Lutein Inhibits the Migration of Retinal Pigment Epithelial Cells via Cytosolic and Mitochondrial Akt Pathways (Lutein Inhibits RPE Cells Migration)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Lutein Can Inhibit PDGF-Induced Migration of RPE Cells via an Akt Pathway
2.2. Ad-Mito-Akt Can Transduce RPE Successfully with Constitutively Activated Mitochondrial Akt
2.3. Overexpressed Mitochondrial Akt Can Increase Migration of RPE Cells. Lutein Can Successfully Inhibit Migration of Rpe Cells with or without Activation of Mitochondrial Akt
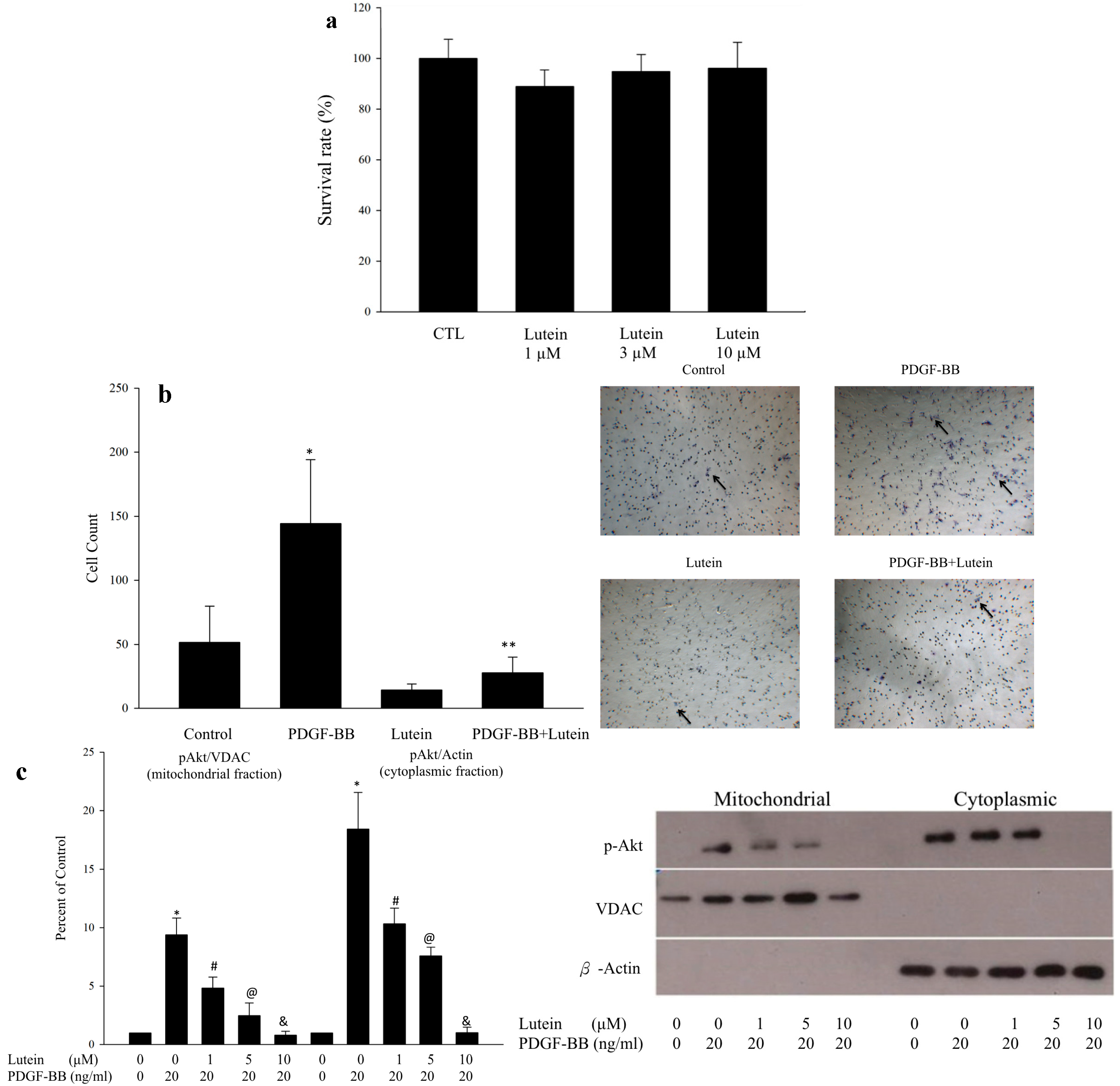
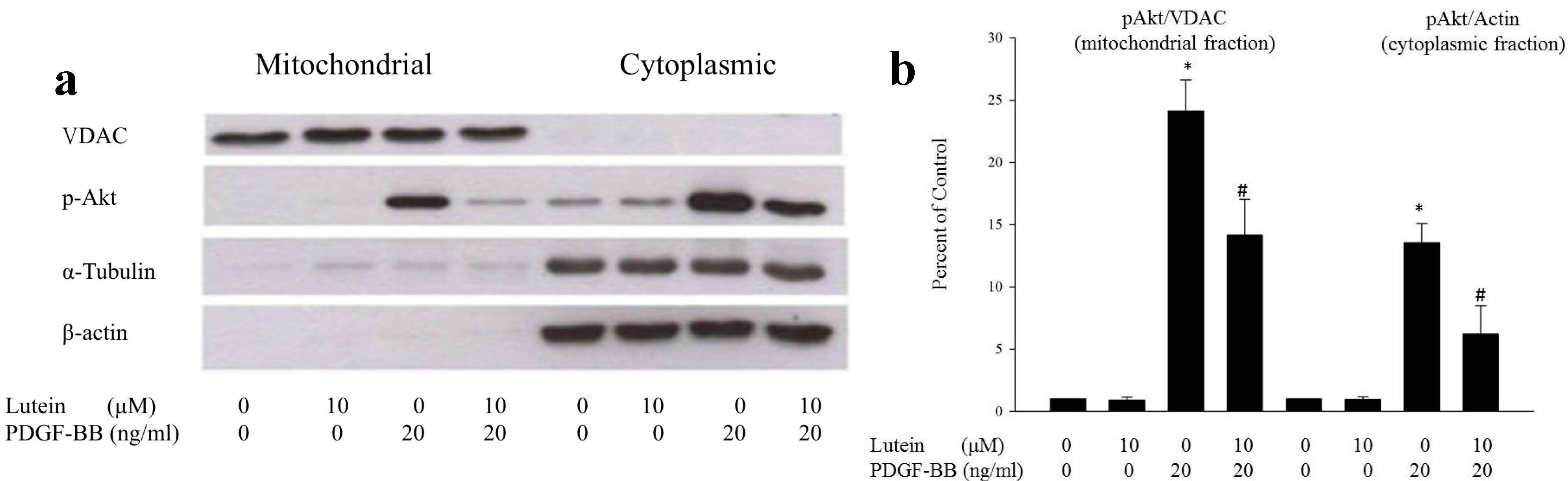

2.4. Discussion
3. Experimental Section
Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ryan, S.J. The pathophysiology of proliferative vitreoretinopathy in its management. Am. J. Ophthalmol. 1985, 100, 188–193. [Google Scholar]
- Simo, R.; Villarroel, M.; Corraliza, L.; Hernandez, C.; Garcia-Ramirez, M. The retinal pigment epithelium: Something more than a constituent of the blood-retinal barrier—implications for the pathogenesis of diabetic retinopathy. J. Biomed. Biotechnol. 2010. [Google Scholar] [CrossRef]
- Pastor, J.C. Proliferative vitreoretinopathy: An overview. Surv. Ophthalmol. 1998, 43, 3–18. [Google Scholar] [CrossRef]
- Andrews, A.; Balciunaite, E.; Leong, F.L.; Tallquist, M.; Soriano, P.; Refojo, M.; Kazlauskas, A. Platelet-derived growth factor plays a key role in proliferative vitreoretinopathy. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2683–2689. [Google Scholar]
- Wiedemann, P. Growth factors in retinal diseases: proliferative vitreoretinopathy, proliferative diabetic retinopathy, and retinal degeneration. Surv. Ophthalmol. 1992, 36, 373–384. [Google Scholar] [CrossRef]
- Robbins, S.G.; Mixon, R.N.; Wilson, D.J.; Hart, C.E.; Robertson, J.E.; Westra, I.; Planck, S.R.; Rosenbaum, J.T. Platelet-derived growth factor ligands and receptors immunolocalized in proliferative retinal diseases. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3649–3663. [Google Scholar]
- Ikuno, Y.; Leong, F.L.; Kazlauskas, A. PI3K and PLCgamma play a central role in experimental PVR. Investig. Ophthalmol. Vis. Sci. 2002, 43, 483–489. [Google Scholar]
- Lei, H.; Velez, G.; Kazlauskas, A. Pathological signaling via platelet-derived growth factor receptor {alpha} involves chronic activation of Akt and suppression of p53. Mol. Cell. Biol. 2011, 31, 1788–1799. [Google Scholar] [CrossRef]
- Hollborn, M.; Bringmann, A.; Faude, F.; Wiedemann, P.; Kohen, L. Signaling pathways involved in PDGF-evoked cellular responses in human RPE cells. Biochem. Biophys. Res. Commun. 2006, 344, 912–919. [Google Scholar]
- Campochiaro, P.A. Pathogenic mechanisms in proliferative vitreoretinopathy. Arch. Ophthalmol. 1997, 115, 237–241. [Google Scholar] [CrossRef]
- Yang, J.Y.; Yeh, H.Y.; Lin, K.; Wang, P.H. Insulin stimulates Akt translocation to mitochondria: implications on dysregulation of mitochondrial oxidative phosphorylation in diabetic myocardium. J. Mol. Cell. Cardiol. 2009, 46, 919–926. [Google Scholar] [CrossRef]
- Khoo, H.E.; Prasad, K.N.; Kong, K.W.; Jiang, Y.; Ismail, A. Carotenoids and their isomers: Color pigments in fruits and vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar]
- Sies, H.; Stahl, W.; Sundquist, A.R. Antioxidant functions of vitamins. Vitamins E and C, beta-carotene, and other carotenoids. Ann. N. Y. Acad. Sci. 1992, 669, 7–20. [Google Scholar] [CrossRef]
- Chichili, G.R.; Nohr, D.; Schaffer, M.; von Lintig, J.; Biesalski, H.K. beta-Carotene conversion into vitamin A in human retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3562–3569. [Google Scholar] [CrossRef]
- Verstraeten, T.; Hartzer, M.; Wilcox, D.K.; Cheng, M. Effects of vitamin A on retinal pigment epithelial cells in vitro. Investig. Ophthalmol. Vis. Sci. 1992, 33, 2830–2838. [Google Scholar]
- Chan, C.M.; Fang, J.Y.; Lin, H.H.; Yang, C.Y.; Hung, C.F. Lycopene inhibits PDGF-BB-induced retinal pigment epithelial cell migration by suppression of PI3K/Akt and MAPK pathways. Biochem. Biophys. Res. Commun. 2009, 388, 172–176. [Google Scholar] [CrossRef]
- Kim, J.H.; Na, H.J.; Kim, C.K.; Kim, J.Y.; Ha, K.S.; Lee, H.; Chung, H.T.; Kwon, H.J.; Kwon, Y.G.; Kim, Y.M. The non-provitamin A carotenoid, lutein, inhibits NF-kappaB-dependent gene expression through redox-based regulation of the phosphatidylinositol 3-kinase/PTEN/Akt and NF-kappaB-inducing kinase pathways: role of H(2)O(2) in NF-kappaB activation. Free Radic. Biol. Med. 2008, 45, 885–896. [Google Scholar]
- Ahmad, N.; Wang, Y.; Haider, K.H.; Wang, B.; Pasha, Z.; Uzun, O.; Ashraf, M. Cardiac protection by mitoKATP channels is dependent on Akt translocation from cytosol to mitochondria during late preconditioning. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H2402–H2408. [Google Scholar]
- Su, C.C.; Yang, J.Y.; Leu, H.B.; Chen, Y.; Wang, P.H. Mitochondrial Akt-regulated mitochondrial apoptosis signaling in cardiac muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H716–H723. [Google Scholar]
- Esser, P.; Heimann, K.; Bartz-schmidt, K.U.; Fontana, A.; Schraermeyer, U.; Thumann, G.; Weller, M. Apoptosis in proliferative vitreoretinal disorders: possible involvement of TGF-beta-induced RPE cell apoptosis. Exp. Eye Res. 1997, 65, 365–378. [Google Scholar] [CrossRef]
- Miller, H.; Miller, B.; Ryan, S.J. The role of retinal pigment epithelium in the involution of subretinal neovascularization. Investig. Ophthalmol. Vis. Sci. 1986, 27, 1644–1652. [Google Scholar]
- Chan, C.M.; Huang, J.H.; Chiang, H.S.; Wu, W.B.; Lin, H.H.; Hong, J.Y.; Hung, C.F. Effects of (–)-epigallocatechin gallate on RPE cell migration and adhesion. Mol. Vis. 2010, 16, 586–595. [Google Scholar]
- Chan, C.M.; Chang, H.H.; Wang, V.C.; Huang, C.L.; Hung, C.F. Inhibitory effects of resveratrol on PDGF-BB-induced retinal pigment epithelial cell migration via PDGFRbeta, PI3K/Akt and MAPK pathways. PLoS One 2013, 8, e56819. [Google Scholar]
- Takahashi, K.; Itagaki, T.; Yamagishi, K.; Ohkuma, H.; Uyama, M. [A role of the retinal pigment epithelium in the involution of subretinal neovascularization]. Nippon Ganka Gakkai Zasshi 1990, 94, 340–351. [Google Scholar]
- Sasaki, K.; Sato, M.; Umezawa, Y. Fluorescent indicators for Akt/protein kinase B and dynamics of Akt activity visualized in living cells. J. Biol. Chem. 2003, 278, 30945–30951. [Google Scholar]
- Shiraishi, I.; Melendez, J.; Ahn, Y.; Skavdahl, M.; Murphy, E.; Welch, S.; Schaefer, E.; Walsh, K.; Rosenzweig, A.; Torella, D.; et al. Nuclear targeting of Akt enhances kinase activity and survival of cardiomyocytes. Circ. Res. 2004, 94, 884–891. [Google Scholar] [CrossRef]
- Tsujita, Y.; Muraski, J.; Shiraishi, I.; Kato, T.; Kajstura, J.; Anversa, P.; Sussman, M.A. Nuclear targeting of Akt antagonizes aspects of cardiomyocyte hypertrophy. Proc. Natl. Acad. Sci. USA 2006, 103, 11946–11951. [Google Scholar]
- Bijur, G.N.; Jope, R.S. Rapid accumulation of Akt in mitochondria following phosphatidylinositol 3-kinase activation. J. Neurochem. 2003, 87, 1427–1435. [Google Scholar] [CrossRef]
- Yang, J.Y.; Deng, W.; Chen, Y.; Fan, W.; Baldwin, K.M.; Jope, R.S.; Wallace, D.C.; Wang, P.H. Impaired translocation and activation of mitochondrial Akt1 mitigated mitochondrial oxidative phosphorylation Complex V activity in diabetic myocardium. J. Mol. Cell. Cardiol. 2013, 59, 167–175. [Google Scholar]
- Lai, H.C.; Liu, T.J.; Ting, C.T.; Sharma, P.M.; Wang, P.H. Insulin-like growth factor-1 prevents loss of electrochemical gradient in cardiac muscle mitochondria via activation of PI 3 kinase/Akt pathway. Mol. Cell. Endocrinol. 2003, 205, 99–106. [Google Scholar] [CrossRef]
- Ikuno, Y.; Kazlauskas, A. An in vivo gene therapy approach for experimental proliferative vitreoretinopathy using the truncated platelet-derived growth factor alpha receptor. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2406–2411. [Google Scholar]
- Zheng, Y.; Ikuno, Y.; Ohj, M.; Kusaka, S.; Jiang, R.; Cekic, O.; Sawa, M.; Tano, Y. Platelet-derived growth factor receptor kinase inhibitor AG1295 and inhibition of experimental proliferative vitreoretinopathy. Jpn. J. Ophthalmol. 2003, 47, 158–165. [Google Scholar]
- Krinsky, N.I.; Landrum, J.T.; Bone, R.A. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu. Rev. Nutr. 2003, 23, 171–201. [Google Scholar] [CrossRef]
- Hu, B.J.; Hu, Y.N.; Lin, S.; Ma, W.J.; Li, X.R. Application of Lutein and Zeaxanthin in nonproliferative diabetic retinopathy. Int. J. Ophthalmol. 2011, 4, 303–306. [Google Scholar]
- Murthy, R.K.; Ravi, K.; Balaiya, S.; Brar, V.S.; Chalam, K.V. Lutein protects retinal pigment epithelium from cytotoxic oxidative stress. Cutan. Ocul. Toxicol. 2013, 33, 132–137. [Google Scholar]
- Aimjongjun, S.; Sutheerawattananonda, M.; Limpeanchob, N. Silk lutein extract and its combination with vitamin E reduce UVB-mediated oxidative damage to retinal pigment epithelial cells. J. Photochem. Photobiol. B Biol. 2013, 124, 34–41. [Google Scholar] [CrossRef]
- Lo, H.M.; Tsai, Y.J.; Du, W.Y.; Tsou, C.J.; Wu, W.B. A naturally occurring carotenoid, lutein, reduces PDGF and H(2)O(2) signaling and compromised migration in cultured vascular smooth muscle cells. J. Biomed. Sci. 2012, 19, 18. [Google Scholar] [CrossRef]
- Reynoso-Camacho, R.; Gonzalez-Jasso, E.; Ferriz-Martinez, R.; Villalon-Corona, B.; Loarca-Pina, G.F.; Salgado, L.M.; Ramos-Gomez, M. Dietary supplementation of lutein reduces colon carcinogenesis in DMH-treated rats by modulating K-ras, PKB, and beta-catenin proteins. Nutr. Cancer 2011, 63, 39–45. [Google Scholar]
- Lee, D.K.; Grantham, R.N.; Mannion, J.D.; Trachte, A.L. Carotenoids enhance phosphorylation of Akt and suppress tissue factor activity in human endothelial cells. J. Nutr. Biochem. 2006, 17, 780–786. [Google Scholar]
- Ji, W.T.; Liu, H.J. PI3K-akt signaling and viral infection. Recent Pat. Biotechnol. 2008, 2, 218–226. [Google Scholar]
- Nikolaus, P.; Andreas, G. Versatility of the mitochondrial protein import machinery. Nat. Rev. Mol. Cell Biol. 2001, 2, 339–349. [Google Scholar] [CrossRef]
- Wu, W.C.; Chang, Y.C.; Wu, K.Y.; Chen, S.Y.; Hsieh, M.C.; Wu, M.H.; Wu, H.J.; Wu, W.S.; Kao, Y.H. Pharmacological implications from the adhesion-induced signaling profiles in cultured human retinal pigment epithelial cells. Kaohsiung J. Med. Sci. 2014, 30, 1–11. [Google Scholar]
- Mookherjee, P.; Quintanilla, R.; Roh, M.S.; Zmijewska, A.A.; Jope, R.S.; Johnson, G.V.W. Mitochondrial-targeted active akt protects SH-SY5Y neuroblastoma cells from staurosporine-induced apoptotic cell death. J. Cell. Biochem. 2007, 102, 196–210. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Su, C.-C.; Chan, C.-M.; Chen, H.-M.; Wu, C.-C.; Hsiao, C.-Y.; Lee, P.-L.; Lin, V.C.-H.; Hung, C.-F. Lutein Inhibits the Migration of Retinal Pigment Epithelial Cells via Cytosolic and Mitochondrial Akt Pathways (Lutein Inhibits RPE Cells Migration). Int. J. Mol. Sci. 2014, 15, 13755-13767. https://doi.org/10.3390/ijms150813755
Su C-C, Chan C-M, Chen H-M, Wu C-C, Hsiao C-Y, Lee P-L, Lin VC-H, Hung C-F. Lutein Inhibits the Migration of Retinal Pigment Epithelial Cells via Cytosolic and Mitochondrial Akt Pathways (Lutein Inhibits RPE Cells Migration). International Journal of Molecular Sciences. 2014; 15(8):13755-13767. https://doi.org/10.3390/ijms150813755
Chicago/Turabian StyleSu, Ching-Chieh, Chi-Ming Chan, Han-Min Chen, Chia-Chun Wu, Chien-Yu Hsiao, Pei-Lan Lee, Victor Chia-Hsiang Lin, and Chi-Feng Hung. 2014. "Lutein Inhibits the Migration of Retinal Pigment Epithelial Cells via Cytosolic and Mitochondrial Akt Pathways (Lutein Inhibits RPE Cells Migration)" International Journal of Molecular Sciences 15, no. 8: 13755-13767. https://doi.org/10.3390/ijms150813755



