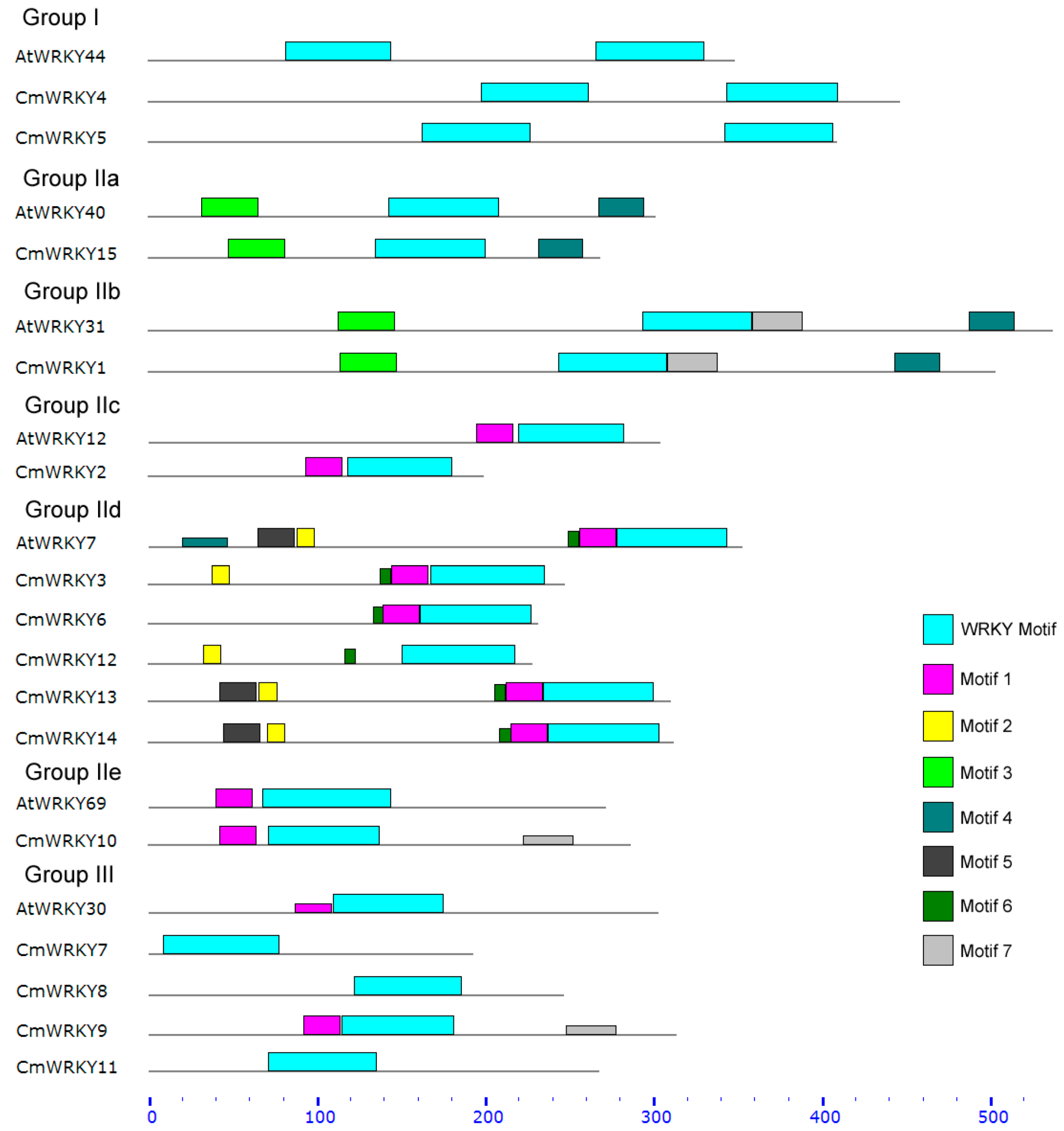
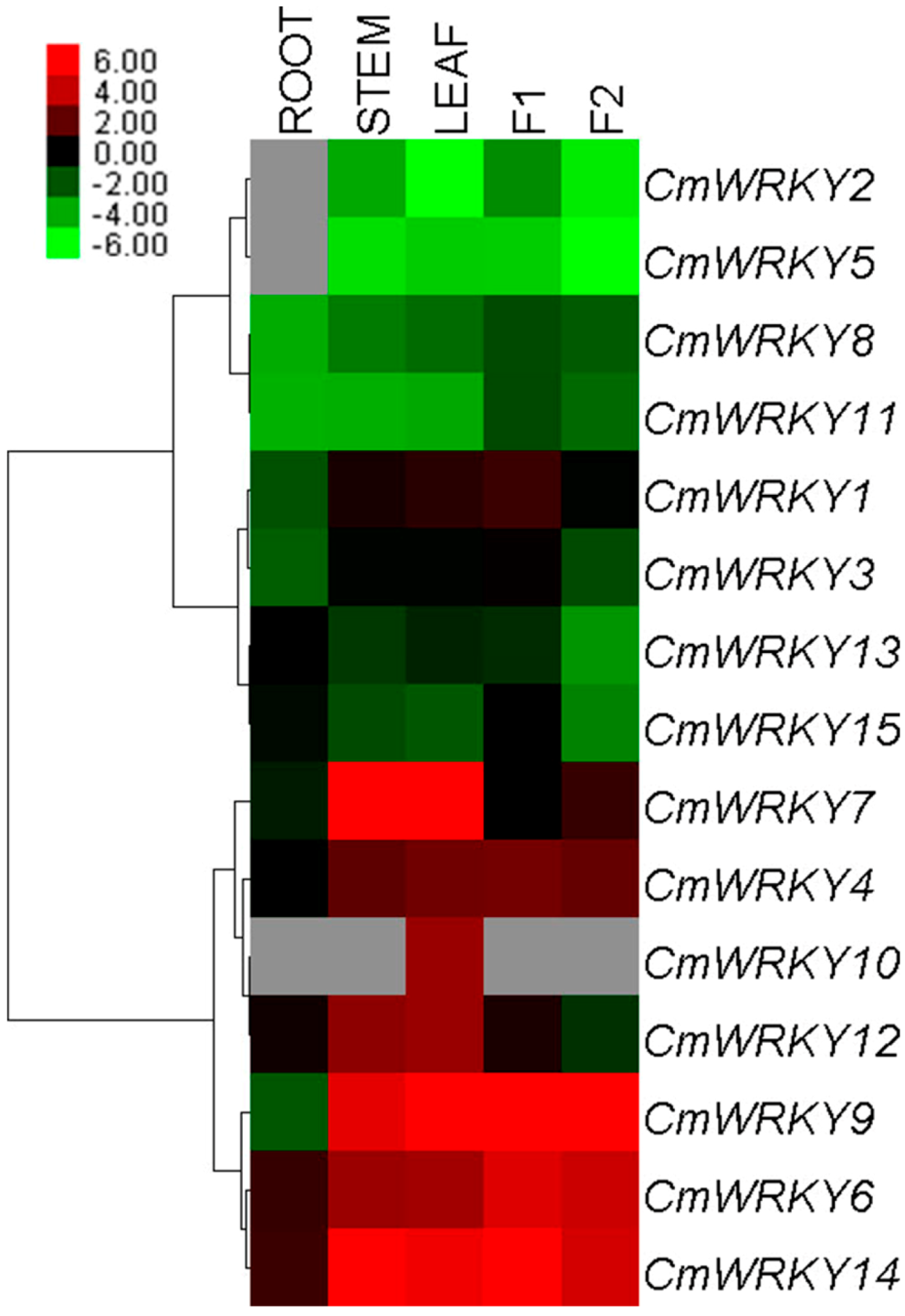
2.2. Transcription Profiling of CmWRKY Genes
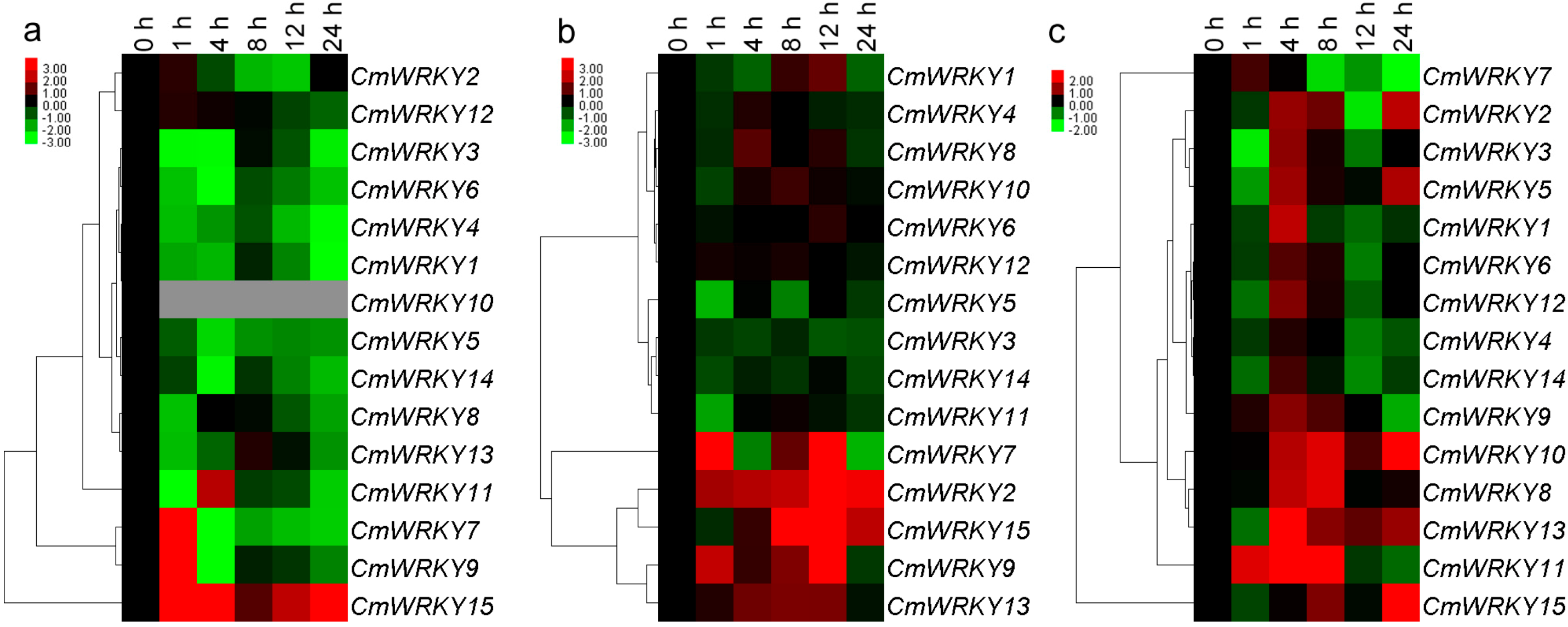
2.3. The Transcription of CmWRKY Genes in Plants Challenged by Phytohormones and Abiotic Stress
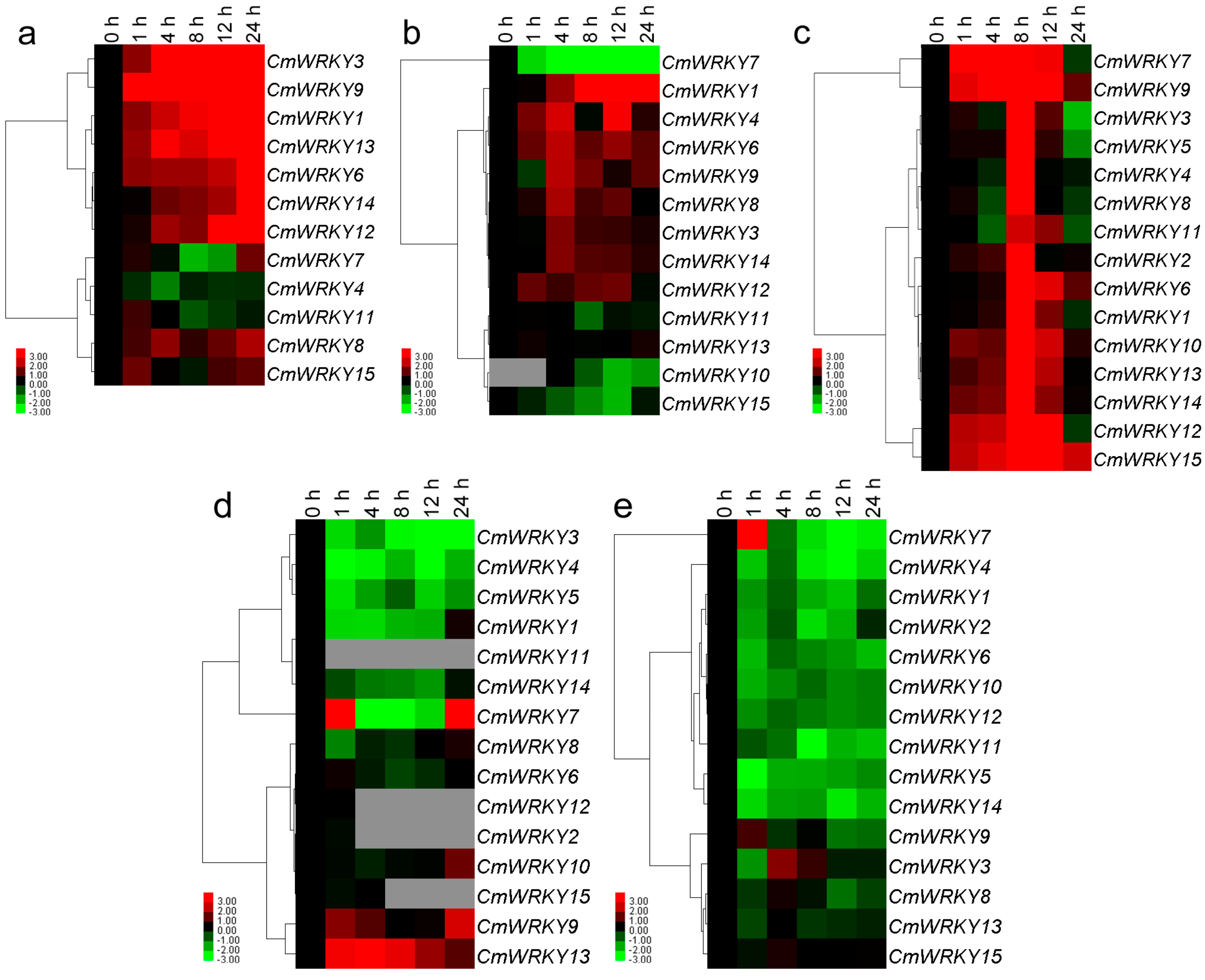
2.4. Differential Responses of the CmWRKY Genes to Biotic Stress
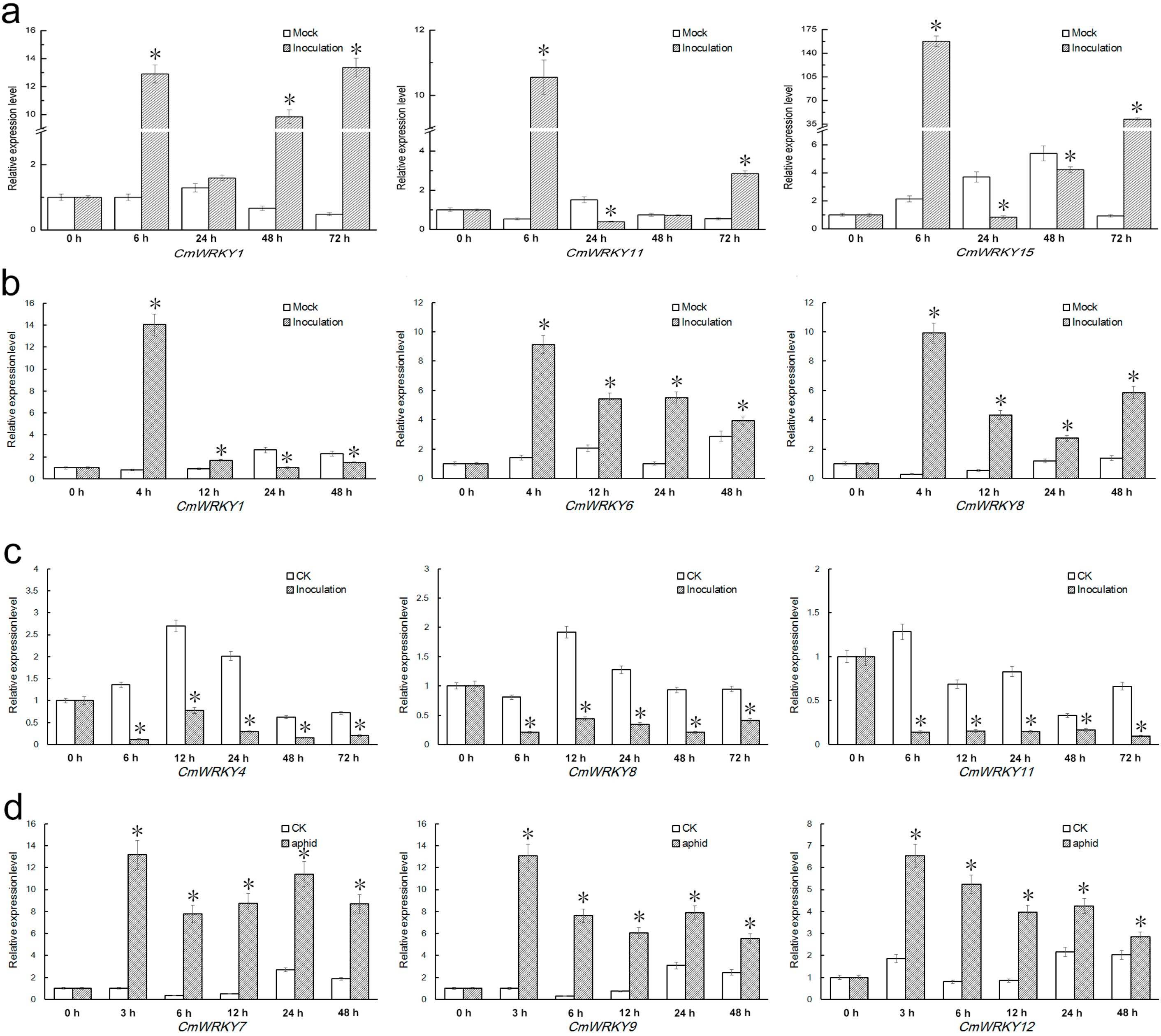
3. Experimental Section
3.1. Plant Materials
3.2. Isolation and Sequencing of Full-Length CmWRKY cDNAs
3.3. Phylogeny of WRKY Sequences
3.4. Plant Treatments
3.5. Real-Time Quantitative PCR (qPCR)
3.6. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Supplementary Files
Supplementary File 1References
- Shinoyama, H.; Aida, R.; Ichikawa, H.; Nomura, Y.; Mochizuki, A. Genetic engineering of chrysanthemum (Chrysanthemum morifolium): Current progress and perspectives. Plant Biotechnol. 2012, 29, 323–337. [Google Scholar]
- Teixeira da Silva, J.A.; Shinoyama, H.; Aida, R.; Matsushita, Y.; Raj, S.K.; Chen, F. Chrysanthemum biotechnology: Quo vadis? Crit. Rev. Plant Sci. 2012, 32, 21–52. [Google Scholar]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY transcription factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar]
- Ishiguro, S.; Nakamura, K. Characterization of a cDNA encoding a novel DNA-binding protein, SPF1, that recognizes SP8 sequences in the 5' upstream regions of genes coding for sporamin and beta-amylase from sweet potato. Mol. Gen. Genet. 1994, 244, 563–571. [Google Scholar]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar]
- Luo, M.; Dennis, E.S.; Berger, F.; Peacock, W.J.; Chaudhury, A. MINISEED3 (MINI3), a WRKY family gene, and HAIKU2 (IKU2), a leucine-rich repeat (LRR) KINASE gene, are regulators of seed size in Arabidopsis. Proc. Natl. Acad. Sci. USA 2005, 102, 17531–17536. [Google Scholar] [CrossRef]
- Pnueli, L.; Hallak-Herr, E.; Rozenberg, M.; Cohen, M.; Goloubinoff, P.; Kaplan, A.; Mittler, R. Molecular and biochemical mechanisms associated with dormancy and drought tolerance in the desert legume Retama raetam. Plant J. 2002, 31, 319–330. [Google Scholar] [CrossRef]
- Johnson, C.S.; Kolevski, B.; Smyth, D.R. TRANSPARENT TESTA GLABRA2, a trichome and seed coat development gene of Arabidopsis, encodes a WRKY transcription factor. Plant Cell 2002, 14, 1359–1375. [Google Scholar] [CrossRef]
- Robatzek, S.; Somssich, I.E. Targets of AtWRKY6 regulation during plant senescence and pathogen defense. Genes Dev. 2002, 16, 1139–1149. [Google Scholar]
- Seki, M.; Narusaka, M.; Ishida, J.; Nanjo, T.; Fujita, M.; Oono, Y.; Kamiya, A.; Nakajima, M.; Enju, A.; Sakurai, T.; et al. Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray. Plant J. 2002, 31, 279–292. [Google Scholar] [CrossRef]
- Kim, K.C.; Lai, Z.; Fan, B.; Chen, Z. Arabidopsis WRKY38 and WRKY62 transcription factors interact with histone deacetylase 19 in basal defense. Plant Cell 2008, 20, 2357–2371. [Google Scholar]
- Mukhtar, M.S.; Deslandes, L.; Auriac, M.C.; Marco, Y.; Somssich, I.E. The Arabidopsis transcription factor WRKY27 influences wilt disease symptom development caused by Ralstonia solanacearum. Plant J. 2008, 56, 935–947. [Google Scholar] [CrossRef]
- Lai, Z.; Vinod, K.; Zheng, Z.; Fan, B.; Chen, Z. Roles of Arabidopsis WRKY3 and WRKY4 transcription factors in plant responses to pathogens. BMC Plant Biol. 2008, 8, 1471–2229. [Google Scholar]
- Higashi, K.; Ishiga, Y.; Inagaki, Y.; Toyoda, K.; Shiraishi, T.; Ichinose, Y. Modulation of defense signal transduction by flagellin-induced WRKY41 transcription factor in Arabidopsis thaliana. Mol. Genet. Genomics 2008, 279, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Pandey, S.P.; Roccaro, M.; Schon, M.; Logemann, E.; Somssich, I.E. Transcriptional reprogrammingregulated by WRKY18 and WRKY40 facilitates powdery mildew infection of Arabidopsis. Plant J. 2010, 64, 912–923. [Google Scholar]
- Bhattarai, K.K.; Atamian, H.S.; Kaloshian, I.; Eulgem, T. WRKY72-type transcription factors contribute to basal immunity in tomato and Arabidopsis as well as gene-for-gene resistance mediated by the tomato R gene Mi-1. Plant J. 2010, 63, 229–240. [Google Scholar]
- Shim, J.S.; Jung, C.; Lee, S.; Min, K.; Lee, Y.W.; Choi, Y.; Lee, J.S.; Song, J.T.; Kim, J.K.; Choi, Y.D. AtMYB44 regulates WRKY70 expression and modulates antagonistic interaction between salicylic acid and jasmonic acid signaling. Plant J. 2013, 73, 483–495. [Google Scholar]
- Gao, Q.-M.; Venugopal, S.; Navarre, D.; Kachroo, A. Low oleic acid-derived repression of jasmonic acid-inducible defense responses requires the WRKY50 and WRKY51 proteins. Plant Physiol. 2011, 155, 464–476. [Google Scholar]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, pp. W202–W208. Available online: http://meme.nbcr.net/meme/intro.html (accessed on 12 March 2013).
- Chen, F.; Mackey, A.J.; Vermunt, J.K.; Roos, D.S. Assessing performance of orthology detection strategies applied to eukaryotic genomes. PLoS One 2007, 2, e383. [Google Scholar]
- Huang, S.; Gao, Y.; Liu, J.; Peng, X.; Niu, X.; Fei, Z.; Cao, S.; Liu, Y. Genome-wide analysis of WRKY transcription factors in Solanum lycopersicum. Mol. Genet. Genomics 2012, 287, 495–513. [Google Scholar]
- Robatzek, S.; Somssich, I.E. A new member of the Arabidopsis WRKY transcription factor family, AtWRKY6, is associated with both senescence- and defence- related processes. Plant J. 2001, 28, 123–133. [Google Scholar] [CrossRef]
- Xu, X.; Chen, C.; Fan, B.; Chen, Z. Physical and functional interactions between pathogen-induced Arabidopsis WRKY18, WRKY40, and WRKY60 transcription factors. Plant Cell 2006, 18, 1310–1326. [Google Scholar] [CrossRef]
- Knoth, C.; Ringler, J.; Dangl, J.L.; Eulgem, T. Arabidopsis WRKY70 is required for full RPP4-mediated disease resistance and basal defense against Hyaloperonospora parasitica. Mol. Plant Microbe Ineract. 2007, 20, 120–128. [Google Scholar]
- Jiang, Y.; Deyholos, M. Comprehensive transcriptional profiling of NaCl-stressed Arabidopsis roots reveals novel classes of responsive genes. BMC Plant Biol. 2006, 6, 25. [Google Scholar] [CrossRef]
- Chen, W.; Provart, N.J.; Glazebrook, J.; Katagiri, F.; Chang, H.-S.; Eulgem, T.; Mauch, F.; Luan, S.; Zou, G.; Whitham, S.A.; et al. Expression profile matrix of Arabidopsis transcription factor genes suggests their putative functions in response to environmental stresses. Plant Cell 2002, 14, 559–574. [Google Scholar]
- Lee, B.-H.; Henderson, D.A.; Zhu, J.-K. The Arabidopsis cold-responsive transcriptome and its regulation by ICE1. Plant Cell 2005, 17, 3155–3175. [Google Scholar]
- Taki, N.; Sasaki-Sekimoto, Y.; Obayashi, T.; Kikuta, A.; Kobayashi, K.; Ainai, T.; Yagi, K.; Sakurai, N.; Suzuki, H.; Masuda, T.; et al. 12-Oxo-Phytodienoic acid triggers expression of a distinct set of genes and plays a role in wound-induced gene expression in Arabidopsis. Plant Physiol. 2005, 139, 1268–1283. [Google Scholar] [CrossRef]
- Li, J.; Brader, G.; Palva, E.T. The WRKY70 transcription factor: A node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell 2004, 16, 319–331. [Google Scholar]
- Journot-Catalino, N.; Somssich, I.E.; Roby, D.; Kroj, T. The transcription factors WRKY11 and WRKY17 act as negative regulators of basal resistance in Arabidopsis thaliana. Plant Cell 2006, 18, 3289–3302. [Google Scholar]
- Li, J.; Brader, G.; Kariola, T.; Tapio Palva, E. WRKY70 modulates the selection of signaling pathways in plant defense. Plant J. 2006, 46, 477–491. [Google Scholar]
- Shang, Y.; Yan, L.; Liu, Z.Q.; Cao, Z.; Mei, C.; Xin, Q.; Wu, F.Q.; Wang, X.F.; Du, S.Y.; Jiang, T.; et al. The Mg-Chelatase H Subunit of Arabidopsis Antagonizes a Group of WRKY Transcription Repressors to Relieve ABA-Responsive Genes of Inhibition. Plant Cell 2010, 22, 1909–1935. [Google Scholar] [CrossRef]
- Guo, A.; He, K.; Liu, D.; Bai, S.; Gu, X.; Wei, L.; Luo, J. DATF: A database of Arabidopsis transcription factors. Bioinformatics. 2005, 21, pp. 2568–2569. Available online: http://datf.cbi.pku.edu.cn/ (accessed on 22 January 2013).
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Song, A.; Lu, J.; Jiang, J.; Chen, S.; Guan, Z.; Fang, W.; Chen, F. Isolation and characterisation of Chrysanthemum crassum SOS1, encoding a putative plasma membrane Na+/H+ antiporter. Plant Biol. (Stuttg.) 2012, 14, 706–713. [Google Scholar]
- Song, A.; Zhu, X.; Chen, F.; Gao, H.; Jiang, J.; Chen, S. A chrysanthemum heat shock protein confers tolerance to abiotic stress. Int. J. Mol. Sci. 2014, 15, 5063–5078. [Google Scholar]
- Ricachenevsky, F.K.; Sperotto, R.A.; Menguer, P.K.; Fett, J.P. Identification of Fe-excess-inducedgenes in rice shoots reveals a WRKY transcription factor responsive to Fe, drought and senescence. Mol. Biol. Rep. 2010, 37, 3735–3745. [Google Scholar]
- War, A.R.; Hussain, B.; Sharma, H.C. Induced resistance in groundnut by jasmonic acid and salicylic acid through alteration of trichome density and oviposition by Helicoverpa armigera (Lepidoptera: Noctuidae). AoB Plants 2013, 5, plt053. [Google Scholar] [CrossRef]
- Alaey, M.; Babalar, M.; Naderi, R.; Kafi, M. Effect of pre-and postharvest salicylic acid treatment on physio-chemical attributes in relation to vase-life of rose cut flowers. Postharvest Biol. Technol. 2011, 61, 91–94. [Google Scholar]
- Song, A.; Zhao, S.; Chen, S.; Jiang, J.; Chen, S.; Li, H.; Chen, Y.; Chen, X.; Fang, W.; Chen, F. The abundance and diversity of soil fungi in continuously monocropped chrysanthemum. Sci. World J. 2013, 2013, 632920. [Google Scholar] [CrossRef]
- Li, H.; Chen, S.; Song, A.; Wang, H.; Fang, W.; Guan, Z.; Jiang, J.; Chen, F. RNA-Seq derived identification of differential transcription in the chrysanthemum leaf following inoculation with Alternaria tenuissima. BMC Genomics 2014, 15, 9. [Google Scholar] [CrossRef]
- Zandvoort, R.; Groenewegen, C.A.M.; Zadoks, J.C. Methods for the inoculation of Chrysanthemum morifolium with Puccinia horiana. Neth. J. Plant Pathol. 1968, 74, 174–176. [Google Scholar]
- He, J.; Chen, F.; Chen, S.; Lv, G.; Deng, Y.; Fang, W.; Liu, Z.; Guan, Z.; He, C. Chrysanthemum leaf epidermal surface morphology and antioxidant and defense enzyme activity in response to aphid infestation. J. Plant Physiol. 2011, 168, 687–693. [Google Scholar]
- Rozen, S.; Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000, 132, 365–386. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar]
- De Hoon, M.J.; Imoto, S.; Nolan, J.; Miyano, S. Open source clustering software. Bioinformatics 2004, 20, 1453–1454. [Google Scholar]
- Eisen, M.B.; Spellman, P.T.; Brown, P.O.; Botstein, D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 1998, 95, 14863–14868. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Song, A.; Li, P.; Jiang, J.; Chen, S.; Li, H.; Zeng, J.; Shao, Y.; Zhu, L.; Zhang, Z.; Chen, F. Phylogenetic and Transcription Analysis of Chrysanthemum WRKY Transcription Factors. Int. J. Mol. Sci. 2014, 15, 14442-14455. https://doi.org/10.3390/ijms150814442
Song A, Li P, Jiang J, Chen S, Li H, Zeng J, Shao Y, Zhu L, Zhang Z, Chen F. Phylogenetic and Transcription Analysis of Chrysanthemum WRKY Transcription Factors. International Journal of Molecular Sciences. 2014; 15(8):14442-14455. https://doi.org/10.3390/ijms150814442
Chicago/Turabian StyleSong, Aiping, Peiling Li, Jiafu Jiang, Sumei Chen, Huiyun Li, Jun Zeng, Yafeng Shao, Lu Zhu, Zhaohe Zhang, and Fadi Chen. 2014. "Phylogenetic and Transcription Analysis of Chrysanthemum WRKY Transcription Factors" International Journal of Molecular Sciences 15, no. 8: 14442-14455. https://doi.org/10.3390/ijms150814442




